 |
 |
| Plant Pathol J > Volume 38(3); 2022 > Article |
|
Abstract
Fusarium incarnatum-equiseti species complex (FIESC) contain over 40 members. The primer pair Smibo1FM/Semi1RM on the RPB2 partial gene has been reported to be able to identify Fusarium semitectum. The F. fujikuroi species complex (FFSC) contains more than 50 members. The F. verticillioides as a member of this complex can be identified by using VER1/VER2 primer pair on the CaM partial gene. In this research, the Smibo1FM/Semi1RM can amplify F. sulawesiense, F. hainanense, F. bubalinum, and F. tanahbumbuense, members of FIESC at 424 bp. The VER1/VER2 can amplify F. verticillioides, F. andiyazi, and F. pseudocircinatum, members of FFSC at 578 bp. Polymerase chain reaction-restriction fragment length polymorphism by using the combination of three restriction enzymes EcoRV, MspI, and HpyAV can differentiate each species of FIESC used. The two restriction enzymes HpaII and NspI can distinguish each species of FFSC used. The proper identification process is required for pathogen control in the field in order to reduce crop yield loss.
Fusarium is a genus known to cause several plant diseases (Leslie and Summerell, 2006). Over the last 100 years, the Fusarium taxonomy has undergone a variety of modifications. The Fusarium incarnatum-equiseti species complex (FIESC) comprises 33 phylospecies and over 40 species members. This species complex is still being developed (Xia et al., 2019). The F. fujikuroi species complex (FFSC) has more than 50 members with a wide host range (OŌĆÖDonnell et al., 2015). Morphological data are insufficient and unreliable as a basis for the identification of a species, as they can lead to incorrect identification. Molecular techniques must be implemented to accurately identify pathogenic fungi up to phylogenetic levels. Molecular observations are needed for fungal identification. This method can be employed in various ways, including using polymerase chain reaction (PCR), species-specific primers, DNA sequencing, or PCR-restriction fragment length polymorphism (RFLP). Therefore, research on accurate identification methods are required.
The molecular identification performed using PCR by species-specific primer becomes an efficient method. Its high sensitivity enables the pathogen to be directly detected even though the fungal mycelia in complex mixtures and invisible under the microscope (Jurado et al., 2006). Mul├© et al. (2004) has designed a species-specific assay based on calmodulin partial (CaM) gene for the identification of F. verticilloides, F. proliferatum, and F. subglutinans which have been widely used by the researchers across the world. Hong et al. (2010) designed Smibo1FM/Semi1RM primer pair for molecular identification of F. semitectum (=F. incarnatum) as quarantine fungal species in Korea. There have been no other reports on specific primers that can identify each species specifically.
The PCR-RFLP has been used to detect intraspecies and interspecies variations. PCR-RFLP is the amplification of the fragment containing the variation (Rasmussen, 2012). This technique involves treating the amplified fragment with an appropriate restriction enzyme (Rasmussen, 2012). The restriction analysis of PCR-RFLP is an appropriate method for taxonomic studies in Fusarium spp. (Konstantinova and Yli-Mattila, 2004; Nicholson et al., 1993). The PCR-RFLP technique has been used to distinguish members of F. graminearum species complex (Hafez et al., 2020; Suga et al., 2008), members of FFSC (Suga et al., 2014), and has also been used to study genetic diversity in F. oxysporum f. sp. fragariae (Kim et al., 2017).
The PCR-RFLP method can be used to group fungal types or select genetic characteristic-based isolates (Diguta et al., 2011). This method began with the amplification of DNA from the gene target area using a PCR machine and then moved on to the RFLP method of cutting DNA amplicons using restriction enzymes (Diguta et al., 2011). The PCR-RFLP technique produces high polymorphism in specific fungal isolates, such as Fusarium, by using species-specific markers (Datta et al., 2011). This marker has also been presented to be inexpensive, simple to use, and does not take a long time to investigate the diversity of fungal species through the fungal isolation process (Diguta et al., 2011) or without the isolation stage (Viaud et al., 2000).
Molecular diagnostics are also important for an accurate identification of pathogens. Therefore, the authors hypothesized that the PCR-RFLP technique would accurately identify FIESC and FFSC members. The objectives of this study are to (1) generate molecular amplification using species-specific primer, and (2) develop a PCR-RFLP technique for identifying several member of FIESC and FFSC. The findings of the research will form the basis for appropriate pathogen control measures in the future.
A total of 27 isolates of FIESC and FFSC were procured from previous research by Pramunadipta et al. (2022). The authors also used the Northern Regional Research Laboratory (NRRL) 22172 Agricultural Research Service Culture Collection isolate as a positive control for F. verticillioides (Table 1). The Fusarium spp. isolates were grown on potato dextrose broth at 25┬░C for 3-4 days. The mycelia were air-dried for 24 h before being powdered with sterilized small steel wire with a vortex in the microtube. The mycelial powder was mixed with 300 ╬╝l of potassium ethyl xanthogenate solution and then incubated at 60┬░C for 30 min as previously described (Suga et al., 2008). The final DNA pellet was resuspended in 400 ╬╝l of water.
The PCR was used to amplify the RPB2 gene region of the FIESC using the Smibo1FM (5ŌĆ▓-GCAAAAGCCTCTCGCCAC-3ŌĆ▓) and Semi1RM (5ŌĆ▓-AGGTGTAGAGATATCGCGG-3ŌĆ▓) primer pair (Hong et al., 2010). CaM gene region of FFSC was amplified using the VER 1 (5ŌĆ▓-CTTCCTGCGATGTTTCTCC-3ŌĆ▓) and VER 2 (5ŌĆ▓-AATTGGCCATTGGTATTATATATCTA-3ŌĆ▓) primer pair (Mul├© et al., 2004). Reactions were performed in the BioRad T100 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under the following PCR conditions: (1) initial denaturation at 94┬░C for 2 min; (2) 30 cycles of denaturation at 94┬░C for 1 min; (3) annealing at 61┬░C for 1 min for Smibo1FM/Semi1RM primer pair; (4) extension at 72┬░C for 2 min; (5) final extension at 72┬░C for 7; and (6) cooling process at 15┬░C. The PCR conditions for the VER1/VER2 primer pair were the same as for the Smibo1FM/Semi1RM primer pair, with the exception that the annealing temperature was set to 56┬░C for 1 min. The PCR products were then visualized using 1% agarose gel (Seakem GTG agarose, Lonza Bioscience, Basel, Switzerland) together with a 100-bp ladder (Geneaid Biotech Ltd, New Taipei City, Taiwan) as a standard size at 100 V for 35 min. Agarose gel was stained with an ethidium bromide solution for 10 min and then visualized under UV light. DNA purification for representative isolates was conducted by using PCR Clean-up Gel Extraction Nucleospin Extract II (Macherey-Nagel, D├╝ren, Germany). The sequence mix was sent to the Life Science Research Center, Gifu University, Japan for sequencing. Sequences were obtained using the ABI PRISM 3100 Genetic Analyzer (Hitachi, Ltd., Tokyo, Japan) as previously described (Suga et al., 2008). Sequences were then deposited in the GenBank with accession number (MW246174-MW246179).
The results of the partial RPB2 gene and partial CaM gene sequences were then aligned using MEGA X software (Kumar et al., 2018). A group of restriction enzymes were selected by computational PCR-RFLP analysis based on the discrimination of numerous species using Genetyx version 4.0 (Genetyx, Tokyo, Japan). The sequences were then subjected to in-silico RFLP analysis using the pDRAW32 DNA analysis software (http://www.acaclone.com/) and the restriction maps were drawn using Photoshop 2020 software. The PCR-RFLP was performed using Smibo1FM/Semi1RM and VER1/VER2 primer pairs. The total volume of the reaction mixture was 20 ╬╝l as previously described (Suga et al., 2008). Cycling parameters used same as above with 35 cycles of denaturation, annealing and extension. The PCR amplicons of 424 bp for RPB2 gene and 578 bp amplicons for CaM gene were confirmed by subjecting 5 ╬╝l of the PCR mixture to 1% agarose gel electrophoresis. The remaining 10 ╬╝l of PCR mixture was digested with 0.5 units of EcoRV, MspI, and HpyAV (New England Biolabs, Ipswich, MA, USA) for RPB2 gene and HpaII and NspI for CaM gene in each 15 ╬╝l reaction volume. The mixtures were incubated for 1 h at 37┬░C and then visualized using electrophoresis in Metaphor agarose gel (Cambrex Bio Science, Rockland, ME, USA) together with a 100-bp ladder (New England Biolabs) as a standard size.
A total of 22 FIESC isolates were used for confirmation of developing PCR-RFLP. PCR amplification for F. sulawesiense, F. hainanense, F. bubalinum, and F. tanahbumbuense by using Smibo1FM/Semi1RM primer pair had amplification at 424 bp (Fig. 1A). The representative of FIESC isolates were then sequenced by using Smibo1FM/Semi1RM primer pair. Different results were shown in the DNA base sequence for the Smibo1FM/Semi1RM primer pair sequencing results, indicating that each fungal species differs. This primer encodes the RPB2 region in FIESC and can be used as a starting point for fungal identification PCR-RFLP design (Fig. 2A).
Three combination of restriction enzymes, i.e., EcoRV, MspI, and HpyAV tested in each FIESC species, showed four types of fragment patterns by in-silico RFLP analysis (Fig. 2B and C). The result of PCR-RFLP amplification in laboratory using combination of three restriction enzyme, showed same pattern as in-silico RFLP analysis (Fig. 2D). F. sulawesiense showed pattern I, F. hainanense showed pattern II, F. bubalinum showed pattern III, and F. tanahbumbuense showed pattern IV (Tables 1 and 2, Fig. 2D). PCR-RFLP fragment between F. bubalinum and F. tanahbumbuense looked the same, but there were differences in some fragments. One of F. bubalinum fragment pattern showed at 141 bp, but not showed at 87-bp fragment. Meanwhile, the one of F. tanahbumbuense fragment pattern showed at 87 bp, but not showed at 141-bp fragment (Table 2, Fig. 2C and D). PCR-RFLP fragments under 100 bp were showed in the F. sulawesiense, F. hainanense, F. bubalinum, and F. tanahbumbuense. The fragments that appear are fragments that support fragments above 100 bp. In this study, PCR-RFLP fragments above 100 bp are enough to distinguish between species in the FIESC.
The specific primer (Smibo1FM/Semi1RM) developed for the identification of F. semitectum by Hong et al. (2010) was found to be non-specific in this study, as this primer also showed amplification in F. sulawesiense, F. hainanense, F. bubalinum, and F. tanahbumbuense. The PCR-RFLP method by using this primer pair and the combination of three restriction enzymes above can display specific patterns in each species. These results indicate that this method can be used as an alternative to identify those members of the FIESC.
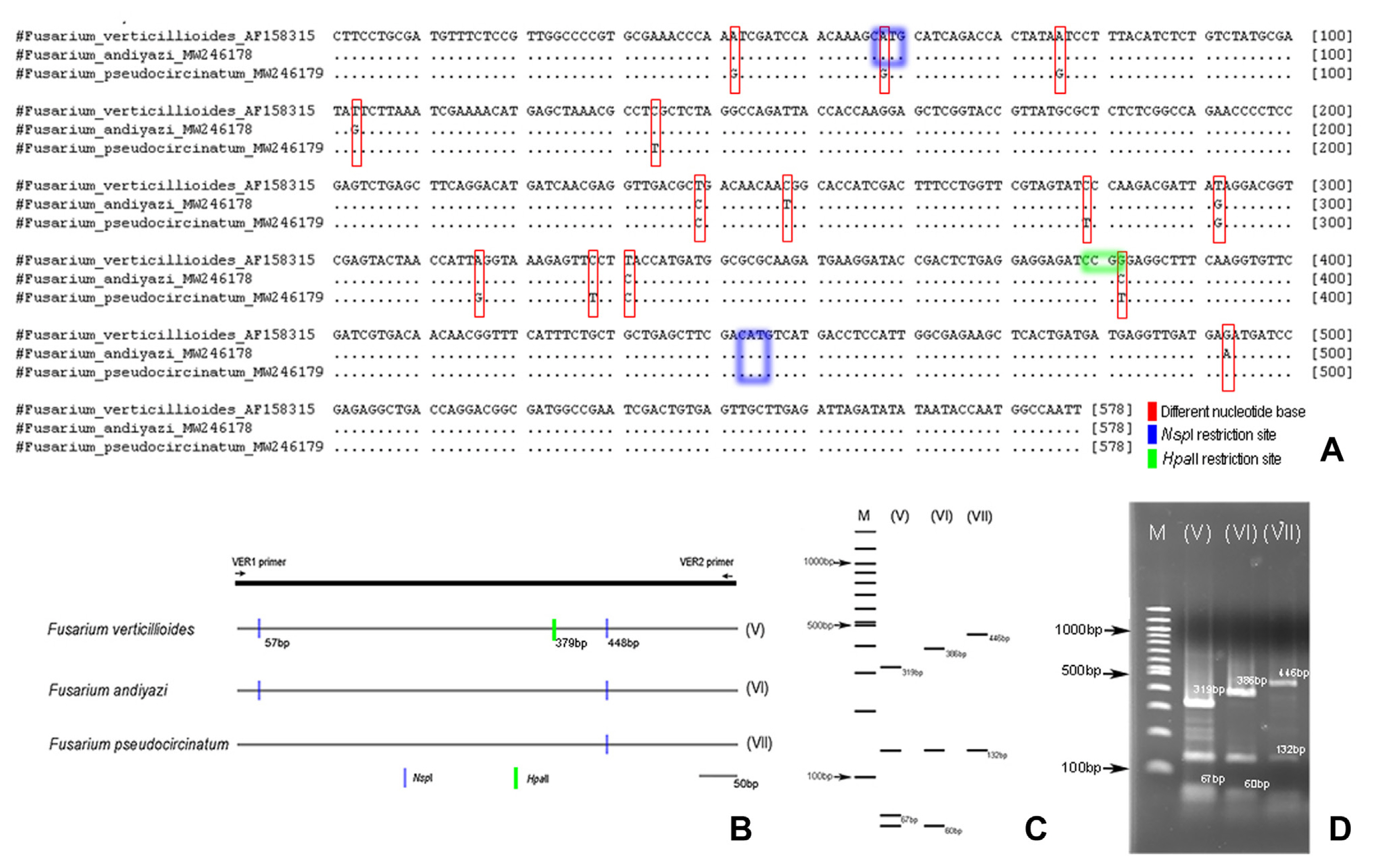
A total of 6 FFSC isolates were used for developing PCR-RFLP. PCR amplification for F. verticillioides, F. andiyazi, and F. pseudocircinatum by using VER1/VER2 primer pair amplified at 578 bp (Fig. 1B). PCR products of the representative of FFSC isolates were then sequenced. The differentiated results were shown in the DNA base sequence for the VER1/VER2 primer pair sequencing results, indicate that each fungal species differed. This primer was used to amplify the partial gene of the calmodulin (CaM) gene region, and could be used as a starting point for fungal identification PCR-RFLP design (Fig. 3A).
Two combination of restriction enzyme i.e., HpaII and NspI in each tested FFSC isolate showed three specific patterns by using in-silico RFLP analysis (Fig. 3B and C). The result of PCR-RFLP amplification using combination of two restriction enzyme for each species showed the same patten as that of the in-silico RFLP analysis (Fig. 3D). F. verticillioides showed pattern V, F. andiyazi showed pattern VI, F. pseudocircinatum showed pattern VII (Tables 1 and 2, Fig. 3D). PCR-RFLP fragments between F. verticillioides, F. andiyazi, and F. pseudocircinatum produced same fragment on 132 bp, but difference for other fragments. F. verticillioides fragment showed at 319 bp, F. andiyazi fragment showed at 386 bp, while F. pseudocircinatum fragment showed at 446 bp (Fig. 3C and D). F. verticillioides and F. andiyazi had PCR-RFLP fragments under 100 bp. The fragments that appear are those that support fragments above 100 bp. In this study, PCR-RFLP fragments above 100 bp were found to be sufficient for distinguishing between species in the FFSC. The specific primers (VER1/VER2) designed for the detection of F. verticilloides by Mul├© et al. (2004) was shown to also provide specific amplification in F. andiyazi and F. pseudocircinatum. These three species were further distinguished using the PCR-RFLP method, which included the use of the VER1/VER2 primer pair and the use of the two restriction enzymes mentioned above to produce different pattern in each species. These findings suggest that this method can be used to identify FFSC members as an alternative method.
Fusarium spp. needed to be identified using molecular techniques (Pramunadipta et al., 2022). The use of a species-specific primer to identify microbes does not always produce accurate results. One of the most important properties of a primer is its target specificity (Ye et al., 2012). In an ideal situation, a primer pair would only amplify the intended target and not the unintended ones. It seems to be unusual for a primer pair designed for one target to bind to another, resulting in non-specific target amplification (Ye et al., 2012). Based on the findings of this study, the PCR-RFLP method can be used to correctly identify fungi as an alternative method. This technique needs to be improved so that the fungi species used in research with other fungi can be identified. This technique needs to be improved in order to identify the fungi species used in research with other fungi.
Acknowledgments
This research was conducted as part of Syafiqa PramunadiptaŌĆÖs doctoral studies at Life Science Research Center, Gifu University as part of the ŌĆ£6-months Sandwich Program in UGSAS-GU 2019-2020ŌĆØ in research collaboration. The assistance of Mrs. Tomomi Katsu and Mrs. Ayako Usui are gratefully acknowledged. This research also funded by RTA UGM 2020.
Fig.┬Ā1
Polymerase chain reaction amplification of Fusarium spp. with Smibo1FM/Semi1RM primer pair (A) and VER1/VER2 primer pair (B). Lane: I, F. sulawesiense; II, F. hainanense; III, F. bubalinum; IV, F. tanahbumbuense; V, F. verticillioides; VI, F. andiyazi; VII, F. pseudocircinatum; N, water; M, 100 bp ladder (Geneaid).
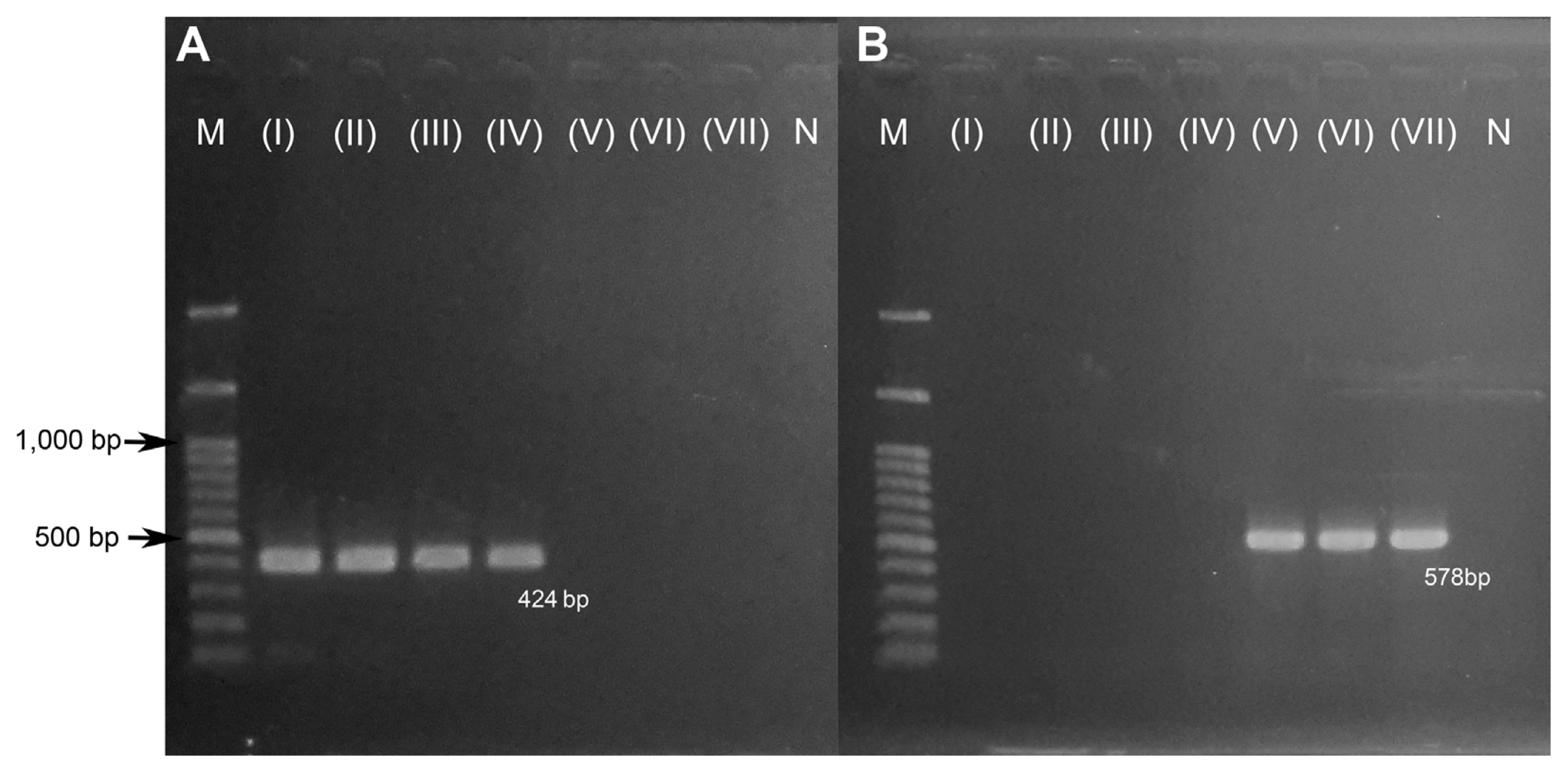
Fig.┬Ā2
Analysis of polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) by using Smibo1FM/Semi1RM primer pair. (A) Sequencing result of Smibo1FM/Semi1RM primer pair and restriction sites of MspI, HpyAV, and EcoRV. (B) Restriction map of three restriction enzymes combination. (C) Gel electropherograms of PCR-RFLP in-silico. (D) Gel electropherograms of PCR-RFLP in laboratory. Lane M, 100 bp ladder (NEB).
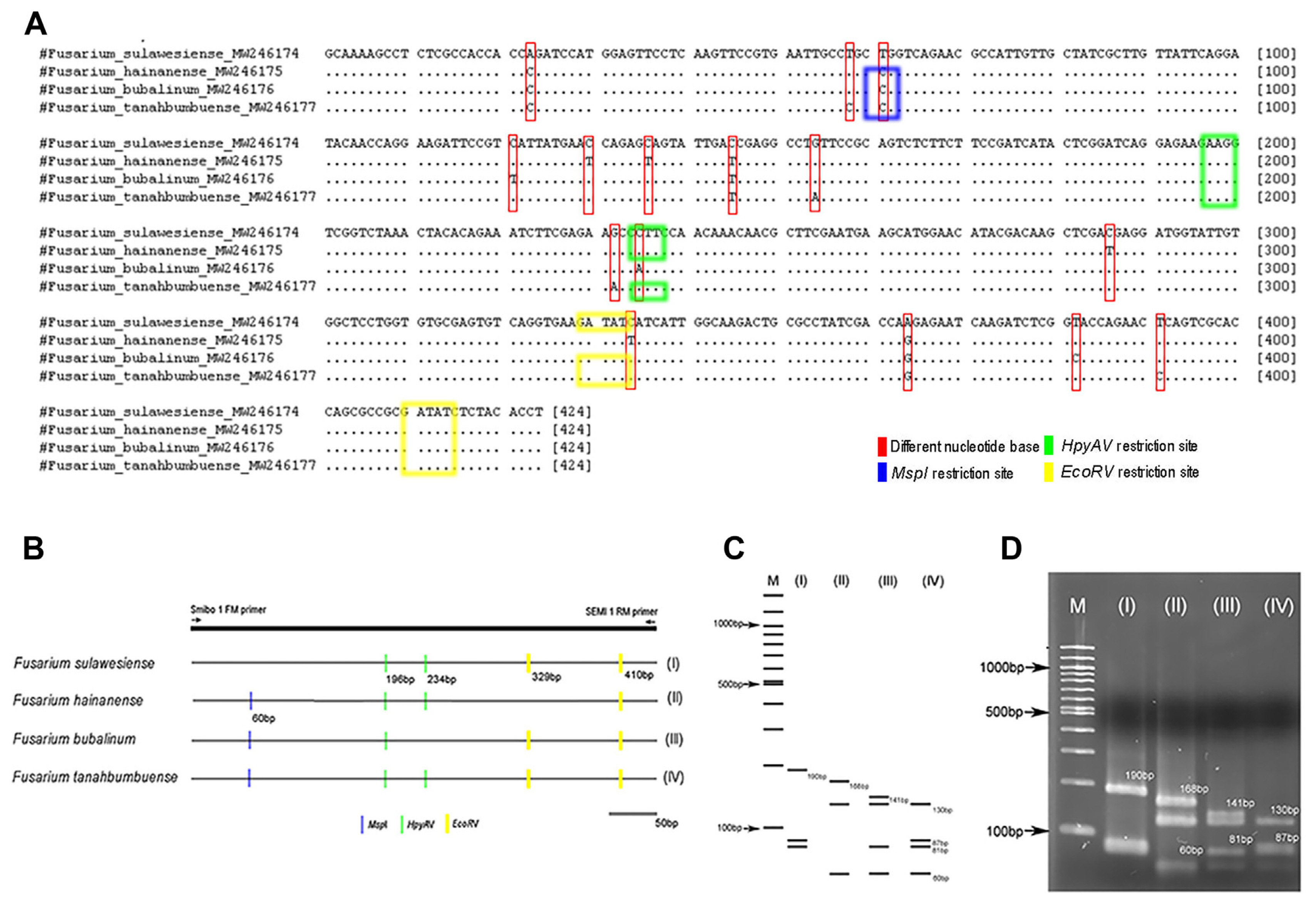
Fig.┬Ā3
Analysis of polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) by using VER1/VER2 primer pair. (A) Sequencing result of VER1/VER2 primer pair and restriction sites of NspI and HpaII. (B) Restriction map of two restriction enzymes combination. (C) Gel electropherograms of PCR-RFLP in-silico. (D) Gel electropherograms of PCR-RFLP in laboratory. Lane M, 100 bp ladder (NEB).
Table┬Ā1
Isolates of the Fusarium spp. used for development of PCR-RFLP technique
| Species | Isolate codea | Host plant | Geographic origin | TEF 1-╬▒ accession no.a | PCR-RFLP pattern | |
|---|---|---|---|---|---|---|
|
|
||||||
| RPB2, EcoRV, MspI, and HpyAVb,c | CaM, HpaII, and NspIb,c | |||||
| Fusarium incarnatum-equiseti species complex | ||||||
| ŌĆāF. sulawesiense | LP 3 | Oryza sativa | Indonesia | MT138454 | I (MW246174) | - |
| JTG 1 | MT138455 | I | - | |||
| JTM 35 | MT138456 | I | - | |||
| DIY 9 | MT138457 | I | - | |||
| NTB 1 | MT138458 | I | - | |||
| NTT 2 | MT138459 | I | - | |||
| ŌĆāF. hainanense | SMU 3 | MT138469 | II (MW246175) | - | ||
| SMU 11 | MT138470 | II | - | |||
| SMU 24 | MT138471 | II | - | |||
| LP 2 | MT138472 | II | - | |||
| BTN 4 | MT138473 | II | - | |||
| JBR 10 | MT138474 | II | - | |||
| DIY 7 | MT138475 | II | - | |||
| ŌĆāŌĆāF. bubalinum | SMB 1 | MT138461 | III (MW246176) | - | ||
| SMB 2 | MT138462 | III | - | |||
| JBR 5 | MT138463 | III | - | |||
| JBR 13 | MT138464 | III | - | |||
| JTG 14 | MT138465 | III | - | |||
| JTM 10 | MT138466 | III | - | |||
| SL 3 | MT138467 | III | - | |||
| SL 4 | MT138468 | III | - | |||
| ŌĆāF. tanahbumbuense | NTT 6 | MT138460 | IV (MW246177) | - | ||
|
|
||||||
| Fusarium fujikuroi species complex | ||||||
| ŌĆāF. andiyazi | JBR B | O. sativa | Indonesia | MT138495 | - | VI (MW246178) |
| ŌĆāF. pseudocircinatum | SMU W | MT138489 | - | VII | ||
| BL O | MT138490 | - | VII (MW246179) | |||
| BL AA | MT138491 | - | VII | |||
| BL AB | MT138492 | - | VII | |||
| ŌĆāF. verticillioides | NRRL22172d | Zea mays | Germany | AF160262 | - | V (AF158315) |
a Isolate and GenBank accession number derived from Pramunadipta et al. (2022).
Table┬Ā2
Size of fragments expected for PCR-RFLP developed in this study
References
Datta, S., Choudhary, R. G., Shamim, M. and Dhar, V. 2011. Polymorphism in the internal transcribed spacer (ITS) region of the ribosomal DNA among different Fusarium species. Arch. Phytopathol. Plant Prot. 44:558-566.

Diguta, C. F., Vincent, B., Guilloux-Benatier, M., Alexandre, H. and Rousseaux, S. 2011. PCR ITS-RFLP: a useful method for identifying filamentous fungi isolates on grapes. Food Microbiol. 28:1145-1154.


Hafez, M., Abdelmagid, A., Adam, L. R. and Daayf, F. 2020. Specific detection and identification of Fusarium graminearum sensu stricto using a PCR-RFLP tool and specific primers targeting the translational elongation factor 1╬▒ gene. Plant Dis. 104:1076-1086.


Hong, S.-Y., Kang, M. R., Cho, E.-J., Kim, H.-K. and Yun, S.-H. 2010. Specific PCR detection of four quarantine Fusarium species in Korea. Plant Pathol. J. 26:409-416.

Jurado, M., V├Īzquez, C., Mar├Łn, S., Sanchis, V. and Gonz├Īlez-Ja├®n, M. T. 2006. PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in maize. Syst. Appl. Microbiol. 29:681-689.


Kim, J.-S., Kang, N. J., Kwak, Y.-S. and Lee, C. 2017. Investigation of genetic diversity of Fusarium oxysporum f. sp. fragariae using PCR-RFLP. Plant Pathol. J. 33:140-147.



Konstantinova, P. and Yli-Mattila, T. 2004. IGS-RFLP analysis and development of molecular markers for identification of Fusarium poae, Fusarium langsethiae, Fusarium sporotrichioides and Fusarium kyushuense. Int. J. Food Microbiol. 95:321-331.


Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35:1547-1549.



Leslie, J. F. and Summerell, B. A. 2006. The Fusarium Laboratory Manual. Blackwell Publishing, Iowa, USA. pp. 388.
Mul├©, G., Susca, A., Stea, G. and Moretti, A. 2004. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 110:495-502.

Nicholson, P., Jenkinson, P., Rezanoor, H. N. and Parry, D. W. 1993. Restriction fragment length polymorphism analysis of variation in Fusarium species causing ear blight of cereals. Plant Pathol. 42:905-914.

OŌĆÖDonnell, K., Ward, T. J., Robert, V. A. R. G., Crous, P. W., Geiser, D. M. and Kang, S. 2015. DNA sequence-based identification of Fusarium: current status and future directions. Phytoparasitica 43:583-595.


Pramunadipta, S., Widiastuti, A., Wibowo, A., Suga, H. and Priyatmojo, A. 2022. Identification and pathogenicity of Fusarium spp. associated with the sheath rot disease of rice (Oryza sativa) in Indonesia. J. Plant Pathol. 104:251-267.


Rasmussen, H. B. 2012. Restriction fragment length polymorphism analysis of PCR-amplified fragments (PCR-RFLP) and gel electrophoresis: valuable tool for genotyping and genetic fingerprinting. In: Gel electrophoresis: principles and basics, eds. by S. Magdeldin, pp. 315-334. InTech Europe, Rijeka, Croatia.
Suga, H., Karugia, G. W., Ward, T., Gale, L. R., Tomimura, K., Nakajima, T., Miyasaka, A., Koizumi, S., Kageyama, K. and Hyakumachi, M. 2008. Molecular characterization of the Fusarium graminearum species complex in Japan. Phytopathology 98:159-166.


Suga, H., Kitajima, M., Nagumo, R., Tsukiboshi, T., Uegaki, R., Nakajima, T., Kushiro, M., Nakagawa, H., Shimizu, M., Kageyama, K. and Hyakumachi, M. 2014. A single nucleotide polymorphism in the translation elongation factor 1╬▒ gene correlates with the ability to produce fumonisin in Japanese Fusarium fujikuroi. Fungal Biol. 118:402-412.


Viaud, M., Pasquier, A. and Brygoo, Y. 2000. Diversity of soil fungi studied by PCR-RFLP of ITS. Mycol. Res. 104:1027-1032.

- TOOLS
-
METRICS

- ORCID iDs
-
Achmadi Priyatmojo

https://orcid.org/0000-0002-8260-6481 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



