An Acidic PATHOGENESIS-RELATED1 Gene of Oryza grandiglumis is Involved in Disease Resistance Response Against Bacterial Infection
Article information
Abstract
Wild rice, Oryza grandiglumis shows hyper-resistance response to pathogen infection. In order to identify genes necessary for defense response in plants, we have carried out a subtractive hybridization coupled with a cDNA macroarray. An acidic PATHOGENESIS-RELATED1 (PR1) gene of the wild rice is highly identical to the acidic PR1 genes of different plant species. The OgPR1a cDNA has an apparent single open reading frame with a predicted molecular mass 40,621 Da and an isoelectic point of 5.14. Both in silico analysis and a transient expression assay in onion epidermal cells revealed that the OgPR1a protein could be localized in intercellular space in plants. The OgPR1a mRNA was strongly transcribed by the exogenous treatment with ethylene and jasmonic acid as well as protein phosphatase inhibitors. Additionally, ectopic expression of the OgPR1a conferred disease resistance on Arabidopsis to the bacterial and fungal infections.
Plants have unique defense mechanisms to continue their life against adverse environmental conditions since they are sessile living-organisms without any specialized immune cells and circulation system. At the phytopathological point of view, they have acquired effective immune responses in order to protect themselves against pathogen infections.
Molecular sensing of microbe-associated molecular patterns (MAMPs) by pattern recognition receptor (PRRs) of host plants can initiate relatively weak defense responses, called pattern-triggered immunity (PTI) in plants (Han and Jung, 2013; Jones and Dangl, 2006). As well, plants have another immune receptors, called race-specific resistance (R) proteins to directly or indirectly recognize pathogen-derived effector proteins in plant cells (Jones and Dangl, 2006; Spoel and Dong, 2012). Consequently, the R gene-mediated response restricts the growth of pathogen at the infected regions to prevent successful colonization in plants. The built-in defense responses include the hormone-mediated defense responses, the rapid accumulation of callose and reactive oxygen species in infected sites, alternation of transcriptome, and so on (De Vos et al., 2005; Katagiri and Tsuda, 2010; Kawahara et al., 2012). Since Van Loon and Van Kammen (1970) had identified different kinds of pathogenesis-related (PR) proteins in leaves of tobacco plants that exhibited hypersensitive response (HR) to the infection of Tobacco Mosaic Virus (TMV), 17 different kinds of PR proteins were isolated in various plant species (Van Loon et al., 2006).
PR1 proteins are one of well-studied defense-related proteins, even if the molecular function of PR1 protein is not obvious yet (Alexander et al., 1993). Based on the isoelectic point of PR1 proteins, they were classified into two groups as acidic or basic forms (Van Loon and Van Strien, 1999). The basic PR1 genes exhibit approximately ∼65% sequence identities to the acidic PR1 (Cornelissen et al., 1987; Payne et al., 1989). They generally have two discrete signal sequences at N-terminal and C-terminal regions to target extracellular space and vacuole, respectively (Nidermann et al., 1995; Payne et al., 1989; Sessa et al., 1995). These features proposed that the basic PR1 proteins could be dually localized to both regions in plants. Unlike the basic PR1 proteins, acidic PR1 proteins seem to be secreted to the intercellular space in infected leaves of plants (Carr et al., 1987; Parent and Asselin, 1984). The acidic PR1 proteins can be sub-divided into three different branches based on sequence identities and their acidity for conferring protein stability in response to acidic pH in the extracellular space and proteolytic attack (Buchel and Linthorst, 1999). Either acidic or basic PR1 mRNAs were strongly transcribed in leaves of plants exposed to biotic and abiotic stresses, such as pathogen infection, UV-treatments, and wounding (Brederode et al., 1991; Mitsuhara et al., 2008). In addition, exogenous treatment with plant hormones, salicylic acid (SA), jasmonate (JA) and ethylene (ET) also stimulated the strong transcription of PR1 mRNA in plants (Kim and Hwang, 2000; Reymond and Farmer, 1998; Zhang et al., 2010). Thus the level of PR1 mRNA was often used as a molecular marker to predict the establishment of immune response, such as HR and systemic acquired resistance (SAR) (Jung and Hwang, 2000; Jung et al., 2009). It was also known that purified or recombinant PR1 proteins had an anti-oomycete activity against Phytophthora infestans (Niderman et al., 1995). In addition, ectopic expression of PR1 gene enabled the transgenic plants to effectively resist against the pathogen infection, although some reports showed contradictory results (Alexander et al., 1993; Linthorst al., 1989).
Sequence analysis of OgPR1a gene
Using SSH (suppression subtractive hybridization) and RACE (rapid amplification of cDNA ends) technology, OgPR1a (an acidic PATHOGENESIS-RELATED1 of O. grandiglumis) cDNA was successfully identified from leaves of wild rice pretreated with wounding and MAMPs (Kim et al., 2005). The EST sequence of OgPR1a gene has been submitted to the GenBank as an accession number CK429151. The OgPR1a cDNA consists of 507 nucleotides with an apparent single open reading frame with a predicted molecular mass 40,621 Da and an isoelectic point of 5.14 (Fig. 1A). The predicted OgPR1a protein has a typical N-terminal hydrophobic signal peptide, which is required to enter into endoplasmic reticulum (Figs. 1A and 1B). The N-terminal signal sequences are completely identical to those of acidic OsPR1a gene (Agrawal et al., 2000). Thus we expected that the acidic OgPR1a protein might be secreted to the extracellular space in leaves of wild rice. The deduced amino acid sequences of OgPR1a were compared to the previously identified acidic PR1 genes of different plant species (Fig. 1B). Not only did the amino acid sequences of OgPR1a have the highest identity to the acidic OsPR1a gene of cultivated rice plants (93.5%), but also show relatively higher identities to those of monocot plants than dicot plants. Additionally, six cysteine residues important for a proper protein folding were well conserved in the OgPR1a protein.
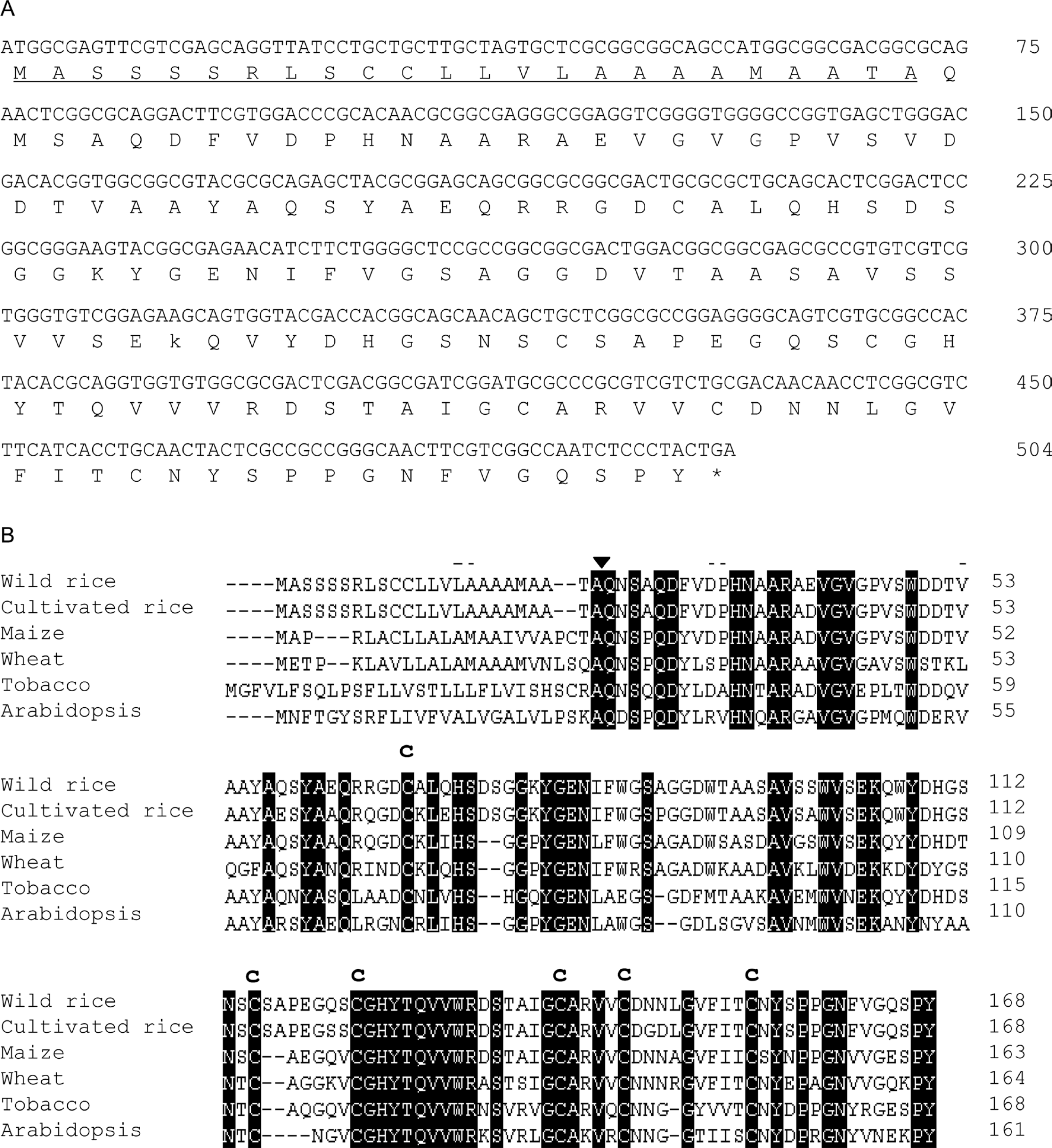
A typical acidic PATHOGENEGENE-RELATED1 (PR1) gene was identified in leaves of wild rice (Oryza grandiglumis). (A) Nucleotide and deduced amino acid sequence of the OgPR1a gene. The deduced amino acid is designated at the bottom of the sequence. An Asterisk represents the stop codon. The underline indicates the signal peptide sequences. (B) A comparative alignment of the deduced OgPR1a amino acid sequences with its homologous genes of different plant species. Identical amino acid residues are highlighted in black. The arrowhead indicates the cleavage site between the signal peptides and the mature proteins The positions of the cysteine residues for disulphide linkage were shown as Cs. Origin of sequences: rice (AJ278436), maize (U82200), wheat (CAA07473), tobacco (X06930), Arabidopsis (M90508), respectively.
Expression of OgPR1a mRNA by treatment of plant hormones
As mentioned in our previous article, OgPR1a mRNA was strongly transcribed in the leaves of wild rice in response to mechanical and chemical stimuli (Kim et al., 2005). Mechanical stresses tended to activate ET- and JA-dependent signaling pathway in plants (Díaz et al., 2002; Li et al., 2002). In addition to SA, these two plant hormones, ET and JA also act as main players for regulating defense responses in plants (Denancé et al., 2013). To check if the plant hormones could regulate transcription of OgPR1a gene, either ethephon (1 mM), which was converted to ethylene and phosphoric acid, or JA (100 μM) was applied on leaf segments of wild rice. The segments were incubated under continuous light condition until harvesting. Total RNAs were isolated from the leaves of wild rice in accordance with the manufacturer’s instruction (Invitrogen, Carlsbad, CA). All the procedures for northern hybridization were described elsewhere (Jeon et al., 2012; Shin et al., 2012). The OgPR1a mRNA was induced within 24 h after ethephon treatment and the level of OgPR1a mRNA were gradually increased until 72 h after treatment (Fig. 2A). Exogenous treatment of JA also stimulated OgPR1a transcription in leaf segments (Fig. 2B). These results indicate that plant hormones can finely tune the level of OgPR1a transcription in response to environmental stimuli, including wounding and pathogen infection. Moreover, the application of two protein phosphatase (PPase) inhibitors, cantharidin (CN, 1 μM) and endothall (EN, 1 μM) also induced the expression of OgPR1a mRNA in leaves of wild rice (Fig. 2B). One of possible explanations is that the transcription of OgPR1a might be regulated by hyperphosphorylated certain cellular proteins. It is also plausible that a certain signal transduction pathway regulated by a receptor protein was affected by the exogenous treatment with the PPase inhibitors, because the PPase inhibitors interfered with the activity of protein phosphatase(s) that works together with the receptor protein (Bajsa et al., 2011). Thus the transcription of PR1 mRNA seemed to be caused by the aberrant function of receptor protein in plants treated with PPase inhibitors. On the contrary, a PPase inhibitor, okadaic acid effectively suppressed PR1 expression in tobacco plants (Conrath et al., 1997). However, the molecular mechanism of the PPase inhibitors to regulate expression of PR1 gene in plants is still unclear.
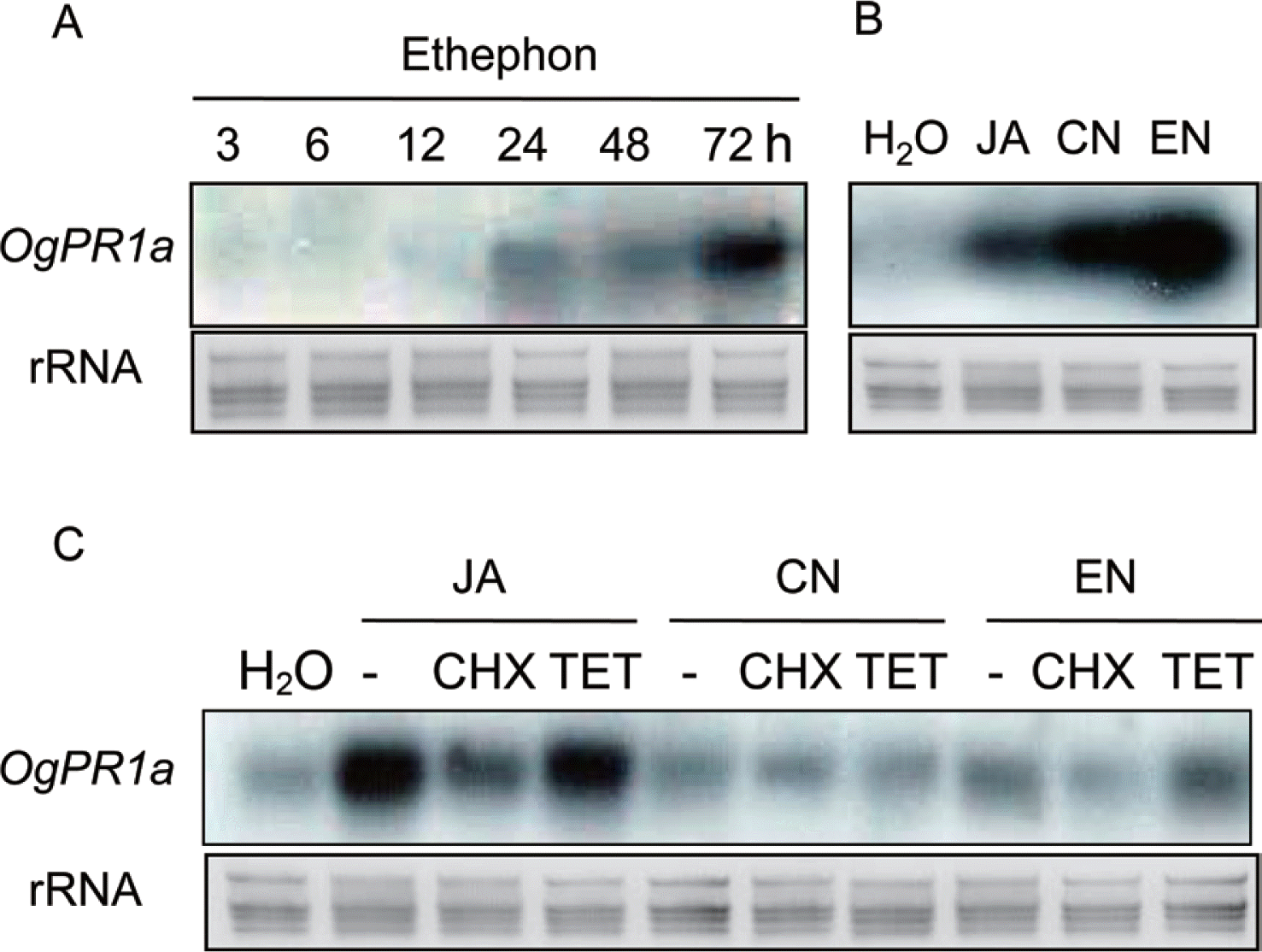
OgPR1a was strongly transcribed in leaves either treated with plant hormones or protein phosphatase inhibitors. (A) Expression of OgPR1a gene in leaves of seedling after ethephon treatment. One mM ethephon was applied on leaves of wild rice, and leaf segments were collected at indicated time points. (B) Exogenous treatment with 100 μM of jasmonic acid (JA), 1 μM of cantharidin (CN) and 1 μM of endothall (EN) also induced OgPR1a expression in the leaf segment. (C) Cycloheximide (CHX) inhibited transcription of OgPR1a gene. Rice seedlings were treated with 100 μM JA, 1 μM CN and 1 μM EN alone, or together with 10 μM CHX or 10 μM tetracycline (TET). H2O was used as mock control. As a representative of the equal RNA loading, the ribosomal RNAs in the membrane were stained with methylene blue.
Cycloheximide (CHX) and tetracycline (TET) are representative inhibitors of protein synthesis in eukaryotic and prokaryotic cells, respectively. Thus the drugs are used in the biochemical approach to study whether or not de novo protein synthesis was required for any given phenomena. To check an inhibitory efficacy of CHX and TET on Og-PR1a mRNA expression, 10 μM of each compound was supplemented to JA (100 μM), CN (1 μM), and EN (1 μM) solutions. Expectedly treatment of CHX effectively hindered the transcription of OgPR1a mRNA triggered by treatment with JA, CN and EN (Fig. 2C). These results show the requirement of de novo protein synthesis to induce OgPR1a mRNA expression under biotic or abiotic stress conditions.
OgPR1a-expressing Arabidopsis plants were resistant against bacterial infection
To examine the biological role of OgPR1a gene during pathogenesis and defense response, we have developed OgPR1a-expressing Arabidopsis plants (A. thaliana ecotype Col-0). A full-length cDNA fragment of OgPR1a was amplified by using gene specific primers (forward primer, 5’-ggatccatggcgagttcgtcgagcagg-3’; reverse primer, 5’-gagctctcagtagggagattggccgac-3’) and cloned into BamH1 and SacI sites of the pBI121 binary vector. The resulting construct was introduced into Arabidopsis genome via the floral dipping procedure in order to generate transgenic plants (Clough and Bent, 1998). The putative OgPR1a-expressing transgenic Arabidopsis plants were initially screened on the half strength of MS (Murashige and Skoog) media supplemented with kanamycin at 100 μg/ml. Most of kanamycin-resistant T1 generation plants exhibited the stable expression of OgPR1a gene in leaves of Arabidopsis plants without any exogenous stimuli (Fig. 3A). Among 20 independent transgenic plants, we have chosen 3 independent transgenic lines (#6, #11, and #16) for further study because of the reasons as following: (1) The OgPR1a mRNA was strongly expressed in the leaves of Arabidopsis and (2) T-DNA was interposed into a single locus in the Arabidopsis genome (Figs. 3A and 3B). To monitor disease resistance response of the OgPR1a-expressing plants against pathogen infection, Pseudomonas syringae pv. tomato DC3000 (OD600=0.0001) was infiltrated into leaves of 4-week old plants using a needless syringe. We counted the number of bacteria in the leaf discs on 3 days after inoculation. As shown in Fig. 3C, the ectopic expression of OgPR1a effectively inhibited the growth of Pseudomonas in leaves of transgenic Arabidopsis plants, compared to those in wild-type plants. Transgenic tobacco plants carrying a basic PR1 gene of Capsicum annuum (CaPR1b) showed enhanced disease resistance response to the infection by Ralstonia solanacearum and P. syringae pv. tabaci (Sarowar et al., 2005). A necrotrophic fungus Botrytis cinerea was also grown on potato dextrose agar under continuous fluorescent light to promote conidial formation. Three-week old wild-type Col-o and OgPR1-expressing Arabidopsis were inoculated with the conidial suspension (5×105 conidia/ml). The infected plants were kept on a high humidity chamber in order to trigger disease development during 3 days, and transferred in a normal growth chamber (Jung et al., 2008). As shown in figure 3D, symptom development was abolished in OgPR1a-expressing Arabidopsis plants, compared with those seen in wild-type Col-o plants. Our results strongly suggest that PR1 proteins have a certain role to inhibit the bacterial and fungal growth at the infected tissues in plants.
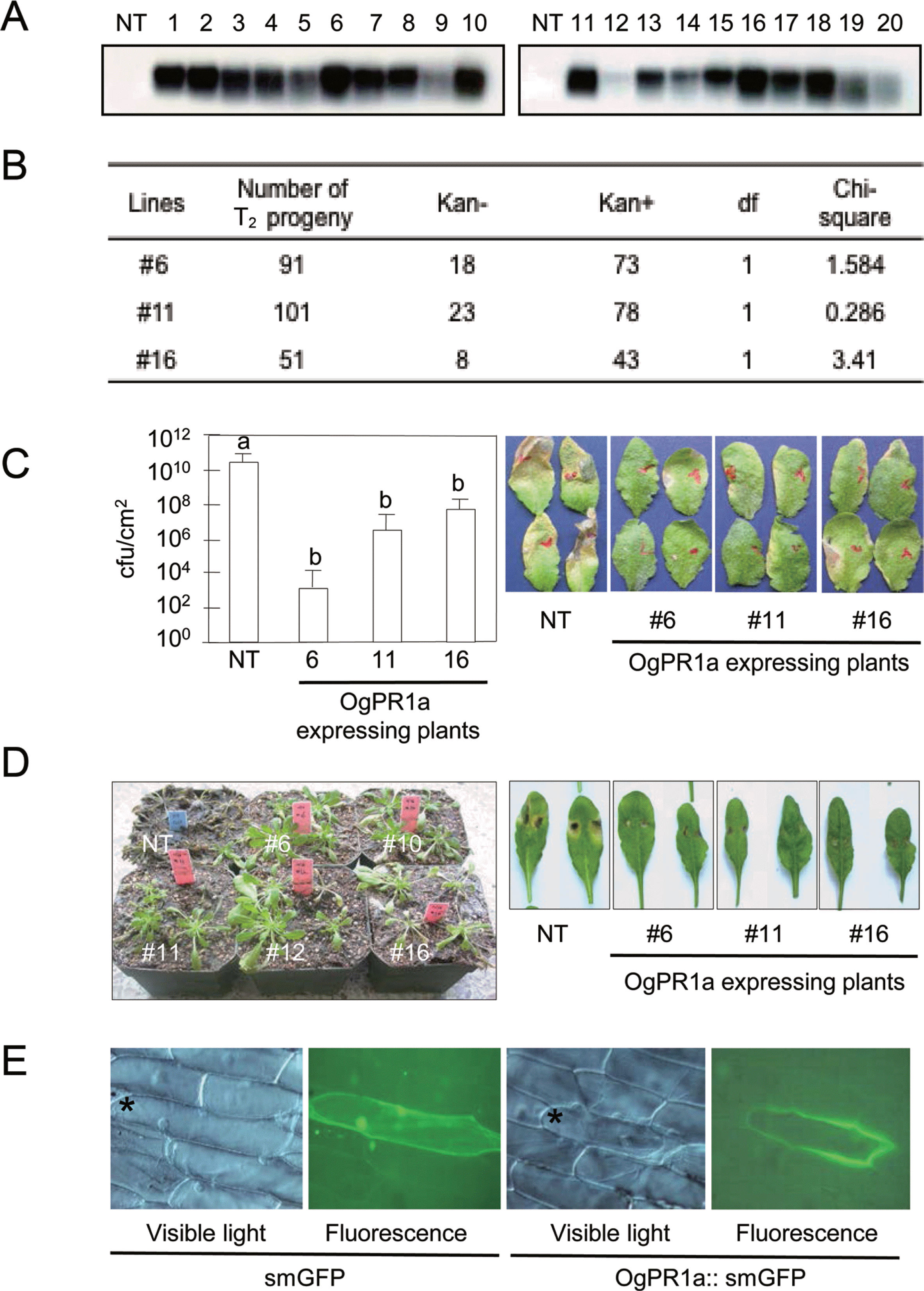
Extracellularly localized OgPR1a proteins conferred disease resistance on Arabidopsis plants against bacterial and fungal infection. (A) Ectopic expression of OgPR1a gene in Arabidopsis plant. Total RNA was isolated from the non-transgenic (NT) and transgenic plants (line #1∼#20) and hybridized with the OgPR1a cDNA in order to check whether or not the OgPR1a gene was expressed in leaves of Arabidopsis. Numbers indicate independent lines of transgenic T1 plants. (B) Progeny analysis of T2 Arabidopsis plants. Sterilized seeds of T2 generation were sowing on the half strength of MS media containing kanamycin (100 μg/ml), and the number of survived seedling was counted for the analysis. (C) Pseudomonas growth in OgPR1a-expressing plants. P. syringae pv. tomato DC3000 (OD600=0.0001) were syringe-infiltrated into leaves of wild-type and OgPR1a-expressing plants. The number of bacteria was monitored on day 3 after inoculation. The photos were also taken 3 days after inoculation. Error bars indicate standard error (n=5). Different letters indicate statistically significant differences (P<0.01, student t-test). The experiments were repeated twice with similar results. (D) Disease symptoms on wild-type Col-o and transgenic plants on 7 days after spray-inoculation of B. cinerea (5×105 conidia/ml) (left panel) and on 4 days after drop-inoculation (5×105 conidia/ml) (right panel). (E) Subcellular localization of OgPR1a protein in onion epidermal cells. OgPR1a::smGFP was introduced and transiently expressed in epidermal cells. The pictures were taken under fluorescence microscope (Zeiss). The asterisks point out the cell showing green fluorescene. Note that images of small GFP were duplicated with those described in our previous article because we had monitored OgPR1a-localization together with the OgPR10 proteins (Shin et al., 2012).
Based on in silico analysis of the deduced amino acid sequences of OgPR1a gene (Fig. 1A), we expected that OgPR1a protein would be an extracellular protein in wild rice. To test whether or not the OgPR1a proteins were secreted out of cells in plants, we expressed GFP-fused Og-PR1a proteins controlled by CaMV 35S promoter in onion epidermal cells. An OgPR1a open reading frame without a stop codon was amplified with two primers containing XbaI and BamH1 sites (forward primer, 5’-gggtctagaatggcgagttcgtcgagcagg-3’; reverse primer, 5′-gggggatccgtagggagattggccgacgaa-3′). Finally, the fragments were inserted into XbaI and BamH1 site of the plasmid psmGFP (David and Vierstra, 1996). The OgPR1a::smGFP chimeric gene was delivered into onion peels by the particle bombardment experiment (Takeuchi et al., 1992). The green fluorescence was monitored in the epidermal tissues under a fluorescence microscope at 8 h after incubation on liquid MS media (Axiophot, Zeiss, Germany). As shown in Fig. 3D, green fluorescence was detected in extracellular space, but not in intracellular area in onion epidermal cells. These studies show the possibility that OgPR1a proteins can act as one of anti-microbial compounds in intercellular spaces in plants.
In conclusion, we showed that OgPR1a gene of wild rice was involved in disease resistance response in plants. Although a exact biochemical function of the acidic PR1 gene is still unclear during plant defense response, it is likely that the PR1 genes are useful as a genetic source for developing disease resistant crop plants, as well as the molecular marker to monitor defense responses in plants.
Acknowledgements
This work was supported by the Wu Jang-Choon Project from the Rural Development Administration (RDA) (PJ007850) and Basic Science Research Program from the National Research Foundation of Korea (2010-0006441) to H.W. Jung, and the Next-generation BioGreen21 program from RDA (PJ007978) to Y.S. Chung.