 |
 |
| Plant Pathol J > Volume 38(2); 2022 > Article |
|
Abstract
Ceratocystis wilt disease has caused significant mortality in duku (Lansium domesticum) since 2014 and has now spread to all districts in South Sumatra, Indonesia. Recently, 16 isolates from duku representing populations from various districts in South Sumatra were isolated. Analysis for the morphological characteristic of the isolate showed that the population has a uniform morphology. Genetic analysis based on internal transcribed spacer (ITS) and β-tubulin sequences verified that the population has being dominated by the ITS5 haplotype of Ceratocystis fimbriata and a new ITS group, the ITS7b haplotype that was localized in Musi Banyuasin. Both haplotypes were highly pathogenic to duku. Inoculation tests on various forest and agroforestry plant hosts showed that both haplotypes were highly pathogenic to Acacia mangium, moderately pathogenic to Acacia carsicarpa, Eucalyptus urophylla, and Melaleuca cajuputi, but weakly pathogenic to Dyera costulata, Hevea brasiliensis, and Alstonia scholaris. Therefore, this pathogen becomes a serious threat to Indonesia’s biodiversity due to its ability to infect forest and agroforestry plants, especially the indigenous ones.
Lansium domesticum belongs to the Meliaceae family and is native to Southeast Asia. In Indonesia, this fruit is called duku (South Sumatra) and langsat (West Kalimantan) (Hanum et al., 2013), ceroring (Bali), dookkoo (Java, Sumatra), and duki (Lim, 2011). Furthermore, it is one of the leading commodity plants and the mascot of flora in South Sumatra, widely known in Indonesia as “duku Palembang or duku Komering” (Rupiah et al., 2018). The central production of L. domesticum in Indonesia is the province of South Sumatra after which it is distributed to various districts, such as Ogan Komering Ulu, East Ogan Komering Ulu, South Ogan Komering Ulu, Ogan Komering Ilir, Muara Enim, Musi Banyuasin, Musi Rawas, and North Musi Rawas.
Additionally, the fruit has high economic value because the selling price is quite expensive and it is liked by the public for its fresh sweet, and very delicious taste. Also, it has other benefits, which include being an ingredient in cancer prevention (Matsumoto and Watanabe, 2020; Tilaar et al., 2008) with the discovery of new compounds in the peel, namely 3-hydroxy-8,14-secogammacera-7, and 14-dien-21-one that exhibits cytotoxic activity that attenuates the MCF-7 breast cancer cell line (Zulfikar et al., 2020). L. domesticum Corr. has also been reported to have benefits as larvicides (Ni’mah et al., 2015; Putranta and Wijaya, 2017), antitumor, anticancer (Khalili et al., 2017), antimalarial, antimelanogenesis, antibacterial, antimutagenic (Hanum et al., 2013), prebiotic Bifidobacteria spp. (Norhayati et al., 2016), organic catalyst (Nishizawa et al., 2010), and cosmetic ingredient due to its antioxidant properties (Subandrate et al., 2016; Tilaar et al., 2008).
Previous studies conducted from 2014 to 2017 (Suwandi et al., 2021) showed that a very severe wilt disease of duku was first discovered in Ogan Komering Ulu District in three locations/villages, namely Belatung, Lubuk Batang Baru, and Lubuk Batang Lama. The death symptoms of the disease of Ceratocystis are characterized by wilting of part or the whole tree, whereby the branches and eventually the entire plant dies. Therefore, this study aims to examine the spread of this disease from the original area to all duku plantation centers in various districts in South Sumatra and the genetic diversity of the pathogen causing it.
Ceratocystis is a pathogen that attacks various plant species, including Acacia mangium and Acacia crassicarpa as its original host (Tarigan et al., 2010), Eucalyptus spp. (Harrington et al., 2014), Mangifera indica (Al Adawi et al., 2013), Dalbergia tonkinensis, and Chukrasia tabularis (Chi et al., 2019a, 2020), Albizia lebbeck (Razzaq et al., 2020), and others. Since the host plant of Ceratocystis is widely spread, and the duku is located around the forest, it is very important to consider the host plants of Ceratocystis that have economic value, such as Acacia carsicarpa, Eucalyptus urophylla, Dyera costulata, Alstonia scholaris, Hevea brasiliensis, and Melaleuca cajuputi. Therefore, this study aims to determine the distribution of disease in various duku production centers in South Sumatra, genetic variation, and host range in forest and agroforestry plants.
Between 2019 to 2021, incidences with disease trees were observed in eight duku plantations in Ogan Komering Ulu District, four in South Ogan Komering Ulu, one in East Ogan Komering Ulu, six in Musi Banyuasin, five in North Musi Rawas, three in Musi Rawas, three in Ogan Komering Ilir, and one in Muara Enim, South Sumatra (Fig. 1). In each plantation, five plots with a size of 10 × 10 m were selected from the center of the diseased tree (Pratama et al., 2021b; Suwandi et al., 2021). Furthermore, the trees are declared infected if some branches or stems show symptoms of the disease. As a result of this, five diseased duku trees were randomly selected from the affected plantations to be isolated in the laboratory.
Isolates were collected from fresh wounds of L. domesticum which showed symptoms of branch wilting, discoloration of vascular tissue, and dead plants caused by Ceratocystis. Furthermore, the samples were performed by making an incision in the bark and cutting a tangential longitudinal section (approximately 50 mm) of the newly infected xylem with the stain. The duku plants which were collected as samples were around 10 to 100 years old, and are therefore prone to infection in the plantation. Symptoms of wilt disease were evaluated as follows, the extent of lesion progression from discoloration of bark and wood, presence of sap flow from the surface of the lesion, the extent of leaf wilting or shedding, and death of the tree. The wood samples were stored in plastic bags and refrigerated before isolation.
Isolation of Ceratocystis was carried out based on carrot bait method (Moller and De Vay, 1968). Discolored wood was placed between two carrot slices that were first treated with streptomycin sulfate (100 mg/l) and incubated at room temperature to induce fungal sporulation on the slices. Wood pieces were sterilized with sodium hypochlorite (NaClO) for 5 min, and rinsed with distilled water. Afterward, there were dried in laminar airflow planted directly on malt extract agar (MEA) media at room temperature (25°C) for 7-10 days to induce direct sporulation in MEA.
Masses of single ascospores which developed at the tips of ascomata on wood slices planted directly on MEA or infected carrots were transferred to 2% MEA (20 g/l malts, 20 g/l agar) (Biolab, Midrand, South Africa) in a new Petri dish, after which these cultures were incubated at 25°C.
The morphological characteristics of the observed fungi were represented by isolates originating from eight regions that were severely affected by Ceratocystis, namely Ogan Komering Ulu (Kepayang; CAL32194), East Ogan Komering Ulu (Bantan Pelita; CAL32367), South Ogan Komering Ulu (Simpang; CAL32164), Ogan Komering Ilir (Pairing; CAL30673), Musi Banyuasin (Sanga Desa; CAL32156), Musi Rawas (Tuah Negri; CAL31663), North Musi Rawas (Lawang Agung; CAL31654), and Muara Enim (Ujan Mas; CAL31351). Morphological observations of Ceratocystis isolate used the structure of the fungus which was cultured on 2% MEA media and incubated for 10 days at 25°C. Samples were prepared by placing fungal structures on glass slides in lactic acid and observing these structures under a light microscope. For each isolate, 100 replicate were established for the measurements of length and width of the base, ascomata neck, ascospores, bacilliform conidia, barrel-shaped conidia, and chlamydospores (Al Adawi et al., 2013).
To determine the growth rate in culture, 4 mm mycelium-covered agar plugs were taken from the outer edge of 10-days-old cultures and placed face down in the center of a 90 mm Petri dish containing 2% MEA. Furthermore, a total of eight isolates were selected which represent the most severely affected areas from each region, namely CAL32194, CAL32156, CAL32164, CAL32367, CAL31654, CAL31663, CAL30673, and CAL31351. Each isolate was replicated four times and planted in an incubator at a temperature of 10-30°C with an interval of 5°C. Also, the diameter of the colony was measured every 2 days for 14 days and the average was calculated.
The pure cultures used for the DNA extraction were 14 isolates that represent each affected area, namely Ogan Komering Ulu (CAL32194, CAL32191, CAL32193, CAL32196, CAL32195, and CAL32192), East Ogan Komering Ulu (CAL32367), South Ogan Komering Ulu (CAL32164), Ogan Komering Ilir (CAL30673), Musi Banyuasin (CAL32156 and CAL32157), Musi Rawas (CAL31663), North Musi Rawas (CAL31654), and Muara Enim (CAL31351). These isolates were grown in potato dextrose broth (PDB) for DNA extraction at 25°C for 10 days. Mycelium from PDB cultures was filtered, dried, and grounded into a fine powder using a mortar. DNA was extracted using the YeaStar Genomic DNA Kit (Zymo Research Corporation, Irvine, CA, USA). The concentration, as well as purity, were measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Montchanin, DE, USA).
Amplification and polymerase chain reaction (PCR) sequencing were obtained from two gene regions, namely beta-tubulin which include βT1a (TTCCCCCGTCTCCACTTCTTCATG) and βT1b (GACGAGATCGTTCATGTTGAACTC) (Glass and Donaldson, 1995) as well as internal transcribed spacer (ITS) which include; ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) (White et al., 1990). Furthermore, the amplification was performed in a 50 μl reaction containing 20 μl Master Mix (Eppendorf, Hamburg, Germany) (25 mM MgCl2, 0.06 U/μl Taq-DNA-polymerase, 0.2 mM of each dNTP), 1 μl of each forward and reverse primer, 1 μl DNA template, and 27 μl sterile water. Also, PCR was performed using a C1000 Touch thermal cycler (Bio-Rad, Hercules, CA, USA). The parameters were initial denaturation for 3 min at 94°C, 30 cycles for 30 seconds at 94°C for 30 s, for 30 s at 52°C, and 1 min at 72°C for. Amplification was completed at 72°C for 10 min and the PCR product was stored at 10°C. The PCR amplicon was sequenced at 1st BASE (Malaysia), while the DNA sequences were compared with the GenBank database through a nucleotide BLAST search located at the National Center for Biotechnology Information (NCBI), Bethesda, MD, USA. The relevant sequences were transferred and then processed using the BioEdit software (Hall, 1999).
Trees were visualized and edited in MEGA v. 7 with maximum parsimony (MP) analysis and bootstrap of 1,000 replicates (Kumar et al., 2016). Branch support for nodes was obtained by performing 1,000 bootstrap replicates of the aligned sequences. For MP analysis, the metrics calculated included tree length, retention index, and consistency index. Also, C. virescens was used as the out-group taxon and the in-group was considered to be monophyletic.
These studies were conducted using ten isolates of C. fimbriata. The isolates were selected from the most severely affected area namely Ogan Komering Ulu and Musi Banyuasin (Table 1) and representing from two different type of haplotype ITS5 and ITS7b. Inoculation was designed using two studies to evaluate the pathogenicity of the isolates. First inoculation was tested their pathogenicity on L. domesticum. Two-year-old L. domesticum plants were collected from local seedlings with a stem diameter of 2-3 cm and a height of 50-60 cm and were put into a 15 cm diameter pot containing peat soil used for the experiment. All the plants were kept in the experimental house and watered twice a day.
The second inoculation test was performed to determine the specificity of the host range in A. mangium, A. carsicarpa, E. urophylla, D. costulata, H. brasiliensis, A. scholaris, and M. cajuputi. The age of the plant used for inoculation was four months with a stem diameter of 2-3 cm and a height of 70-80 cm, which was collected from a forest plant nursery in South Sumatra, planted in the same pot media and maintained as described for the first experiment.
Inoculation was performed using the isolates grown in MEA for 2 weeks. The plants were injured with a sterile scalpel by making an L-shaped (10 mm long) incision on the seedling stem, approximately 10 cm above the soil surface, and inserting agar mycelium (4 mm diam.) into each wound site. Ten host plants were inoculated with each Ceratocystis isolate and the same number of seedlings was inoculated with sterile MEA as a control. The plants were arranged in a randomized block design, and all inoculated wounds were covered with moistened sterile cotton and parafilm.
The inoculated plants were kept in the experimental house and watered twice a day. After 45 days, the peel tissue from the seedlings was incised at the top and bottom of the site and the length of the lesion was measured. The length of lesions in inoculated plants was measured after 45 days. To re-isolate the inoculated pathogens, wood samples were collected from the edges of the lesions and grown on MEA plates or placed between two carrot slices.
Pathogenicity test data were analyzed using the SAS university edition software package (SAS Institute Inc., Cary, NC, USA). Furthermore, the analysis of variance (ANOVA) and Tukey’s honestly significance difference (Tukey’s honestly significant difference) test was used to determine the significant differences in the mean comparisons of the different treatments.
Ceratocystis wilt disease in duku was first reported in 2014 and was found only in 3 villages in Ogan Komering Ulu District, namely Belatung, Lubuk Batang Baru and Lubuk Batang Lama with an incidence of 100% (Suwandi et al., 2021). Currently, the attacked duku plantation has been destroyed and replaced with corn plants, the survey to observe this disease was continued considering the plant has high economic value and as the mascot of fruits in South Sumatra. Recent reports from 2019 to 2021 show that this disease has spread widely across various districts as centers of duku plantations in South Sumatra with varying levels of disease incidence (Table 1). It has spread widely in other plantations in the Ogan Komering Ulu District covering the Kartamulya, Saleman, Pengaringan, Mutual Jiwa, and Kepayang areas with the incidence of the disease reaching 100% in Pengaringan and Kepayang villages (Table 1). In the same year, it was also found that this disease attacks the duku trees sporadically in Musi Banyuasin District, within 271 km from the disease origin of Ogan Komering Ulu, and this has resulted in the death of all trees (100%) in the duku plantations in Sanga Desa and Tanjung Raya (Table 1).
From 2020 to 2021, there were similar disease incidences on the duku plantations in Ogan Komering Ilir, within 158 km from the disease origin, and Muara Enim (within 152 km from the disease origin) with mild infestation with the incidence of less than 28% and 11.5%, respectively. In 2021, Musi Rawas (within 263 km from the disease origin), had a fairly incidence of 40.2%. In 2021, severe infestations were also detected in several villages of North Musi Rawas, within 345 km from the disease origin, especially Beringin Jaya and Lawang Agung with a percentage of 56.1% and 43.6%, respectively (Table 1). Due to the rapid development and spread of this disease in Ogan Komering Ulu and Musi Banyuasin in a short time, it is feared that this attack will kill duku plants in other districts in South Sumatra. Therefore, this disease destroys duku plant, which has high economic value and has become the mascot of the fruit flora of South Sumatra.
Infected duku tree is characterized by wilting leaves on certain twigs or branches. The leaves turn yellow, wilt, and dry, then it eventually dies due to a lack of nutrient supply to the plant. Although, it will take up to four to five months after the first symptoms for it to completely die. Ceratocystis disease attacks have resulted in the death of duku trees that are between 10 to 100 years old (Fig. 2A and B). Pathogen development on stems causes staining of vascular tissue and cankers on stems, and the initial symptoms shown are black streaks on the vascular tissue of the plant, as well as discoloration of the sapwood (Fig. 2C and D). There is a wound on the diseased tree caused by a squirrel scratch (Fig. 2E). In general, holes will appear on the infected duku stem caused by Hypocryphalus mangiferae (Fig. 2F) which is a vector insect for Ceratocystis (Fig. 2G).
Isolation of symptomatic xylem tissue in L. domesticum using carrot bait and direct planting into MEA media resulted in 16 isolates which represent Ogan Komering Ulu, East Ogan Komering Ulu, South Ogan Komering Ulu, Ogan Komering Ilir, Musi Banyuasin, Musi Rawas, North Musi Rawas, and Muara Enim areas which were severely affected by this disease. Meanwhile, the overall isolation percentage of L. domesticum samples from each region was 65%, 53.3%, 56%, 80%, 64%, 80%, 53.3%, and 60% for Ogan Komering Ulu, Musi Banyuasin, South Ogan Komering Ulu, East Ogan Komering Ulu, North Musi Rawas, Musi Rawas, Ogan Komering Ilir, and Muara Enim, respectively (Table 2).
Sixteen selected Ceratocystis isolates were collected from diseased duku plants, and there include CAL32194, CAL32191, CAL32196, CAL32195, and CAL32192 from Ogan Komering Ulu; CAL32159, CAL32156, CAL32157, and CAL32158 from Musi Banyuasin; CAL32164 from South Ogan Komering Ulu; CAL32367 from East Ogan Komering Ulu; CAL31654 from North Musi Rawas, CAL31663 from Musi Rawas; CAL30673 from Ogan Komering Ilir; and CAL31351 from Muara Enim. The isolate cultures obtained in this study were preserved in the Culture Collection (CMW), Laboratory of Phytopathology, Department of Plant Protection, Faculty of Agriculture, Sriwijaya University.
The isolates obtained had similar morphological characteristics when grown on MEA media. All isolates had light gray mycelia and dark gray to greenish colors, they also had black ascomata bases that were globose to subglobose (Fig. 3A) and produced an ascomata neck with divergent ostiolar hyphae at the ends (Fig. 3B). This fungus also produced chained barrel-shaped conidia (Fig. 3C), and chlamydospores (Fig. 3D), it also had hat-shaped ascospores (Fig. 3E). Cylindrical conidia (Fig. 3F) were generated from the primary phialidic conidiophore (Fig. 3G).
All morphological characteristics of the isolates studied were similar to the description of C. fimbriata which is isolated from M. indica (Van Wyk et al., 2007), Prosopis cineraria (Ghaf) in Oman, Dalbergia sissoo (Shisham) in Pakistan (Al Adawi et al., 2013), and the diseased A. mangium (Tarigan et al., 2011). However, there were no significant differences in the structural dimensions of all isolates for ascomata, ascospores, and chlamydospores (Table 3). All reported isolates were in the range of C. fimbriata and showed relatively similar growth responses. They did not grow at 10°C and optimal growth for all Ceratocystis isolates occurred between 25°C and 30°C (Table 3).
For the ITS and β-tubulin gene regions, PCR amplification showed a fragment size of about 550 base pairs, and the product sequences were then stored in the GenBank database where it was compared with other Ceratocystis (Supplementary Table 1). A BLAST search using the β-tubulin gene in GenBank showed that isolates of the species C. fimbriata sensu stricto were grouped with 99% identical sequences (Fig. 4). Meanwhile, using ITS gene data, the isolates were dominated by the ITS5 which was 100% similar to that of WRC previously isolated from the duku plant where the disease originated, and a new ITS haplotype (ITS7b) of C. fimbriata (Fig. 5).
The phylogenetic relationships of these selected isolates with related taxa were analyzed using the MP method, and the result showed that isolates of C. fimbriata in L. domesticum were closely related to C. fimbriata in Eucalyptus grandis in Zimbabwe, Camellia sinensis, Colocasia esculenta, and Punica granatum in China, Acacia in Vietnam and Indonesia as well as Mangifera indica in Oman, Pakistan, and Indonesia. The phylogeny was assessed and analyzed using bootstrap analysis with 1,000 replications, as well as β-tubulin sequence respectively, and the result of the analysis showed that all isolates belonged to the Latin American Clade of C. fimbriata sensu lato. The similarity of this sequence to the previous case of C. fimbriata and the identification with phenotypic characteristics showed that the causative agent of sudden wilt disease in L. domesticum in Indonesia is classified as C. fimbriata.
L. domesticum seedlings inoculated in the first experiment showed discoloration in the bundle vessels, whereby 90% and 100% of it dies 45 days, as well as 70 days after pathogen inoculation respectively (Fig. 6A and B). ANOVA for lesion length in duku showed that there was no significant difference among all isolates inoculated to this host. All inoculated isolates resulted in lesion lengths of 6.86 to 19.81 cm in L. domesticum seedlings (Table 4). Statistical analysis showed a significant difference in lesion length between inoculated L. domesticum and control seedlings. Re-isolation of inoculated seedlings resulted in C. fimbriata and no fungus was found in the control nurseries.
The A. mangium seedlings inoculated with C. fimbriata showed typical symptoms of wilt disease, which include extensive vascular discoloration in all inoculated seedlings (Fig. 6C-F), and wilt was noted to reach 100% of all seedlings at day 70 after inoculation (Table 5). There was no significant difference in the length of lesion produced by the Ceratocystis isolate used in the inoculation. The average length of lesions produced by all isolates of C. fimbriata inoculated to A. mangium seedlings was 9.94 to 20.93 cm (Table 5). Lesion and Ceratocystis fungus was not discovered in the control seedlings after re-isolation.
The isolates from C. fimbriata that were inoculated on other test seedlings, caused death and infection in plants which were characterized by the formation of significant lesions. In A. crassicarpa, E. urophylla, and M. leucadendra seedlings, all isolates caused moderately pathogenic symptoms with lesion lengths of 5.97-12.59 cm, 8.80-11.92 cm, and 1.94-5.17 cm, respectively. However, in D. costulata, H. brasiliensis, and A. scholaris plants, these isolates caused weakly symptoms with lesion lengths of 3.05-5.39 cm, 1.62-7.56 cm, and 3.36-6.51 cm, respectively, compared to controls with an average lesion length of 0.1 cm (the scar with a knife at the time of inoculation) (Table 5).
The members of the ITS5 and ITS7 haplotypes tested on all duku and other agroforestry plants showed approximately the same pathogenic ability to infect the tested plants. The re-isolation of the eight inoculated test plants resulted in a C. fimbriata culture, that confirmed Koch’s postulate test. None of Ceratocystis isolates grew from control seedlings.
Based on a survey conducted from 2019 to 2021, Ceratocystis has spread widely from its place of origin in the Ogan Komering Ulu District (Suwandi et al., 2021). Currently, the wilt disease has been found to affect the duku plants in other locations. Ceratocystis has been discovered to attack extensive areas with a radius of 345 km from its origin to South Ogan Komering Ulu, Musi Banyuasin, Ogan Komering Ilir, Muara Enim, Musi Rawas, and North Musi Rawas, with various severity levels, whereby it is very severe in Musi Banyuasin with a percentage of 100% the same as in Ogan Komering Ulu. Meanwhile, attacks in North Musi Rawas and other districts reached 56.1% and less than 30%, respectively.
The widespread of the disease in L. domesticum is closely related to the wood-boring insect H. mangiferae that comes from Southeast Asia, but it is well-known as a vector of Ceratocystis disease on mango plants in Oman and Pakistan (Al Adawi et al., 2006, 2013). H. mangiferae were seen in the field which has holes formed by this insect in L. domesticum plants, especially in the lesion area on wood. Squirrel rodents are also always seen on infected duku plants and cause the disease to spread widely by biting the infected stems and branches before moving to healthy plants (Suwandi et al., 2021). Additionally, the pruning of branches that have been infected with Ceratocystis through the use of agricultural tools without sterilization exacerbates the spread of this disease (Chi et al., 2019b) which is also caused by wind (Harrington, 2007). Ceratocystis is also transmitted from infected wild acacia around duku plantations or other plants that are hosts of this pathogen.
Field observations show that attacks from this disease occur from the trunk or branches at the top and go down to the stem, which is spread by squirrels and insects. This disease also occur from the root and continues up to the base of the stem. The infection from these roots is caused by the spread of pathogenic inoculum through rainwater flow or splashes. In some locations in a district affected by the disease, the plants were able to grow healthy, while in other places the attacks were very severe. The variety of disease severity at each location and district is probably due to the various levels of resistance offered by the planted varieties of duku and the degree of soil fertility, which affects the growth and resistance of the plants. There was no correlation between the polyculture and monoculture systems of duku with the attack rate because Ceratocystis wilt disease was discovered in duku, which was grown in both polyculture and monoculture.
The identity of C. fimbriata as a pathogen associated with wilt disease in L. domesticum was determined based on morphological characteristics and a comparison of DNA sequences which include CAL32194, CAL32191, CAL32193, CAL32196, CAL32195, CAL32192, CAL32164, CAL32367, CAL31654, CAL31663, CAL30673 and CAL31351 with reference isolates CMW38737, C1345, A59662, YM061, P20053, C1, CMW22563, WRC while isolates CAL32156, CAL32157 with reference isolates CMW13851, CMW23634, CMW22579 were identified as belonging to C. fimbriata which was collected from L. domesticum in South Sumatra is part of C. fimbriata s.l. complex grouped into C. fimbriata sensu stricto. Comparison of ITS and β-tubulin gene sequences in each isolate obtained showed similarities to C. fimbriata which was reported to attack duku (Suwandi et al., 2021), jackfruit (Pratama et al., 2021b), and bullet wood (Pratama et al., 2021a) plants.
In a previous study, there were two variations of the ITS rDNA sequence from two isolates, namely ITS5 and ITS6z haplotype of C. fimbriata (Suwandi et al., 2021). In this study, there were also two variations of the ITS rDNA sequence, namely the ITS5 and ITS7b haplotype. ITS5 haplotype was the most common genotype since it recovered from seven out of eight district in South Sumatra. ITS7b haplotype was the new genotype of C. fimbriata that affected L. domesticum in South Sumatra localized in Musi Banyuasin District. ITS6z was not isolated from this study. It might be due to the haplotype having a weak pathogenicity (Suwandi et al., 2021). From this and previous study, there are three the ITS haplotype C. fimbriata group isolated from L. domesticum (Meliaceae) including ITS5, ITS6z, and ITS7b that was the same as the haplotype C. fimbriata group from acacia, jackfruit, and bullet wood in Indonesia (Pratama et al., 2021a, 2021b; Tarigan et al., 2011). This shows that the genetic similarity of Ceratocystis in L. domesticum (Meliaceae) with Ceratocystis in Acacia is the result of crossing the ITS5, ITS6z, and ITS7b haplotypes. Therefore, it appears that the Ceratocystis pathogen that attacks L. domesticum (Meliaceae) in South Sumatra originates from Acacia which was first discovered in Riau.
This Ceratocystis wilt disease causes the death of duku plants in South Sumatra, and the symptoms include progressive loss of canopy which leads to the death of the tree, and the bark around the lesions and the wood turn dark blue to brown in the diseased trunk. In general, these symptoms are similar to those of C. fimbriata described in Acacia plants (Tarigan et al., 2010, 2011). C. fimbriata is a severe wilt pathogen that infects jackfruit (Pratama et al., 2021a) and causes a sudden decline in bullet wood disease (Pratama et al., 2021b), hence it has the potential to cause damage and destruction to duku in Indonesia.
C. fimbriata is best known for its severe damage inflicted on various plant families and has a wide host range, such as Myrtaceae represented by Eucalyptus (Li et al., 2014); Actinidiaceae represented by Actinidia spp. (Piveta et al., 2016); Araceae represented by C. esculenta (Oliveira et al., 2017); and Meliaceae represented by L. domesticum (Suwandi et al., 2021). However, recently it has been reported that C. fimbriata kills A. heterophyllus, Moraceae family in Indonesia (Pratama et al., 2021a). This supports the perspective that C. fimbriata has a wide host range, therefore having the potential of infecting other trees not previously mentioned.
Wilt disease of L. domesticum appears to be serious and it can devastate native trees like never before through host transfer (Roy, 2001; Wingfield et al., 2010). Pathogenicity test on duku showed that a very high attack intensity of 100% causes wilting and death of plants. Also, inoculation tests on various forest and agroforestry plant hosts showed that C. fimbriata derived from L. domesticum has a very aggressive on A. mangium (Suwandi et al., 2021), moderately pathogenic to A. carsicarpa, E. urophylla, and M. cajuputi, as well as weakly pathogenic to D. costulata, A. scholaris, and H. brasiliensis. This was shown by the formation of lesions on the stems which leads to the death of the inoculated seedlings.
The most pathogenic isolate from L. domesticum (CAL32193) resulted in the death of seedlings 25 days after inoculation. Furthermore, the death of acacia and eucalyptus plants showed similar symptoms, which include leaf wilting, and discoloration of the vascular tissue until the plant finally dies as found by Tarigan et al. (2011); and Roux et al. (2020). Ceratocystis is a very serious economical disease that has attacked L. domesticum in all duku production centers in South Sumatra hence it damages the income sources of farmers in this province. Also, the verification of M. cajuputi as an endogenous wetland plant that is infected and causes death, becomes a threat to the indigenous ones. Given the very wide host of Ceratosystis, the attack of this pathogen poses a serious threat to the biodiversity of Indonesia.
Sudden wilt disease on L. domesticum caused by C. fimbriata has spread widely to duku production centers in various districts of South Sumatra. Furthermore, the population consisted of individuals with uniform morphology dominated by ITS5 and ITS7b which were still localized in Musi Banyuasin, as well as being highly pathogenic in duku. Ceratocystis was also pathogenic to all forest test plants including wetland indigenous, posing a serious threat to the biodiversity of Indonesia.
Acknowledgments
All authors would like to thank the Research Institutions and Community Service, Sriwijaya University for funding thie reasearch with fiscal year of 2021 in accordace with the Competitive Leading grants contract number: 0107.043/UN9/SB3.LP2M.PT/2021.
Electronic Supplementary Material
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig. 2
Symptoms of wilt and die-back on Lansium domesticum. (A, B) Trees affected by Ceratocystis fimbriata experience rapid and simultaneous wilting of the leaves on the main branch or the entire canopy until it finally dies. (C, D) Dispersal pattern of discoloration in cross-section and the cambium area of wilted tree trunks (yellow arrows). (E) Squirrel bite caused peeled-off bark on diseased tree (yellow arrows). (F) A beetle hole on affected diseased wood (yellow arrow). (G) Hypocryphalus mangiferae as a vector for the spread of Ceratocystis.

Fig. 3
Morphological characteristics of Ceratocystis fimbriata isolated from Lansium domesticum stem lesion: (A) globose ascomata with a long neck, (B) divergent ostiolar hyphae, (C) barrel-shaped conidia, (D) chlamydospores, (E) hat-shaped ascospores, (F) cylindrical conidia, and (G) conidiophore/phialide. Scale bars: A = 100 μm, B-E, G = 10 μm, F = 5 μm.
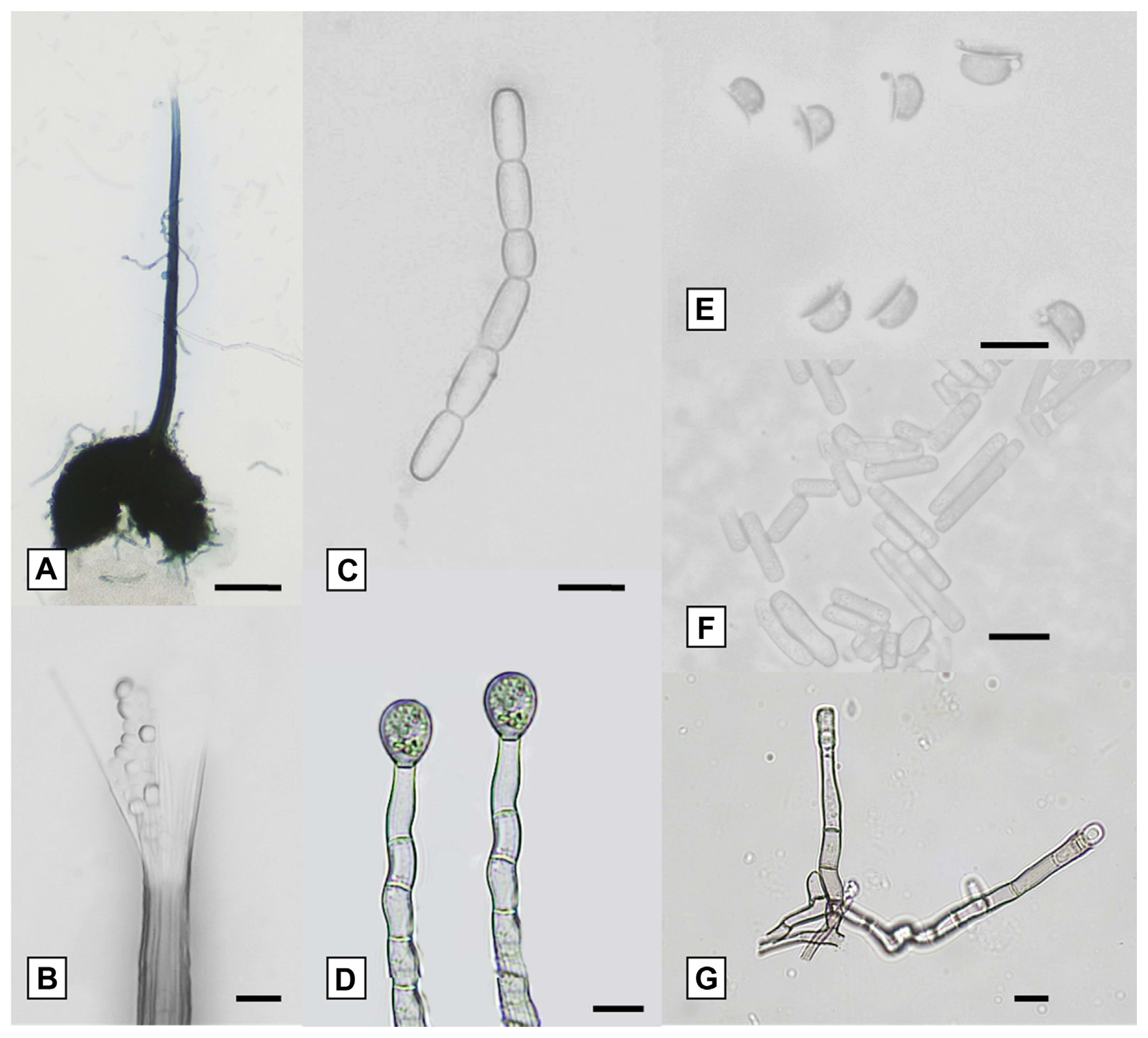
Fig. 4
The phylogenetic tree resulting from the maximum parsimony analysis of the β-tubulin sequence shows the relationship between Ceratocystis fimbriata from the Lansium tree in Indonesia (marked in bold) and other species in the Latin American and Asian clade of the C. fimbriata species complex. C. variospora is used as an outgroup.
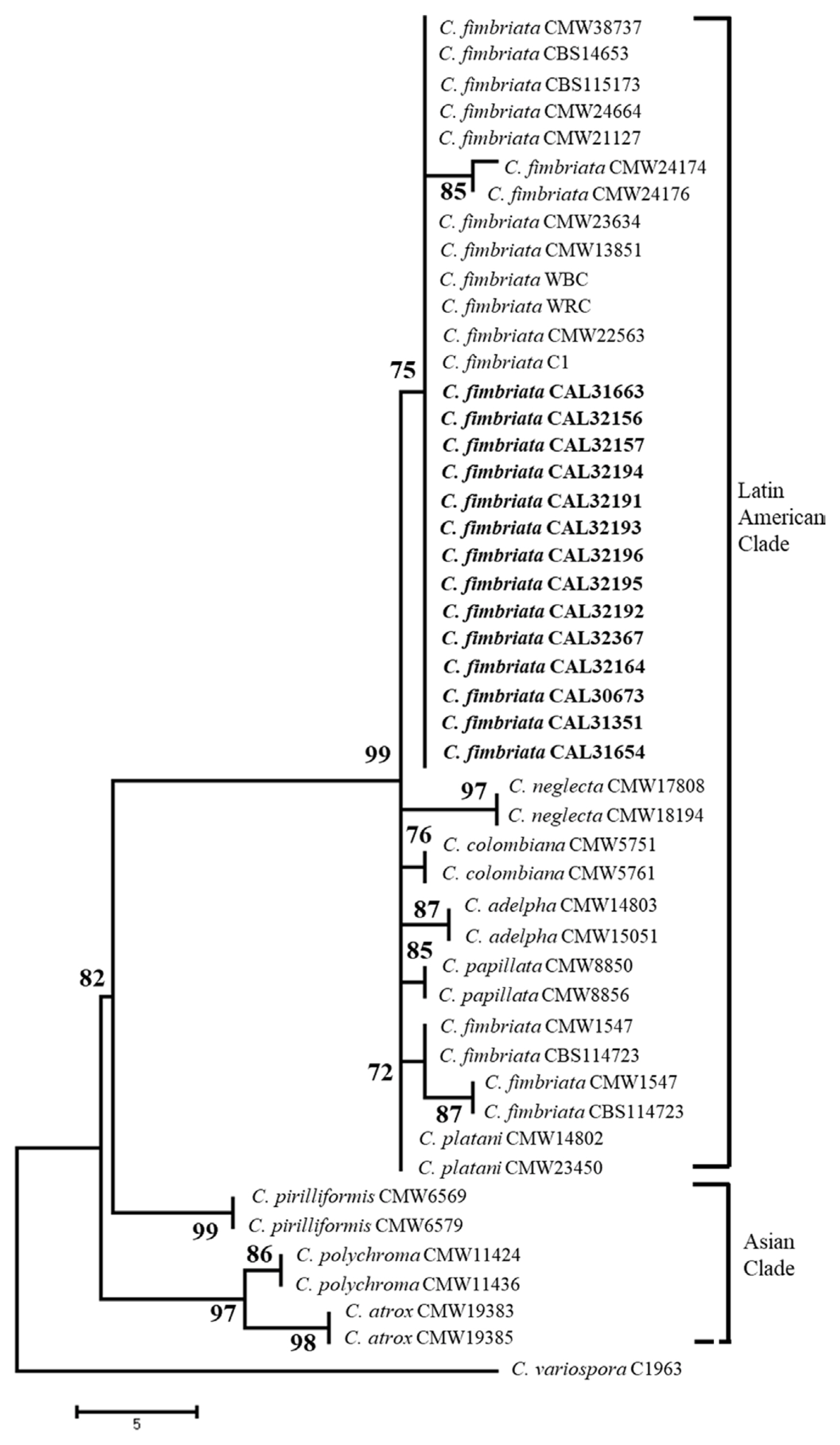
Fig. 5
The dendrogram formed from the maximum parsimony analysis shows the genetic linkage of the representative rDNA internal transcribed spacer (ITS) genotype in Ceratocystis fimbriata sensu stricto. Isolates from Lansium domesticum in Indonesia are marked in bold. The ITS haplotypes of C. fimbriata are numbered following the numerical designation of Harrington et al. (2014). C. variospora is used as an outgroup taxon.
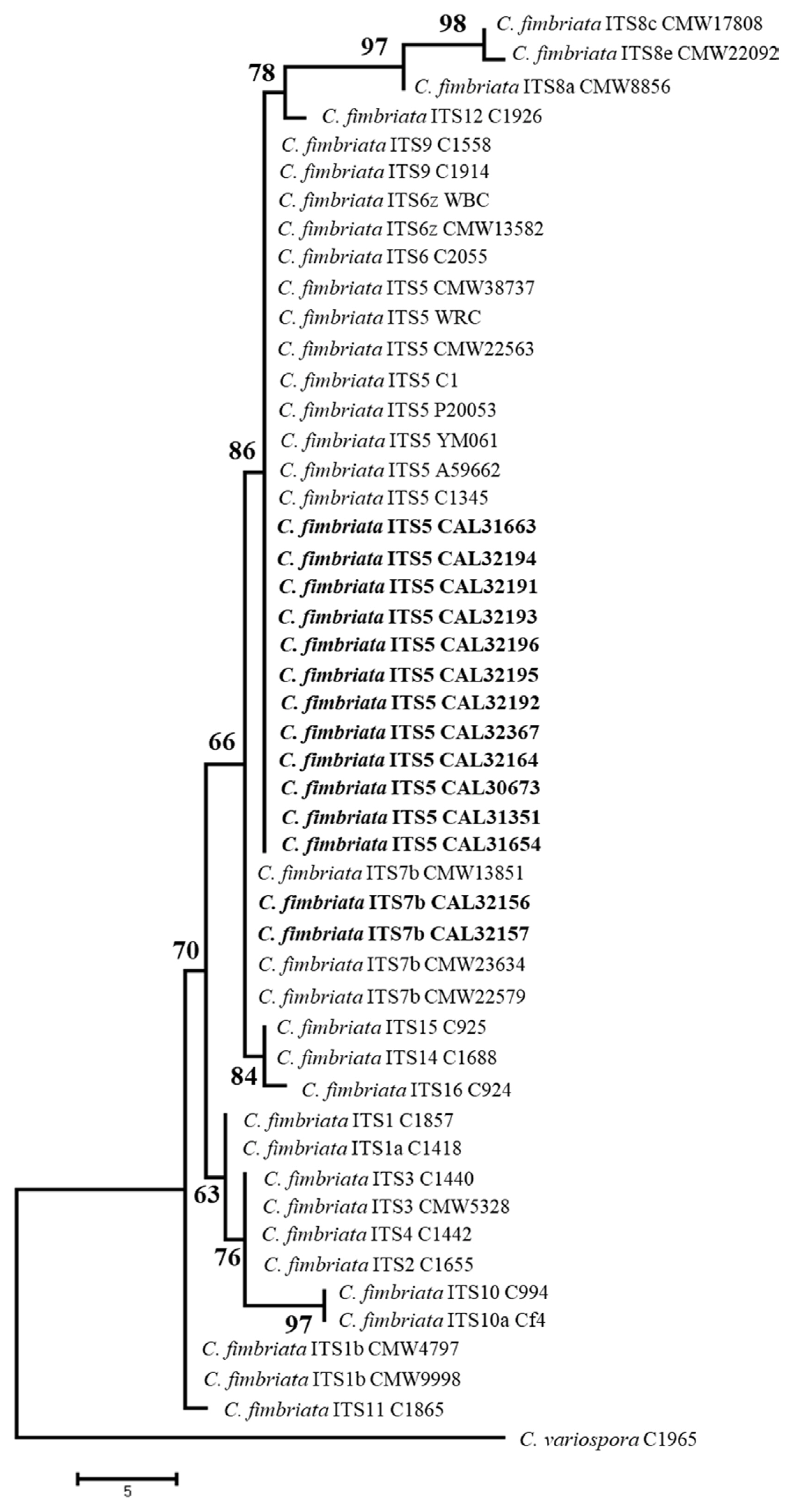
Fig. 6
Symptoms of mycelial plug inoculation with Ceratocystis fimbriata isolates (CAL32194 and CAL32159) from Lansium domesticum 45 days after inoculation. (A) Symptoms on 2-year-old duku seedlings (L. domesticum) inoculated with malt agar plug (control) (I), duku plants experienced complete wilting and finally died after being inoculated with CAL32194 (II) and CAL32159 (III). (B) The formation of an upward lesion from the inoculation site (red arrows) on duku plants after being inoculated by CAL32194 (I) and CAL32159 (II). (C, D) 4-month-old Acacia plants show symptoms of wilting and formation of upward lesions from the inoculation site (red arrow) after being inoculated by CAL32194 (I) and CAL32159 (II). (E) The formation of an upward lesion from the inoculation site (red arrow) on 4-month-old Eucalyptus, at 45 days of observation did not show any signs of wilting. (F) The formation of an upward lesion from the inoculation site (red arrow) on 4-month-old Acacia crassicarpa, at 45 days of observation did not show any signs of wilting.

Table 1
Incidence of Ceratocystis wilt in duku orchards of South Sumatra
Table 2
Recovery of Ceratocystis fimbriata from carrot baiting and direct isolation of wood onto the malt extract agar from samples collected from dying Lansium domesticum trees in Ogan Komering Ulu and Musi Banyuasin
Table 3
Morphology of selected Ceratocystis fimbriata isolates from a different district in South Sumatra
| Morphological charactersa | Isolates | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| CAL32194 | CAL32156 | CAL32164 | CAL32367 | CAL31654 | CAL31663 | CAL30673 | CAL31351 | |
| Ascomatal bases | ||||||||
| Shape | Globose | Globose | Globose | Globose | Globose | Globose | Globose | Globose |
| Ascomatal base (w) | 134.3-312.4 | 122.9-291.4 | 135.7-325.2 | 141.3-317.1 | 137.9-321.1 | 132.1-334.9 | 137.9-346.1 | 122.1-316.9 |
| Ascomatal base (l) | 153.1-404.4 | 131-315.4 | 148.1-398.4 | 151.1-411.4 | 143.1-398.4 | 152.4-394.1 | 139.1-421.8 | 157.1-412.1 |
| Ascomatal necks | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight |
| Neck (l) | 415.4-768.4 | 354.9-677.7 | 413.7-798.8 | 439.9-736.4 | 475.8-813.6 | 484.6-790.9 | 463.8-723.6 | 484.6-780.9 |
| Neck (w) top | 11.5-26.8 | 7.06-18.4 | 11.3-21.9 | 11.1-25.4 | 10.1-17.9 | 11.3-21.7 | 11.1-22.9 | 11.3-21.7 |
| Neck (w) bottom | 24.8-47.9 | 20.3-39.7 | 23.6-42.6 | 22.6-51.2 | 23.7-43.8 | 22.67-42.9 | 23.7-43.6 | 22.67-44.8 |
| Ostiolar hyphae | ||||||||
| Shape | Divergent | Divergent | Divergent | Divergent | Divergent | Divergent | Divergent | Divergent |
| Ostiolar hyphae (l) | 32.2-43.5 | 30.4-40.1 | 32.7-44.7 | 32.7-42.2 | 33.5-43.9 | 33.7-44.8 | 33.5-42.9 | 31.7-44.8 |
| Ascospores | ||||||||
| Hat-shaped ascospores (l) | 3.4-5.7 | 3.3-5.2 | 3.2-5.4 | 3.4-4.9 | 3.2-4.4 | 3.1-5.1 | 3.1-4.3 | 3.3-4.9 |
| Ascospores (w) without sheath | 3.4-5.1 | 3.1-4.1 | 3.3-4.7 | 3.4-4.4 | 3.3-4.1 | 3.4-4.5 | 3.3-4.1 | 3.5-4.4 |
| Ascospores (w) with sheath | 5-6.8 | 4.1-6.1 | 5.1-6.7 | 5.3-6.4 | 5.2-6.5 | 5.5-6.7 | 5.2-6.3 | 5.4-6.6 |
| Primary conidia (l) | 12.1-27.5 | 10.6-18.9 | 13.8-23.8 | 12.2-29.3 | 13.2-25.7 | 14.9-24.8 | 12.5-21.6 | 13.7-24.6 |
| Primary conidia (w) | 3.5-7.4 | 3.2-4.3 | 3.1-5.1 | 3.4-4.1 | 3.2-5.1 | 3.4 -4.4 | 3.4-4.1 | 3.5-4.7 |
| Secondary conidia (l) | 6.3-11.6 | 5.7-10.1 | 6.6-11.8 | 7.9-11.8 | 6.7-11.9 | 6.8-11.5 | 6.5-11.5 | 6.2-11.3 |
| Secondary conidia (w) | 4.5-7.6 | 4.1-7.4 | 4.7-7.5 | 5.6-7.9 | 4.3-7.8 | 4.3-7.8 | 4.3-7.1 | 4.1-7.8 |
| Chlamydospores | ||||||||
| Shape | Globose to pyriform | Globose to pyriform | Globose to pyriform | Globose to pyriform | Globose to pyriform | Globose to pyriform | Globose to pyriform | Globose to pyriform |
| Chlamydospores (l) | 10.7-15.1 | 8.7-15.1 | 11.3-15.6 | 9.7-17.8 | 10.7-15.4 | 10.1-16.5 | 10.3-14.6 | 10.4-14.5 |
| Chlamydospores (w) | 7.9-13.9 | 8.3-11.1 | 6.9-14.2 | 6.8-13.6 | 7.6-11.8 | 7.7-12.5 | 7.6-11.8 | 7.6-12.9 |
| Culture growth rateb | ||||||||
| 10°C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15°C | 3.3-3.5 | 2.2-2.5 | 3.2-3.5 | 2.2-2.7 | 3.2-3.4 | 2.2-2.8 | 2.3-2.9 | 2.4-2.8 |
| 20°C | 3.2-3.7 | 3.1-2.9 | 3.2-3.9 | 3.3-3.9 | 4.2-4.4 | 3.2-3.5 | 4.2-4.4 | 3.2-3.5 |
| 25°C | 5.1-5.3 | 4.1-4.5 | 4.7-5.1 | 4.4-4.7 | 4.4-4.9 | 4.1-4.5 | 4.4-4.9 | 4.1-4.5 |
| 30°C | 3.3-3.6 | 3.1-3.9 | 3.5-4.6 | 3.5-4.2 | 3.8-4.2 | 3.1-3.4 | 3.8-4.2 | 3.1-3.4 |
Table 4
Pathogenicity of Ceratocystis isolates on Lansium domesticum under nursery condition
| Isolates | Host test | Lansium domesticum | ||
|---|---|---|---|---|
|
|
||||
| Lesion lengtha (cm) | Wilting and death at 45 days post inoculation | Wilting and death at 70 days post inoculation | ||
| CAL32156 | 10 | 16.35 f | 7/10 | 10/10 |
| CAL32157 | 10 | 15.49 ef | 7/10 | 8/10 |
| CAL32158 | 10 | 12.29 cd | 5/10 | 5/10 |
| CAL32159 | 10 | 11.02 c | 2/10 | 5/10 |
| CAL32191 | 10 | 11.73 cd | 2/10 | 3/10 |
| CAL32192 | 10 | 13.83 def | 7/10 | 8/10 |
| CAL32193 | 10 | 19.81 g | 9/10 | 10/10 |
| CAL32194 | 10 | 6.86 b | 2/10 | 2/10 |
| CAL32195 | 10 | 12.89 cde | 5/10 | 6/10 |
| CAL32196 | 10 | 11.19 cde | 5/10 | 7/10 |
| Control (MEA) | 10 | 0.01 a | 0/10 | 0/10 |
| P-value | <0.001 | |||
Table 5
Host range test of Ceratocystis isolates on forest and agroforestry plants under nursery condition
| Isolates | Host test | Acacia mangium | Acacia carsicarpa | Eucalyptus urophylla | Dyera costulata | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Lesion lengtha (cm) | Wilting and death at 45 dpi | Wilting and death at 70 dpi | Lesion lengtha (cm) | Wilting and death at 45 dpi | Wilting and death at 70 dpi | Lesion lengtha (cm) | Wilting and death at 45 dpi | Wilting and death at 70 dpi | Lesion lengtha (cm) | Wilting and death at 45 dpi | Wilting and death at 70 dpi | ||
| CAL32156 | 10 | 18.25 ef | 10/10 | 10/10 | 9.86 de | 0/10 | 1/10 | 11.32 b | 0/10 | 1/10 | 4.25b | 0/10 | 0/10 |
| CAL32157 | 10 | 16.32 de | 10/10 | 10/10 | 10.16 de | 0/10 | 2/10 | 11.81 b | 0/10 | 1/10 | 3.91b | 0/10 | 0/10 |
| CAL32158 | 10 | 14.49 cde | 8/10 | 10/10 | 9.39 cd | 0/10 | 1/10 | 9.33 b | 0/10 | 0/10 | 3.63b | 0/10 | 0/10 |
| CAL32159 | 10 | 13.59 bcd | 8/10 | 10/10 | 8.26 bcd | 0/10 | 1/10 | 9.86 b | 0/10 | 0/10 | 3.83b | 0/10 | 0/10 |
| CAL32191 | 10 | 11.73 bc | 7/10 | 10/10 | 7.96 bcd | 0/10 | 0/10 | 9.82 b | 0/10 | 0/10 | 3.57b | 0/10 | 0/10 |
| CAL32192 | 10 | 15.54 cde | 10/10 | 10/10 | 6.57 bc | 0/10 | 0/10 | 10.59 b | 0/10 | 0/10 | 5.15b | 0/10 | 0/10 |
| CAL32193 | 10 | 20.93 f | 10/10 | 10/10 | 12.59 e | 0/10 | 5/10 | 11.92 b | 0/10 | 3/10 | 5.39b | 0/10 | 0/10 |
| CAL32194 | 10 | 9.943 b | 5/10 | 10/10 | 5.97 b | 0/10 | 0/10 | 8.80 b | 0/10 | 0/10 | 3.05b | 0/10 | 0/10 |
| CAL32195 | 10 | 15.39 cde | 9/10 | 10/10 | 7.82 bcd | 0/10 | 2/10 | 11.20 b | 0/10 | 2/10 | 4.02b | 0/10 | 0/10 |
| CAL32196 | 10 | 14.64 cde | 8/10 | 10/10 | 8.64 bcd | 0/10 | 1/10 | 11.15 b | 0/10 | 1/10 | 3.60b | 0/10 | 0/10 |
| Control (MEA) | 10 | 0.01 a | 0/10 | 0/10 | 0.01 a | 0/10 | 0/10 | 0.01 a | 0/10 | 0/10 | 0.01a | 0/10 | 0/10 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||
| Hevea brasiliensis | Alstonia scholaris | Melaleuca leucadendra | |||||||||||
| CAL32156 | 10 | 5.23 e | 0/10 | 0/10 | 5.21 b | 0/10 | 0/10 | 5.81 e | 0/10 | 2/10 | |||
| CAL32157 | 10 | 4.05 de | 0/10 | 0/10 | 4.75 b | 0/10 | 0/10 | 5.17 de | 0/10 | 2/10 | |||
| CAL32158 | 10 | 2.83 bcd | 0/10 | 0/10 | 3.70 ab | 0/10 | 0/10 | 3.15 bc | 0/10 | 0/10 | |||
| CAL32159 | 10 | 2.58 bcd | 0/10 | 0/10 | 3.50 ab | 0/10 | 0/10 | 2.63 bc | 0/10 | 0/10 | |||
| CAL32191 | 10 | 1.92 bc | 0/10 | 0/10 | 3.43 ab | 0/10 | 0/10 | 2.32 b | 0/10 | 0/10 | |||
| CAL32192 | 10 | 3.87 de | 0/10 | 0/10 | 3.98 ab | 0/10 | 0/10 | 4.23 cde | 0/10 | 1/10 | |||
| CAL32193 | 10 | 7.56 f | 0/10 | 0/10 | 6.51 b | 0/10 | 0/10 | 5.06 de | 0/10 | 4/10 | |||
| CAL32194 | 10 | 1.62 ab | 0/10 | 0/10 | 3.36 ab | 0/10 | 0/10 | 1.94 b | 0/10 | 0/10 | |||
| CAL32195 | 10 | 3.47 cde | 0/10 | 0/10 | 3.86 ab | 0/10 | 0/10 | 3.79 bcd | 0/10 | 1/10 | |||
| CAL32196 | 10 | 3.19 bcd | 0/10 | 0/10 | 3.83 ab | 0/10 | 0/10 | 3.42 bcd | 0/10 | 0/10 | |||
| Control (MEA) | 10 | 0.01 a | 0/10 | 0/10 | 0.01 a | 0/10 | 0/10 | 0.01 a | 0/10 | 0/10 | |||
| P-value | <0.001 | <0.001 | <0.001 | ||||||||||
References
Al Adawi, A. O., Barnes, I., Khan, I. A., Al-Subhi, A. M., Al-Jahwari, A. A., Deadman, M. L., Wingfield, B. D. and Wingfield, M. J. 2013. Ceratocystis manginecans is associated with a serious wilt disease of two native legume trees in Oman and Pakistan. Australas. Plant Pathol. 42:179-193.

Al Adawi, A. O., Deadman, M. L., Al Rawahi, A. K., Al-Maqbali, Y. M., Al Jahwari, A. A., Al-Saadi, B. A., Al-Amri, I. S. and Wingfield, M. J. 2006. Aetiology and causal agents of mango sudden decline disease in the sultanate of Oman. Eur. J. Plant Pathol. 116:247-254.

Chi, N. M., Nhung, N. P., Trang, T. T., Thu, P. Q., Hinh, T. X., Nam, N. V., Quang, D. N. and Dell, B. 2019a. The first report of wilt disease in Dalbergia tonkinensis caused by Ceratocystis manginecans. Australas. Plant Pathol. 48:439-445.

Chi, N. M., Thu, P. Q., Hinh, T. X. and Dell, B. 2019b. Management of Ceratocystis manginecans in plantations of Acacia through optimal pruning and site selection. Australas. Plant Pathol. 48:343-350.

Chi, N. M., Trang, T. T., Nhung, N. P., Quang, D. N., Son, V. M., Tuan, T. A., Mai, L. T., Hung, T. X., Nam, N. V., Thu, P. Q. and Dell, B. 2020. Ceratocystis wilt in Chukrasia tabularis in Vietnam: identification, pathogenicity and host tolerance. Australas. Plant Pathol. 50:17-27.

Glass, N. L. and Donaldson, G. C. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 61:1323-1330.



Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95-98.
Hanum, L., Kasiamdari, R. S., Santoso, S. and Rugayah, R. 2013. The phylogenetic relationship among varieties of Lansium domesticum Correa based on ITS rDNA sequences. Indones J. Biotechnol. 18:123-132.

Harrington, T. C. 2007. The genus Ceratocystis: where does the oak wilt fungus fit? In: Proceedings of the 2nd National Oak Wilt Symposium, eds. by R. F. Billings and D. N. Appel, pp. 21-35. Texas Forest Servie Publication, College Station, TX, USA.
Harrington, T. C., Kazmi, M. R., Al-Sadi, A. M. and Ismail, S. I. 2014. Intraspecific and intragenomic variability of ITS rDNA sequences reveals taxonomic problems in Ceratocystis fimbriata sensu stricto. Mycologia 106:224-242.


Khalili, R. M. A., Noratiqah, J. M., Norhaslinda, R., Norhayati, A. H., Amin, B. A., Roslan, A. and Zubaidi, A. L. A. 2017. Cytotoxicity effect and morphological study of different duku (Lansium domesticum Corr.) extract toward human colorectal adenocarcinoma cells line (HT-29). Pharmacogn J. 9:757-761.

Kumar, H., Savaliya, M., Biswas, S., Nayak, P. G., Maliyakkal, N., Setty, M. M., Gourishetti, K. and Pai, K. S. R. 2016. Assessment of the in vitro cytotoxicity and in vivo anti-tumor activity of the alcoholic stem bark extract/fractions of Mimusops elengi Linn. Cytotechnology 68:861-877.


Li, J., Zhang, Y., Xu, K. C., Yang, J. Y., Han, Y. H., Sun, Y. X. and Huang, Q. 2014. First report of wilt of Eucalyptus caused by Ceratocystis fimbriata in China. Plant Dis. 98:1744.

Lim, T. K. 2011. Edible medicinal and non-medicinal plants. 3:Fruits. Springer, Dordrecht, Germany. pp. 265-268.
Matsumoto, T. and Watanabe, T. 2020. Isolation and structure elucidation of constituents of Citrus limon, Isodon japonicus, and Lansium domesticum as the cancer prevention agents. Genes Environ. 42:17.




Moller, W. J. and De Vay, J. E. 1968. Carrot as a species-selective isolation medium for Ceratocystis fimbriata. Phytopathology 58:123-124.
Ni’mah, T., Oktarina, R., Mahdalena, V. and Asyati, D. 2015. Potensi ekstrak biji duku (Lansium domesticum Corr) terhadap Aedes aegypti. Bull Penelit Kesehat 43:131-136.
Nishizawa, M., Imagawa, H. and Yamamoto, H. 2010. A new catalyst for organic synthesis: mercuric triflate. Org Biomol Chem. 8:511-521.


Norhayati, A. H., Mohd, A. K. R., Zetty, H. M. Z., Intan, S. M. M. A., Atif, A. B., Muralidhara, D. V. and Ahmad, Z. L. 2016. Potential effects of duku (Lansium domesticum Corr) and langsat (Lansium domesticum Jack) extracts on the growth of Bifidobacteria spp. Int. J. Pharm Pharm Sci. 8:69-74.

Oliveira, L. S. S., Harrington, T. C., Ferreira, M. A., Freitas, R. G. and Alfenas, A. C. 2017. Populations of Ceratocystis fimbriata on Colocasia esculenta and other hosts in the Mata Atlantica region in Brazil. Plant Pathol. 67:97-106.

Piveta, G., Ferreira, M. A., Muniz, M. F. B., Valdetaro, D., Valdebenito-Sanhuezad, R., Harrington, T. and Alfenas, A. C. 2016. Ceratocystis fimbriata on kiwifruit (Actinidia spp.) in Brazil. N. Z. J. Crop Hortic Sci. 44:13-24.

Pratama, R., Muslim, A., Suwandi, S., Damiri, N. and Soleha, S. 2021a. First report of characterization and pathogenicity of bullet wood (Mimusops elengi) sudden decline disease by Ceratocystis in Indonesia. Biodivers Biodiversitas 22:2636-645.
Pratama, R., Muslim, A., Suwandi, S., Damiri, N. and Soleha, S. 2021b. Jackfruit (Artocarpus heterophyllus), a new host plant of Ceratocystis wilt in South Sumatra, Indonesia. Aust Plant Dis Notes 16:24.


Putranta, N. R. and Wijaya, S. M. 2017. Efektifitas ekstrak kulit duku (Lansium domesticum corr) sebagai larvasida Aedes aegypti. Medula 7:165-170.
Razzaq, K., Rehman, A., Anjum, R., Hanif, S. and Sultan, A. 2020. First report of Ceratocystis manginecans causing Siris (Albizia lebbeck) wilt in Pakistan. Plant Dis. 104:1738.

Roux, J., Wingfield, M. J., Fourie, A., Noeth, K. and Barnes, I. 2020. Ceratocystis wilt on Eucalyptus: first record from South Africa. South For J. For Sci. 82:24-31.

Roy, B. A. 2001. Patterns of association between crucifers and their flower-mimic pathogens: host jumps are more common than coevolution or cospeciation. Evolution 55:41-53.


Rupiah Hanum, L., Negara, Z. P., Dahlan, Z. and Yustian, I. 2018. Morphological diversity of Lansium domesticum Corr in South Sumatra. Sci. Technol. Indones 3:41-44.

Subandrate Sinulingga, S., Wahyuni, S., Altiyan, M. F. and Fatmawati, 2016. Antioxidant potential of Lansium domesticum Corr. seed extract in white male rat (Rattus novergicus) induced by alcohol. Molekul 11:1-8.

Suwandi, S., Irsan, C., Hamidson, H., Umayah, A. and Asriyani, K. D. 2021. Identification and characterization of Ceratocystis fimbriata causing lethal wilt on the Lansium tree in Indonesia. Plant Pathol. J. 37:124-136.



Tarigan, M., Roux, J., Van Wyk, M., Tjahjono, B. and Wingfield, M. J. 2011. A new wilt and die-back disease of Acacia mangium associated with Ceratocystis manginecans and C. acaciivora sp. nov. in Indonesia. S Afr J. Bot. 77:292-304.

Tarigan, M., Roux, J., Wingfield, M. J., Van Wyk, M. and Tjahjono, B. 2010. Three new Ceratocystis spp. in the Ceratocystis moniliformis complex from wounds on Acacia mangium and A. crassicarpa. Mycoscience 51:53-67.

Tilaar, M., Wih, W. L., Ranti, A. S., Wasitaatmadja, S. M., Suryaningsih Junardy, F. D. and Maily, 2008. Review of Lansium domesticum Corrêa and its use in cosmetics. Bol Latinoam Caribe Plantas Med Aromat 7:183-189.
Van Wyk, M., Al Adawi, A. O., Khan, I. A., Deadman, M. L., Al Jahwari, A. A., Wingfield, B. D., Ploetz, R. and Wingfield, M. J. 2007. Ceratocystis manginecans sp. nov., causal agent of a destructive mango wilt disease in Oman and Pakistan. Fungal Divers 27:213-230.
White, T. J., Bruns, T., Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications, eds. by M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, pp. 315-322. Academic Press, New York, USA.

Wingfield, M. J., Slippers, B. and Wingfield, B. D. 2010. Novel association between pathogens, insects, and tree species threaten world forests. N. Z. J. For Sci. 40(Suppl):S95-S103.
- TOOLS
-
METRICS

- ORCID iDs
-
Ahmad Muslim

https://orcid.org/0000-0002-3973-7443 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print



