 |
 |
| Plant Pathol J > Volume 38(3); 2022 > Article |
|
Abstract
Bacterial wilt, which is a major soil-borne disease with widespread occurrence, poses a severe danger in the field of tobacco production. However, there is very limited knowledge on bacterial wilt-induced microecological changes in the tobacco root system and on the interaction between Ralstonia solanacearum and fungal communities in the rhizosphere soil. Thus, in this study, changes in fungal communities in the rhizosphere soil of tobaccos with bacterial wilt were studied by 18S rRNA gene sequencing. The community composition of fungi in bacterial wilt-infected soil and healthy soil in two tobacco areas (Gengma and Boshang, Lincang City, Yunnan Province, China) was studied through the paired comparison method in July 2019. The results showed that there were significant differences in fungal community composition between the rhizosphere soil of diseased plants and healthy plants. The changes in the composition and diversity of fungal communities in the rhizosphere soil of tobaccos are vital characteristics of tobaccos with bacterial wilt, and the imbalance in the rhizosphere microecosystem of tobacco plants may further aggravate the disease.
The rhizosphere refers to the soil microzone affected by the root system and mainly involves the thin layer (1-2 cm) around the surface of roots (Philippot et al., 2013). Due to its high nutrient density, this region is the most dynamic location for microbial interactions, and it hosts beneficial microorganisms, soil-borne pathogens, and competition among them (Kinkel et al., 2011; Raaijmakers et al., 2009). The rhizosphere not only contains thousands of species that are beneficial to plant growth and health (such as nitrogen-fixing bacteria, mycorrhizal fungi, plant growth-promoting rhizobacteria, biocontrol microorganisms, mycoparasitic fungi, and protozoa), but also massive amounts of plant pathogenic microorganisms, which can destroy the protective microbial barrier, overcome the innate defense mechanism of plants, and settle in the rhizosphere to cause plant diseases (Liu et al., 2016; Mendes et al., 2013). The microbial imbalance in the plant-soil-microorganism ecosystem is a crucial contributor to diseases (Classen et al., 2016), and some soil-borne pathogens, such as Rhizoctonia solani, Ralstonia solanacearum, and Fusarium oxysporum, are confirmed to possess close correlations with the microecological imbalance in the soil (Huang et al., 2020; Navarrete et al., 2013). The complexity and diversity of microbial communities in the rhizosphere are essential for maintaining the dynamic balance of the ecosystem (She et al., 2017). Healthy soil with balanced soil microbial communities can better respond to stress, which is conducive to promoting plant growth and reducing soil-borne diseases (Janvier et al., 2007; Nannipieri et al., 2003).
Ranking second among bacterial plant pathogens throughout the world, R. solanacearum is capable of infecting over 200 plants in 54 families (Kim et al., 2016; Salanoubat et al., 2002). R. solanacearum-induced tobacco bacterial wilt occurs throughout the world (including in China) and results in considerable yield and economic losses to tobacco production every year (Li et al., 2020; Zhou et al., 2012). Lincang tobacco-growing areas, which represent one of the key tobacco areas recently developed in China, are located in the southwestern border region of Yunnan, China, and are subjected mainly to tropical and subtropical monsoon climates (tropical area), with clear dry and rainy seasons, sufficient rainfall, and abundant solar radiation. Bacterial wilt is also a major soil-borne disease in Lincang tobacco-growing areas, with an incidence rate of 9.7-15.3%, or even >70.8% in some serious plots; thus, bacterial wilt becomes a crucial factor hindering the development of the tobacco industry in these areas. In previous studies, tobacco bacterial wilt has been mainly prevented and controlled by virtue of the utilization of planting systems, chemical agents, and organic fertilizers that unquestionably exhibit certain side effects (Liu et al., 2014; Yuliar et al., 2015). Currently, the regulation of natural microbial communities is deemed as one of the most promising strategies for improving soil health to achieve a comprehensive and sustainable disease management (Chaparro et al., 2012; Liu et al., 2016). Some rhizosphere microbial communities have been shown to be antagonistic to soil-borne pathogens. For example, Trichoderma spp. is a class of important biocontrol fungi, which can inhibit target pathogens through competition, parasitism and inducing of host resistance (Zheng et al., 2021). Penicillium played an important role in secreting cellulase and antibiotic (Martins et al., 2008). More than 30 parasitic fungi (Paecilomyces lilacinus, Pochonia chlamydosporia, Coprinus comatus, Gliocladium roseum, etc.) of root knot nematode have been reported (Liu, 2011; Yang et al., 2004). Although these previous studies precisely and comprehensively illustrated the involvement of soil fungus in antagonistic interaction with soil-borne pathogens, works in identifying the specific group of soil fungus associated with the occurrence of bacterial wilt in the field are still limited. For this reason, a deep understanding of the changes in microbes in the rhizosphere soil during the development of soil-borne diseases is of great significance to clarify the interaction between microbes in the rhizosphere soil and soil-borne diseases. In this study, the difference in fungal community composition between R. solanacearum-infected soil and healthy soil was analyzed via Illumina MiSeq high-throughput sequencing technology in order to examine the associations of fungal community composition and diversity in the rhizosphere soil of tobacco plant with bacterial wilt and to render a scientific basis for the effective prevention or ecological prevention and control of bacterial wilt.
The standards used to examine flue-cured tobacco bacterial wilt disease were based on the tobacco pest classification and survey methods (GB/T 23222-2008), P.R. China. Disease incidence was calculated by the percentage of diseased tobaccos in each field. Disease index was evaluated using a disease score method: 0 = plants without visible symptoms, 1 = chlorotic spots on stems occasionally or less than half of the leaves wilted on unilateral stems, 3 = (30-50%) black streak less than half the height of the stem or between half to two-thirds of the leaves wilted on unilateral stems, 5 = (50-70%) black streak over half the height of the stem but not reaching to the top of the stem or more than two-thirds of the leaves wilted on unilateral stems, 7 = (70-90%) black streak reaching the top of the stem or all leaves wilted, and 9 = death of plants. Disease index was calculated using the formula:
r is the disease severity; N is the number of infected tobaccos with a rating of r; n is the total number of tobaccos tested; and R is the value of the highest disease severity in each field.
The soil samples were collected in Mengsa Town, Gengma County, Lincang City, Yunnan (23°33′40″N, 99°23′22″E) and Boshang Town, Linxiang District, Lincang City, Yunnan in July 2019. In the test areas, tobacco fields having typical and serious bacterial wilt (Ralstonia solanacearum) levels were selected. In the same plot, with similar conditions and soil properties, plants with typical symptoms of bacterial wilt (R. solanacearum) and healthy plants were sampled, with three plants per group.
The topsoil was removed and whole plants were removed. The loose soil was shaken off, and the soil attached to the root at 0-4 mm was collected as rhizosphere soil. The samples were named as Gengma infested soil (GM_D), Gengma uninfested soil (GM_H), Boshang infested soil (BS_D), and Boshang uninfested soil (BS_H). After removing the impurities and residual fine roots, the soil samples were put separately into sterile self-sealed bags, immediately placed in liquid nitrogen, transported back to the laboratory and stored at −80°C.
Total DNA in soil samples was extracted in accordance with the instructions of a FastDNA SPIN Kit for Soil (MP Biomedicals, Union City, CA, USA). DNA concentration and purity were detected using a NanoDrop2000, and the DNA quality was determined using 1% agarose gel electrophoresis.
Primers ITS1 F (5′-ACTTGGTCATTTAGGAAGTAA-3′) and ITS2 R (5′-BGCTGCGTTCTTCATCGATGC-3′) were used to amplify the fungal 18S rRNA gene. The amplification procedure was as follows: pre-denaturation at 95°C for 3 min, 36 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s, followed by a final extension at 72°C for 10 min. The amplification system was as follows: 4 μl 5× FastPfu buffer, 2 μl 2.5 mmol/l dNTPs, 0.8 μl forward primer (5 μmol/l), 0.8 μl reverse primer (5 μmol/l), 0.4 μl FastPfu polymerase, 0.2 μl bovine serum albumin, 10 ng DNA template and water added ddH2O to a final 20-μl volume.
The polymerase chain reaction products were recovered by 2% agarose gel electrophoresis and further purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Then, they were sequenced by Shanghai Majorbio Bio-pharm Technology Co., Ltd. using MiSeq.
Sequence quality control and analysis. The original sequence were spliced using FLASH (Caporaso et al., 2011) software. They were then filtered using Usearch (Edgar, 2013), and the chimeric sequences were removed to obtain the effective sequences. The operational taxonomic units (OTUs) were clustered using UPARSE at a 97% sequence similarity level. The sequences were annotated by RDP classifier (Wang et al., 2007) and the Silva database (Altschul et al., 1990), and the dilution curve was made using Mothur. The library coverage, Shannon, Simpson, ACE and Chao1 indexes were calculated, and the species diversity and richness indexes were evaluated. A principal coordinate analysis was performed with a Bray-Curtis dissimilarity matrix using Qiime. The rarefaction curves, veen diagram and community composition histogram and heatmap were generated from the R software.
Table 1 shows the number of OTUs and diversity index of fungal communities in the rhizosphere soil samples that were subjected to different treatments at a similar level of 97%. The fungi in the rhizosphere soil of flue-cured tobaccos in different test plots exhibited the same trend for number of OTUs, and the number of OTUs was higher in bacterial wilt-infected soil than in healthy soil. In particular, the number of OTUs of fungi in diseased soil from Boshang (BS_D) and diseased soil from Gengma (GM_D) was increased by 13.82% and 0.87%, respectively, compared with that in healthy soil from Boshang (BS_H) and healthy soil from Gengma (GM_H). According to the Venn diagram of the OTU distribution of fungi (Fig. 1), only 404 OTUs were shared by BS_D and BS_H, which accounted for only 21.23% of the total OTUs (1,903) in the areas. The common OTUs (579) in GM_D and GM_H accounted for only 22.67% of the total OTUs (2,553) in the areas. These results suggest that bacterial wilt affects the number of OTUs of fungi.
The diversity of fungal communities in the rhizosphere soil of flue-cured tobaccos was analyzed, and the results showed that compared with those in BS_H, the Shannon, ACE and Chao1 index in BS_D increased by 3.03%, 10.52%, and 10.73%, respectively. In comparison with GM_H, GM_D presented an increase (3.04%) in the Shannon index and decreases of 3.90% and 2.71% in the ACE and Chao1 indices, respectively. In general, bacterial wilt has certain influences on the number of OTUs, as well as the diversity and richness, of the fungal community in the rhizosphere soil of flue-cured tobacco.
Fig. 2 shows the relative abundance of fungi in the rhizosphere soil samples under each treatment at the phylum level. The mycobiota in flue-cured tobacco rhizosphere soil under different treatments mainly included Ascomycota, Basidiomycota, and Mortierellomycota, and the contents of these three fungi accounted for more than 88% of the total amount of fungi in the soil under different treatments. Ascomycota, Basidiomycota, and Mortierellomycota displayed a similar trend in relative abundance in bacterial wilt-infected soil and healthy soil in the different test plots. The relative abundances of Ascomycota and Mortierellomycota were higher in bacterial wilt-infected soil than in healthy soil; their relative abundances in BS_D were increased by 7.46% and 12.64%, respectively, when compared to those in BS_H, whereas their relative abundances in GM_D were increased by 14.14% and 11.48% in comparison with those in GM_H. In contrast, Basidiomycota showed a decreased relative abundance in bacterial wilt-infected soil when compared with that in healthy soil; when comparing BS_D with BS_H and GM_D with GM_H, the relative abundances decreased by 21.43% and 7.27%, respectively.
At the genus level, the mycobiota in flue-cured tobacco rhizosphere soil under different treatments was also significantly different (Fig. 3). Fusarium, Trichoderma, and Mortierella had a higher relative abundance in bacterial wilt-infected soil than in healthy soil in the two test plots; when comparing BS_D with BS_H, the relative abundance was increased by 28.95%, 5.90%, and 19.18%, respectively, and when comparing GM_D with GM_H, the relative abundance was increased by 33.97%, 38.04%, and 20.59%. Comparing BS_D with BS_H and GM_D with GM_H, the relative abundance of Talaromyces was decreased by 35.97% and 5.75%, respectively. The relative abundance of Penicillium was 18.50% higher in BS_D than that in BS_H, whereas no significant difference was found in the relative abundance of Penicillium between GM_D and GM_H. The relative abundance of Aspergillus was reduced by 53.31% in BS_D when compared with that in BS_H, but GM_D exhibited an increase of 55.56% in the relative abundance of Aspergillus in comparison with GM_H.
According to the species annotation and relative abundance information of each sample at the species level, the top 50 species (in terms of relative abundance) were selected to generate a community heatmap (Fig. 4). Among accurately classified and named species, the relative abundance of Trichoderma asperellum followed the order of GM_D > GM_H > BS_D > BS_H, whereas the relative abundance of Penicillium ochrochloron and Mortierella elongata followed the order of BS_D > BS_H > GM_D > GM_H. These results indicate that tobacco bacterial wilt disease greatly affects the distribution and composition of fungal communities in the rhizosphere soil of flue-cured tobaccos.
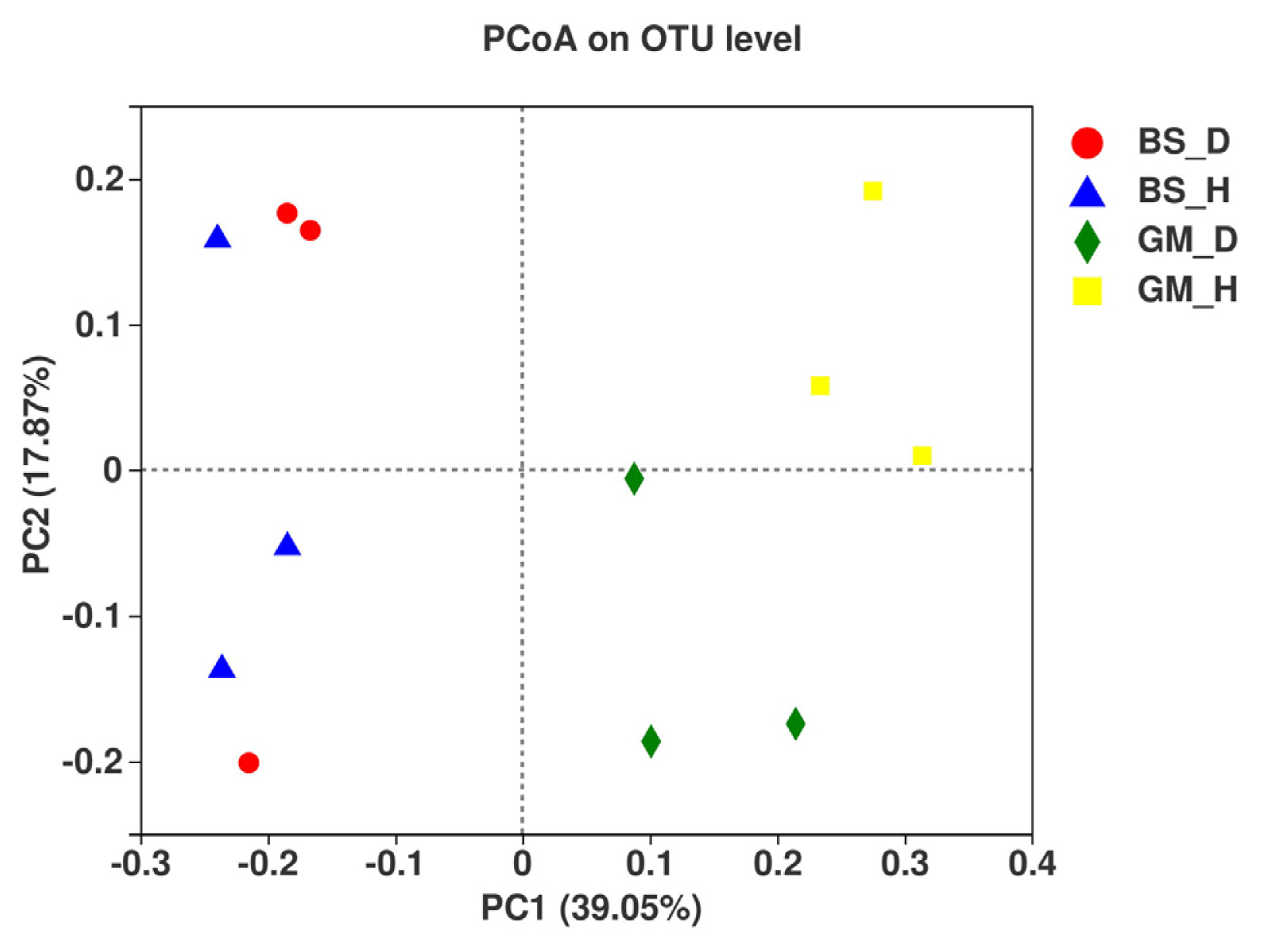
Principal coordinate analysis was conducted using the Bray-Curtis distance method. The degree of explanation of principal coordinate 1 (PC1) and PC2 for the soil sample differences was 39.05% and 17.87%, respectively, with a total of 56.92% (Fig. 5). In the Boshang test plots (BS_D and BS_H), fungal communities in the rhizosphere soil of flue-cured tobaccos were mainly distributed on the left side of the PC1 axis, whereas in the Gengma test plots (GM_D, GM_H), they were mainly distributed on the right side of the PC1 axis. The two fungal communities were far apart, which indicated that the community composition of fungi in the rhizosphere soil of flue-cured tobaccos is significantly different among the different test areas. However, bacterial wilt-infected soil and healthy soil samples of the two test plots were obviously separated and clustered differently. In particular, GM_H was distributed on the upper side of the PC2 axis, whereas GM_D was distributed on the lower side of the PC2 axis, thus suggesting that the community composition of fungi in bacterial wilt-infected soil is significantly different from that in healthy soil. These results show that bacterial wilt has a great impact on the distribution and composition of fungal communities in the rhizosphere soil of flue-cured tobaccos.
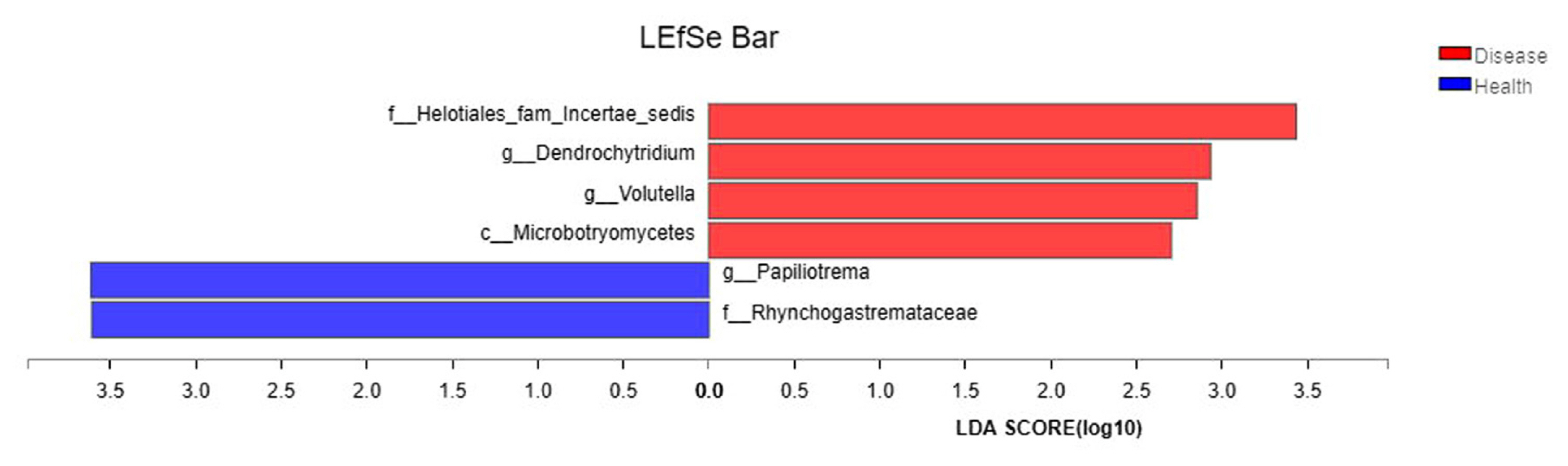
To determine the flora causing the difference in fungal communities between diseased soil and healthy soil, the samples from Boshang and Gengma were divided into the bacterial wilt-infected soil group and the healthy soil group. Then, linear discriminant analysis (LDA) effect size (LEfSe) was employed for multilevel species difference analysis, and samples in the different groups were subjected to LDA, according to taxonomic composition, and communities or species causing significant differences in the division of the samples were identified. The results revealed that among the species with an LDA value greater than 2.5, Helotiales_fam_Incertae_sedis, Dendrochytridium, Volutella, and Microbotryomycetes were enriched mainly in bacterial wilt-infected soil, whereas Papiliotrema and Rhynchogastremataceae were located mainly in healthy soil (Fig. 6). These microbes enriched in bacterial wilt-infected soil and healthy soil may be important groups contributing to the difference in community composition.
Tobacco bacterial wilt is a severe soil-borne disease, in which its onset is correlated with the growth and pathogenicity of pathogenic bacteria and other factors, such as the resistance of host plants, environmental factors, soil physical and chemical properties, and community composition of rhizosphere microbes (Cai et al., 2021; He and Xue, 2005). In particular, soil microbes exhibit the strongest biological activity in the soil microecosystem and are vital determining factors of soil biological characteristics. The abundance and composition of soil microbial communities have a close correlation with the resistance of plants to soil-borne diseases (van Elsas et al., 2002). In this study, the fungal communities in the rhizosphere soil of flue-cured tobaccos at the Boshang and Gengma test plots were distributed on both sides of the PC1 axis and far apart from each other, thus indicating that the fungal community composition at the two test plots is quite different. The soil microbial communities are often affected by many factors, such as plant types, climate, soil properties, and agricultural practices (Garbeva et al., 2004; Wieland et al., 2001). Interestingly, fungal communities in the bacterial wilt-infected soil and healthy soil displayed a similar trend in the two experimental plots. The bacterial wilt-infected soil and healthy soil samples of the two experimental plots were seemingly separated and clustered differently, thus indicating that the fungal community composition is significantly different between bacterial wilt-infected soil soil and healthy soil, which illustrates that bacterial wilt may give rise to changes in microbial communities in the rhizosphere soil of tobaccos. However, changes in the community composition of rhizosphere microbes may also make the roots more susceptible to pathogen infection.
The differences in the dominant flora and relative abundance of fungal communities in the bacterial wilt-infected soil and healthy soil were further analyzed at the phylum, genus, and species levels. The results showed that the relative abundance of Ascomycota was higher in the bacterial wilt-infected soil than that in healthy soil, whereas Basidiomycota showed a reverse trend in terms of relative abundance. Studies have demonstrated that there are numerous pathogens in Ascomycota, but these pathogens are not pathogenic to crops; instead, they usually accumulate in the roots of plants and damage the surface of roots, creating conditions for the infection of some pathogens (Wei, 2012). Ascomycota exhibited a high abundance in the rhizosphere soil of diseased tobacco plants, likely providing conditions for the invasion of R. solanacearum. Basidiomycota serves as a crucial decomposer in the carbon cycle, and it can secrete digestive enzymes to break down organic substances (such as cellulose, lignocellulose, and lignin in plant litters) into smaller molecules (Purahong et al., 2016). In addition, some fungi of Tremellomycetes in Basidiomycota play an important role in inhibiting plant rhizosphere soil-borne diseases, representing biocontrol fungi that can effectively contain diseases (Sharma-Poudyal et al., 2017). Therefore, Basidiomycota exhibited an increased relative abundance in healthy soil, which is conducive to the decomposition of plant residues in the soil and the promotion of the carbon cycle and plays a positive role in improving the rhizosphere microecological environment. Moreover, the relative abundances of Fusarium and Trichoderma in bacterial wilt-infected soil were higher than those in healthy soil in the two test plots. Fusarium is a filamentous fungus that is most harmful to plants, and its secreted toxin can cause over 100 animal and plant diseases (Zhang et al., 2013). The increase in its relative abundance may promote the development of tobacco bacterial wilt. Additionally, Trichoderma is a major biocontrol fungus with functions including solubilizing phosphorus and potassium and fixing nitrogen, which can impede target pathogens via competition, mycoparasitism, antibiosis, and the induction of host resistance, thereby exerting antibacterial effects (Zheng et al., 2021). The increase in the relative abundance of Trichoderma may be a result of the resistance response of the rhizosphere microecosystem to the disease during the infection of pathogens in the plant (Raghavandra et al., 2017). Furthermore, LEfSe analysis showed that Helotiales_fam_Incertae_sedis, Dendrochytridium, Volutella, and Microbotryomycetes were enriched mainly in bacterial wilt-infected soil, whereas Papiliotrema and Rhynchogastremataceae were located mainly in healthy soil. Previous studies have demonstrated that key microbial groups capable of suppressing pathogens are found in all crops, and some of them are also important players in maintaining the structure and function of the entire ecosystem (Berendsen et al., 2012; Lawson et al., 2019). Generally, these different species in diseased and healthy soil may be important groups causing the differences in community composition and are closely related to the occurrence of the disease.
Soil microbial diversity is very important for maintaining soil health and inhibiting soil-borne plant diseases. The loss of soil microbial diversity can easily lead to an increase in plant soil-borne diseases, whereas high microbial diversity and activity promotes plant growth, enhances plant defense, and inhibits the occurrence of soil-borne diseases (Mendes et al., 2015). However, the results of this study revealed that the number of OTUs and Shannon index were higher in bacterial wilt-infected soil than those in healthy soil in the two test plots, which is contrary to the findings in previous studies; i.e., the OTU number and Shannon index are always higher in healthy soil than those in bacterial wilt-infected soil at all stages of tobacco growth (Wang et al., 2017). However, the results are similar to the research conclusion that the numbers of bacteria and fungi are positively correlated with the severity of bacterial wilt in the soil (Kuang et al., 2003). Various factors, such as the physical and chemical properties of the soil, climatic conditions, and cultivation management measures, in different experimental areas are different, and further studies should be performed on the specific reasons underlying these factors.
In conclusion, the occurrence of bacterial wilt has a close correlation with the community composition and diversity of fungi in the rhizosphere soil of flue-cured tobaccos. The rhizosphere soil of diseased tobacco plant exhibits superior fungal diversity and richness, but pathogenic fungi, such as Ascomycota and Fusarium, have a significantly increased relative abundance. The imbalance of the rhizosphere microecosystem of the tobacco plant may further aggravate the disease. Therefore, enhancing the resistance of plant rhizosphere microecosystem by reshaping a stable and more diversified rhizosphere microbial community composition through effective measures is one of the ways to prevent and control tobacco bacterial wilt.
Acknowledgments
This study was supported by the Project of China National Tobacco Corporation Yunnan Branch (No. 2020530000242028, 2021, 2023, 2020, 2010) and Shanghai Tobacco Group Co., Ltd.
Fig. 1
Venn graph of fungus operational taxonomic units distribution in rhizosphere soils at different treatments.
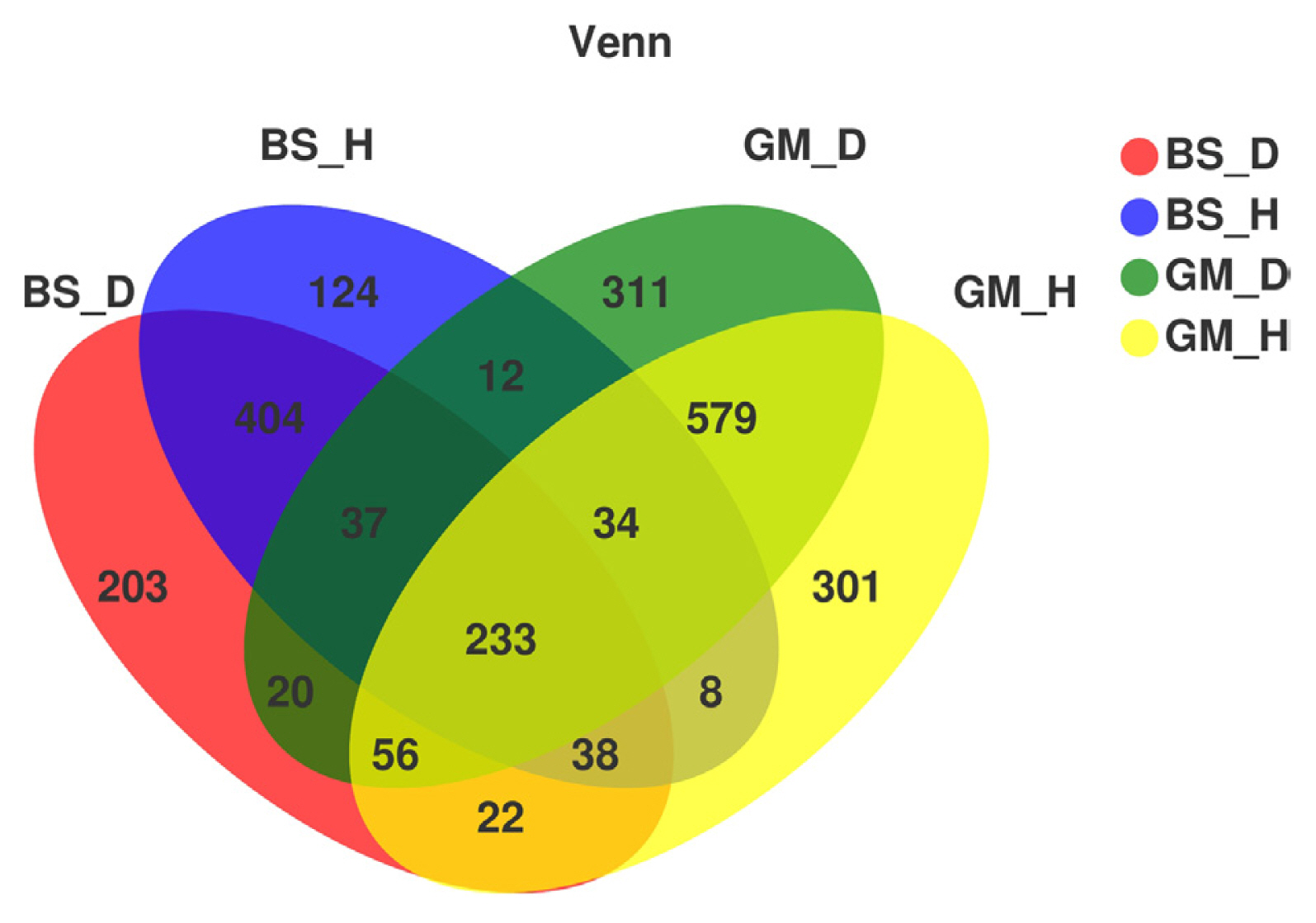
Fig. 2
The relative abundance of fungus on phylum level in rhizosphere soils at different treatments.
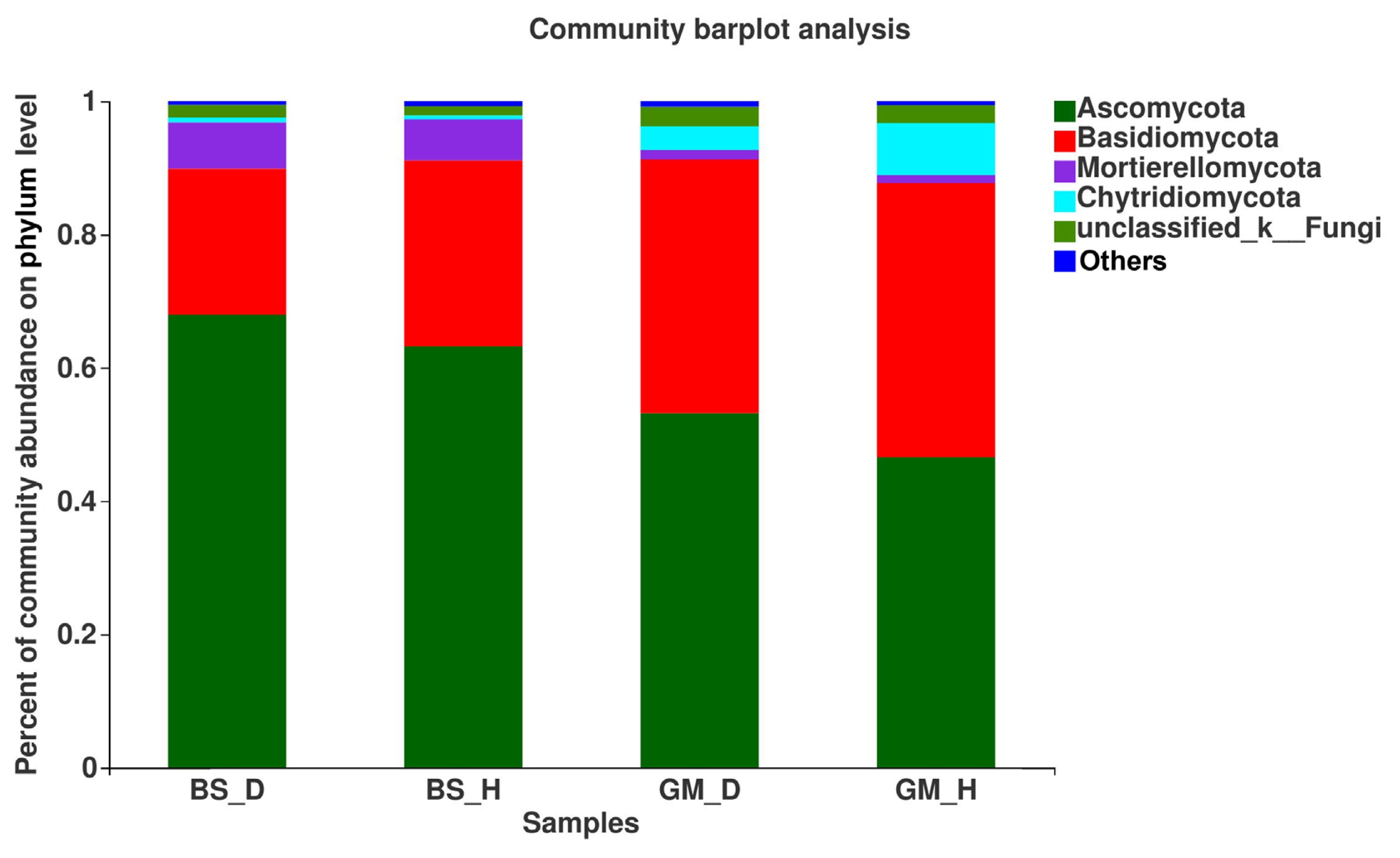
Fig. 3
The relative abundance of fungus on genus level in rhizosphere soils at different treatments.
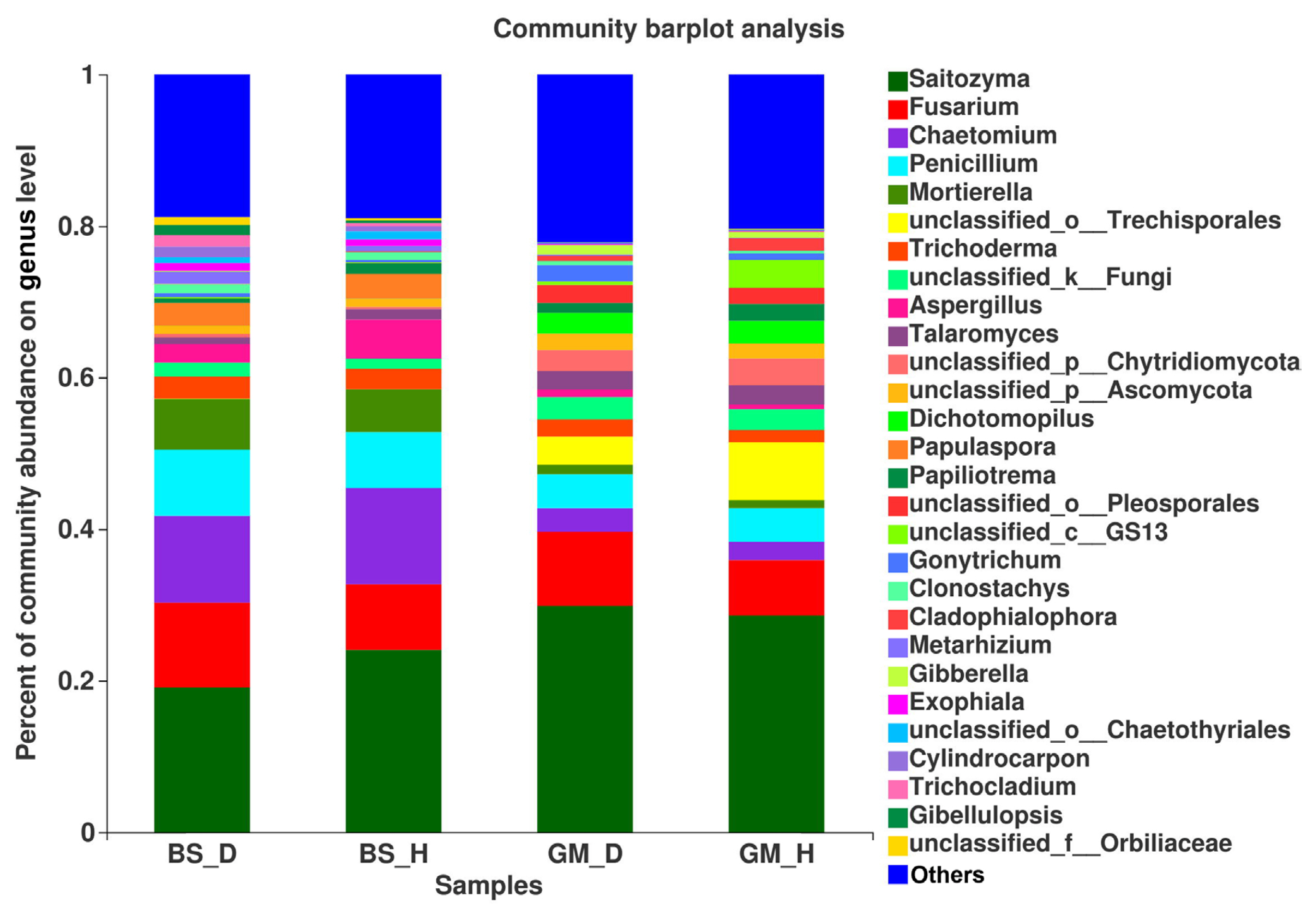
Fig. 4
The microbial community heatmap on species level in rhizosphere soils at different treatments.

Fig. 5
Principal coordinate analysis cluster analysis of fungus community in rhizosphere soils at different treatments. OTU, operational taxonomic unit.
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. and Lipman, D. J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410.


Berendsen, R. L., Pieterse, C. M. J. and Bakker, P. A. H. M. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci. 17:478-486.


Cai, Q., Zhou, G., Ahmed, W., Cao, Y., Zhao, M., Li, Z. and Zhao, A. 2021. Study on the relationship between bacterial wilt and rhizospheric microbial diversity of flue-cured tobacco cultivars. Eur. J. Plant Pathol. 160:265-276.


Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., Fierer, N. and Knight, R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U. S. A. 108:Suppl 1. 4516-4522.



Chaparro, J. M., Sheflin, A. M., Manter, D. K. and Vivanco, J. M. 2012. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 48:489-499.


Classen, A. T., Sundqvist, M. K., Henning, J. A., Newman, G. S., Moore, J. A. M., Cregger, M. A., Moorhead, L. C. and Patterson, C. M. 2016. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6:1-21.

Edgar, R. C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10:996-998.



Garbeva, P., van Veen, J. A. and van Elsas, J. D. 2004. Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42:243-270.


He, Y. and Xue, L. 2005. Biological effects of rare earth elements and their action mechanisms. J. Appl. Ecol. 16:1983-1989 (in Chinese).
Huang, K., Jiang, Q., Liu, L., Zhang, S., Liu, C., Chen, H., Ding, W. and Zhang, Y. 2020. Exploring the key microbial changes in the rhizosphere that affect the occurrence of tobacco root-knot nematodes. AMB Express 10:72.




Janvier, C., Villeneuve, F., Alabouvette, C., Edel-Hermann, V., Mateille, T. and Steinberg, C. 2007. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 39:1-23.

Kim, B.-S., French, E., Caldwell, D., Harrington, E. J. and Iyer-Pascuzzi, A. S. 2016. Bacterial wilt disease: host resistance and pathogen virulence mechanisms. Physiol. Mol. Plant Pathol. 95:37-43.

Kinkel, L. L., Bakker, M. G. and Schlatter, D. C. 2011. A coevolutionary framework for managing disease-suppressive soils. Annu. Rev. Phytopathol. 49:47-67.


Kuang, C. F., He, Z. M., Tang, R. Y., Huang, S. Y. and Deng, F. X. 2003. Determination of microbial number and physiological strains in soil infected with bacterical wilt. Chin. Tob. Sci. 24:43-45 (in Chinese).
Lawson, C. E., Harcombe, W. R., Hatzenpichler, R., Lindemann, S. R., Löffler, F. E., O’Malley, M. A., García Martín, H., Pfleger, B. F., Raskin, L., Venturelli, O. S., Weissbrodt, D. G., Noguera, D. R. and McMahon, K. D. 2019. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 17:725-741.




Li, Y. Y., Wang, L., Peng, W. X. and Li, X. H. 2020. Analysis of the changing trend of microbial community in rhizosphere soil during different stages of tobacco bacterial wilt infection. Chin. Tob. Sci. 41:73-78 (in Chinese).
Liu, X. L. 2011. Infection mechanism of Paecilomyces lilacinus strains on Meloidogyne incognita. PhD thesis. Anhui Agricultural University, Hefei, China. (in Chinese).
Liu, X., Zhang, S., Jiang, Q., Bai, Y., Shen, G., Li, S. and Ding, W. 2016. Using community analysis to explore bacterial indicators for disease suppression of tobacco bacterial wilt. Sci. Rep. 6:36773.




Liu, Y. X., Li, X., Cao, Y., Ning, L. U. and Shi, J. X. 2014. Field control efficiency of tobacco specific bio-organic fertilizer on tobacco bacterial wilt. J. Plant Nutr. Fertil. 20:1203-1211 (in Chinese).
Martins, L. F., Kolling, D., Camassola, M., Dillon, A. J. P. and Ramos, L. P. 2008. Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour. Technol. 99:1417-1424.


Mendes, L. W., Tsai, S. M., Navarrete, A. A., de Hollander, M., van Veen, J. A. and Kuramae, E. E. 2015. Soil-borne microbiome: linking diversity to function. Microb. Ecol. 70:255-265.



Mendes, R., Garbeva, P. and Raaijmakers, J. M. 2013. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37:634-663.


Nannipieri, P., Ascher, J., Ceccherini, M. T., Landi, L., Pietramellara, G. and Renella, G. 2003. Microbial diversity and soil functions. Eur. J. Soil Sci. 54:655-670.


Navarrete, A. A., Kuramae, E. E., de Hollander, M., Pijl, A. S., van Veen, J. A. and Tsai, S. M. 2013. Acidobacterial community responses to agricultural management of soybean in Amazon forest soils. FEMS Microbiol. Ecol. 83:607-621.


Philippot, L., Raaijmakers, J. M., Lemanceau, P. and van der Putten, WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11:789-799.



Purahong, W., Wubet, T., Lentendu, G., Schloter, M., Pecyna, M. J., Kapturska, D., Hofrichter, M., Krüger, D. and Buscot, F. 2016. Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 25:4059-4074.


Raaijmakers, J. M., Paulitz, T. C., Steinberg, C., Alabouvette, C. and Moënne-Loccoz, Y. 2009. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341-361.

Raghavendra, A. K. H., Bissett, A. B., Thrall, P. H., Morin, L., Steinrucken, T. V., Galea, V. J., Goulter, K. C. and van Klinken, R. D. 2017. Characterisation of above-ground endophytic and soil fungal communities associated with dieback-affected and healthy plants in five exotic invasive species. Fungal Ecol. 26:114-124.

Salanoubat, M., Genin, S., Artiguenave, F., Gouzy, J., Mangenot, S., Arlat, M., Billault, A., Brottier, P., Camus, J. C., Cattolico, L., Chandler, M., Choisne, N., Claudel-Renard, C., Cunnac, S., Demange, N., Gaspin, C., Lavie, M., Moisan, A., Robert, C., Saurin, W., Schiex, T., Siguier, P., Thébault, P., Whalen, M., Wincker, P., Levy, M., Weissenbach, J. and Boucher, C. A. 2002. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497-502.



Sharma-Poudyal, D., Schlatter, D., Yin, C., Hulbert, S. and Timothy, P. 2017. Long-term no-till: a major driver of fungal communities in dryland wheat cropping systems. PLoS ONE 12:e0184611.



She, S., Niu, J., Zhang, C., Xiao, Y., Chen, W., Dai, L., Liu, X. and Yin, H. 2017. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch Microbiol. 199:267-275.



van Elsas, J. D., Garbeva, P. and Salles, J. 2002. Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13:29-40.

Wang, Q., Garrity, G. M., Tiedje, J. M. and Cole, J. R. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261-5267.




Wang, R., Zhang, H., Sun, L., Qi, G., Chen, S. and Zhao, X. 2017. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 7:343.




Wei, Z. 2012. Effect and mechanism of bio organic fertilizer on controlling soil borne tomato bacterial wilt. PhD thesis. Nanjing Agricultural University, Nanjing, China. (in Chinese).
Wieland, G., Neumann, R. and Backhaus, H. 2001. Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl. Environ. Microbiol. 67:5849-5854.




Yang, S. J., Lei, L. P., Zhu, M. L., Xia, Z. Y. and Li, Y. H. 2004. Screening of the parasitical fungi of root knot nematode in tobacco. Southwest Chin. J. Agric. Sci. 17:151-154 (in Chinese).
Yuliar Nion, Y. A. and Toyota, K. 2015. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 30:1-11.


Zhang, W. N., Jia, J., Lu, X. Y., Chen, Z. J., Kong, Q. and Chen, Z. 2013. Research progress of Fusarium mycotoxins. Guangdong Agric. Sci. 40:130-133 (in Chinese).
Zheng, Y. X., Yang, M., Wang, J. M., Xu, Y. L., Cai, X. J., Huang, F. Y., Tong, W. J., Chen, X. L., Yu, L. and He, Y. S. 2021. Effects of tobacco root rot on fungal community structure in tobacco rhizosphere soil. Chin. Tob. Sci. 42:50-55 (in Chinese).
Zhou, X. J., Wang, J., Yang, Y. W., Zhao, T. C. and Gao, B. D. 2012. Advances in tobacco bacterial wilt disease. Microbiol. China 39:1479-1486 (in Chinese).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




