Altered Cultivar Resistance of Kimchi Cabbage Seedlings Mediated by Salicylic Acid, Jasmonic Acid and Ethylene
Article information
Abstract
Two cultivars Buram-3-ho (susceptible) and CR-Hagwang (moderate resistant) of kimchi cabbage seedlings showed differential defense responses to anthracnose (Colletotrichum higginsianum), black spot (Alternaria brassicicola) and black rot (Xanthomonas campestris pv. campestris, Xcc) diseases in our previous study. Defense-related hormones salicylic acid (SA), jasmonic acid (JA) and ethylene led to different transcriptional regulation of pathogenesis-related (PR) gene expression in both cultivars. In this study, exogenous application of SA suppressed basal defenses to C. higginsianum in the 1st leaves of the susceptible cultivar and cultivar resistance of the 2nd leaves of the resistant cultivar. SA also enhanced susceptibility of the susceptible cultivar to A. brassicicola. By contrast, SA elevated disease resistance to Xcc in the resistant cultivar, but not in the susceptible cultivar. Methyl jasmonate (MJ) treatment did not affect the disease resistance to C. higginsianum and Xcc in either cultivar, but it compromised the disease resistance to A. brassicicola in the resistant cultivar. Treatment with 1-aminocyclopropane-1-carboxylic acid (ACC) ethylene precursor did not change resistance of the either cultivar to C. higginsianum and Xcc. Effect of ACC pretreatment on the resistance to A. brassicicola was not distinguished between susceptible and resistant cultivars, because cultivar resistance of the resistant cultivar was lost by prolonged moist dark conditions. Taken together, exogenously applied SA, JA and ethylene altered defense signaling crosstalk to three diseases of anthracnose, black spot and black rot in a cultivar-dependent manner.
Cultivar resistance has been considered to be reliable and durable during crop production, and a variety of commercial cultivars and accessions were evaluated to determine genetic resources of disease resistance for economically important crops, such as pepper to anthracnose, rapeseed to Sclerotinia rot and watermelon to Phytophthora blight (Kim et al., 2010, 2013; Mei et al., 2012). More efficient screening methods including plant growth stage and inoculation procedure have been newly developed to find cultivar resistance of radish to clubroot (Jo et al., 2011). In vitro screening of anthracnose-resistant cassava cultivars on potato dextrose agar media amended with stem cortex extracts from different cassava cultivars corresponded well to cultivar resistance demonstrated in the greenhouse and under field conditions (Fokunang et al., 2002). A system of rapid seedling assay was also established for screening large numbers of onion cultivars using different isolates of Fusarium oxysporum f. sp. cepae that causes basal rot (Taylor et al., 2013). Resistant or moderately resistant cultivars can be employed in integrated disease management programs accompanied with other biological and chemical controls, because fungicides, plant defense activators, and antagonistic microbes were often more effective to reduce diseases in the resistant cultivars (Banani et al., 2014; Walters et al., 2011).
Cultivar resistances in many crops are closely associated with augmented cellular and biochemical defenses against pathogen infection compared to susceptible responses. Higher antioxidant enzyme activities of peroxidase, ascorbate peroxidase and glutathione reductase were reported to be found in anthracnose-resistant melon cultivar infected by Colletotrichum lagenarium (Ge et al., 2013). Anthracnose-resistant ripe blueberry fruits produces significantly more anthocyanins and non-anthocyanin flavonoids compared to susceptible ones (Miles et al., 2013). Recently transcriptome and proteome profiles of resistant cultivars from tomato and blueberry were investigated to reveal molecular and genetic clues of the resistance traits (Chen et al., 2013; Dahal et al., 2010; Miles et al., 2011).
In recent decades, crosstalk among distinct defense signaling pathways has been suggested in Arabidopsis plants inoculated by several bacterial and fungal pathogens triggering SA, JA and ethylene-mediated defense reactions. The hemibiotrophic bacterium Pseudomonas syringae pv. tomato and the biotrophic fungus Erysiphe orontii activated SA-dependent defense signaling pathways, whereas defenses to two necrotrophic fungi A. brassicicola and Botrytis cinerea were mainly mediated by JA/ethylene signaling in Arabidopsis (Lorenzo et al., 2003; Reuber et al., 1998; Thomma et al., 1998; Vlot et al., 2009). In general, SA and JA/ethylene-mediated defense signaling pathways are mutually antagonistic, and a reducing defense reaction(s) of one pathway often increases activation of the other (Boatwright and Pajerowska-Mukhtar, 2013; Derksen et al., 2013). Altered disease resistance of crops treated with SA, JA and ethylene as defense elicitors have been demonstrated. Accumulation of SA and JA increased with a distinct temporal profile during oilseed rape leaves by Sclerotinia sclerotiorum infection, and lesion development on the inoculated leaves was arrested by SA and JA treatments (Wang et al., 2012). However, defense signaling crosstalk in crops during pathogenesis or cell death-mediated resistance has been rarely described.
In our previous study, two cultivars of kimchi cabbage (Brassica rapa var. glabra Regel) seedlings demonstrated differential disease resistance to infections by the hemibiotrophic fungus C. higginsianum, the necrotrophic A. brassicicola and the hemibiotrophic bacterium Xcc (Lee and Hong, 2014). PR gene expressions were much more induced in the resistant cultivar CR-Hagwang treated with SA compared to the susceptible cultivar Buram-3-ho. In this study, we aimed to investigate whether exogenous application of the three defense-related chemicals SA, MJ and ACC altered the disease resistance of susceptible and resistant cultivars to anthracnose, black spot and black rot diseases or not. This may provide better understanding of defense signaling crosstalk in the two different kimchi cabbage cultivars, and further suggest agriculturally practical approaches to improve broad spectrum disease resistance. Pathogen growth, plant inoculation and disease assessment were performed as previously described (Lee and Hong, 2014). To analyze pretreatment effect of the defense hormones on the disease resistance of kimchi cabbage seedlings, SA (0.5 mM), MJ (0.1 mM) and ACC (0.1 mM) solutions were sprayed on foliage 3 h, 3 h and 9 h, respectively, prior to fungal and bacterial inoculations, at which most PR gene expressions in the resistant cultivar were highly activated in our previous study (Lee and Hong, 2014). All experiments were repeated four times. Pretreatment with SA, MJ and ACC differentially modulated disease resistance to the fungal and bacterial infections in susceptible and resistant cultivars of kimchi cabbage seedlings.
Changes in anthracnose resistance derived by SA pretreatment were differently demonstrated in the 1st and 2nd leaves of both cultivars (Fig. 1A). Basal disease resistance to C. higginsianum was reduced by SA in the 1st leaves of susceptible cv. Buram-3-ho, but not in the resistant cv. CR-Hagwang. In the 2nd leaves, SA pretreatment enhanced susceptibility only in the resistant cultivar CR-Hagwang, but no change occurred in disease resistance of susceptible cv. Buram-3-ho. Synergistic and/or antagonistic interactions between several defense signaling pathways have been shown to confer disease resistance to invading pathogens (Gimenez-Ibanez and Solano, 2013; Pieterse et al., 2009). SA may antagonistically suppress the JA-mediated defenses in kimchi cabbage seedlings as demonstrated in several plant species including Arabidopsis and tomato plants. SA application enlarged the decayed area of tomato fruit infected by C. coccodes, whereas JA reduced the lesion area, indicating that SA lowered JA-mediated defenses of tomato fruits to C. coccodes (Alkan et al., 2012).
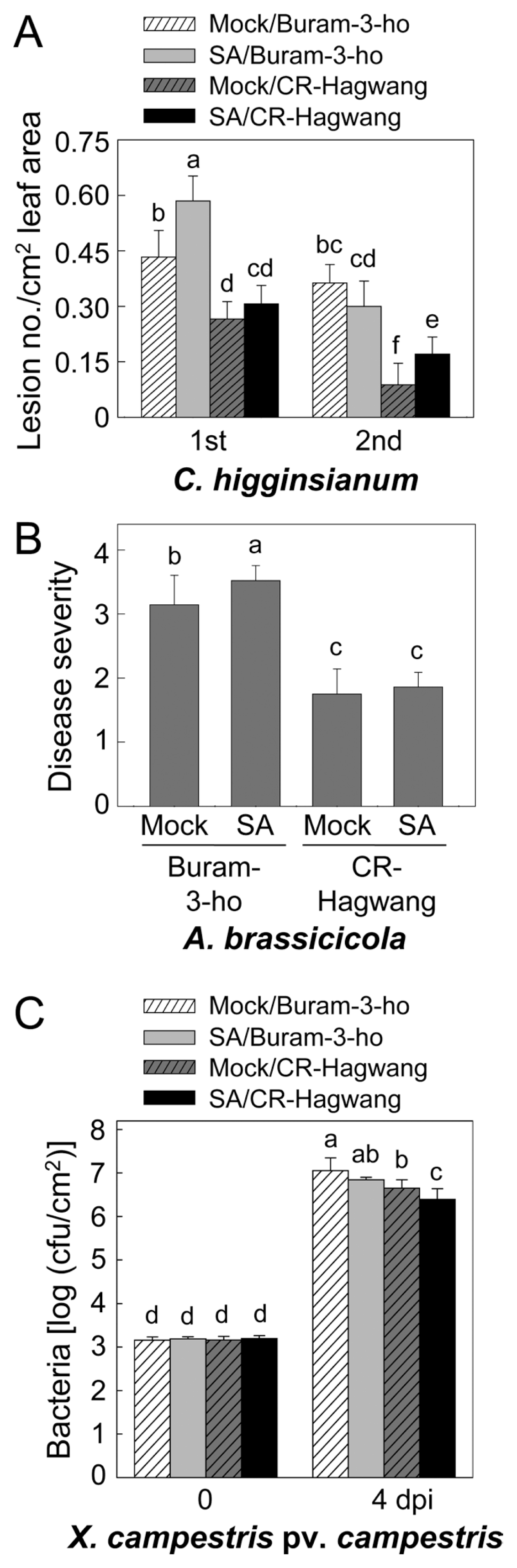
Altered disease resistance of kimchi cabbage seedlings to fungal and bacterial infections in susceptible (cv. Buram-3-ho) and resistant (cv. CR-Hagwang) plants pretreated with 0.5 mM of salicylic acid (SA). (A) Lesion numbers on the inoculated leaves of both kimchi cabbage cultivars pretreated with SA were counted after C. higginsianum inoculation. 1st, 1st true leaves; 2nd, 2nd true leaves. (B) Black spot disease severity of both kimchi cabbage cultivars pretreated with SA. (C) Xcc bacterial growth in both kimchi cabbage cultivars pretreated with SA. dpi, days post-inoculation. Data points are the mean ± standard errors of four independent experiments. The means with different letter were significant at the 5% level as determined by Duncan’s multiple range test.
SA-enhanced disease susceptibility was also observed in the susceptible kimchi cabbage cultivar inoculated with A. brassicicola. Pretreatment of susceptible cv. Buram-3-ho with SA heightened susceptibility to A. brassicicola infection, but did not change the disease resistance of resistant cv. CR-Hagwang (Fig. 1B). SA treatment reduced JA-inducible PDF1.2 gene expression and increased lesion size in Arabidopsis inoculated by A. brassicicola suggesting antagonistic interaction between SA and JA during the resistance response (Leon-Reyes et al., 2009, 2010; Spoel et al., 2007). Disease lesions were progressive much more in SA-pretreated tomato leaves inoculated by Alternaria solani (El Rahman et al., 2012). These results may be due to suppression of JA-dependent defenses by SA treatment in susceptible host plants. However, SA was defense-inducing elicitor in pear fruit inoculated with Alternaria alternata (Tian et al., 2006). We suggested that the resistant cv. CR-Hagwang might activate SA, JA and ethylene-mediated defenses all together to combat pathogens having different infection strategies and to achieve broad spectrum disease resistance in our previous study (Lee and Hong, 2014). Although SA application more or less reduced disease resistance to C. higginsianum and A. brassicicola in the susceptible cultivar, it did not occur in the resistant cultivar. Exogenously applied SA maybe cannot suppress JA- and ethylene-mediated defenses in the resistant cultivar, because JA and ethylene-signaling were also highly up-regualted by these fungal infections.
In this study, we also evaluated disease response of 2nd leaves of the seedlings pretreated with SA. Enhanced disease susceptibility in the susceptible cultivar was not found in the 2nd leaves infected by C. higginsianum, whereas high level of resistance in the 2nd leaves was decreased by SA. These are contrary to the facts found in the 1st leaves. SA-triggered differential molecular responses in the 1st and 2nd leaves against C. higginsianum infection remain to be elucidated.
In contrast, SA application led to enhanced disease resistance to Xcc only in the resistant cv. CR-Hagwang, but not in the susceptible cv. Buram-3-ho (Fig. 1C). This suggests that SA-enhanced disease resistance was only marginally activated in the resistant cultivar. Resistant cv. CR-Hagwang may have sensitive mechanisms to endogenously activated SA signals induced by Xcc infection as well as may produce more SA by Xcc infection. It is in line with the fact that SA application activated an array of PR genes related to resistance to Xcc preferentially in cv. CR-Hagwang (Lee and Hong, 2014). SA-mediated induced resistance has been often demonstrated in various plant species infected by viral (Cucumber mosaic virus, Turnip crinkle virus), bacterial (Erwinia carotovora subsp. carotovora) and fungal (Erysiphe cichoracearum, Fusarium oxysporum f. sp. lycopersici, Gaeumannomyces graminis var. tritici) pathogens (Mandal et al., 2009; Mayers et al., 2005; Sari and Etebarian., 2009; Shang et al., 2011; Vimala and Suriachandraselvan, 2009). Current study is the first report of SA-induced disease resistance of kimchi cabbage plants to a bacterial infection. Meanwhile, it is noteworthy that SA could not induce resistance to Xcc in the susceptible cultivar of kimchi cabbage seedlings. Pivotal components for SA-mediated defense activation might be deficient in the susceptible cultivar of kimchi cabbage seedlings as demonstrated in a variety of SA-related Arabidopsis mutants (Moreau et al., 2012; Ng et al., 2011).
MJ treatment reduced lesion size on peach fruits inoculated by postharvest diseases Monilinia fructicola and Penicillium expansum, but permitted cell-to-cell movement of Tobacco mosaic virus in tobacco leaves (Oka et al., 2013; Yao and Tian, 2005). Ethylene conferred disease resistance of tobacco plants to C. destructivum, but disease susceptibility to loquat fruit to C. acutatum (Cao and Zheng, 2010; Chen et al., 2003). These findings have suggested that involvement of JA and ethylene in plant defenses and protections occurred differently in diverse conditions. Pretreatment with MJ and ACC did not affect the disease resistance to C. higginsianum, A. brassicicola and the bacterial Xcc infection in either kimchi cabbage cultivar (Figs. 2 and 3). Surprisingly, cultivar resistance of CR-Hagwang to A. brassicicola was diminished even in the mock-inoculated seedlings (Figs. 2B and 3B). In the present study, we placed both kimchi cabbage seedling cultivars under moist dark conditions using black plastic covers for 3 h and 9 h to investigate effect of MJ and ACC, respectively, and additional moist dark conditions were treated for the fungal disease development after inoculation. Interestingly, longer exposure to the moist dark conditions prior to A. brassicicola infection seemed to further compromise innate cultivar resistance of cv. CR-Hagwang. Difference in disease severity of the two cultivars to A. brassicicola was reduced by 3 h-mock treatment and 9 h-moist/dark periods completely eliminated black spot resistance of cv. CR-Hagwang. It is maybe caused by enhanced vulnerability of the host plants to challenging pathogens via microclimate change like limited light and increased humidity on the plant surface. Adverse effect of environmental changes on the establishment of disease resistance of cv. CR-Hagwang should be further elucidated. Cultivar CR-Hagwang contained relatively higher chlorophyll contents accumulated (Lee and Hong, 2014). We suppose that it could confer at least in part disease resistance of cv. CR-Hagwang shown in our previous study, although more sophisticated experimental evidence needed (Lee and Hong, 2014). Reduced chlorophyll contents in cv. CR-Hagwang by inhibition of photosynthesis could lead to vulnerability status of kimchi cabbage seedlings to A. brassicicola infection. Closed lids in this study may affect ethylene concentration in the ambient condition of the kimchi seedlings and negatively regulate disease resistance to A. brassicicola in cv. CR-Hagwang (Leon-Reyes et al., 2009). By contrast, prolonged moist dark conditions did not severely affect cultivar resistance of cv. CR-Hagwang against C. higginsianum, suggesting differentiated defense signaling pathways against both fungal pathogens.

Altered disease resistance of kimchi cabbage seedlings to fungal and bacterial infections in susceptible (cv. Buram-3-ho) and resistant (cv. CR-Hagwang) plants pretreated with 0.1 mM of methyl jasmonate (MJ). (A) Lesion numbers on the inoculated leaves of both kimchi cabbage cultivars pretreated with methyl jasmonate (MJ) were counted after C. higginsianum inoculation. 1st, 1st true leaves; 2nd, 2nd true leaves. (B) Black spot disease severity of both kimchi cabbage cultivars pretreated with MJ. (C) Xcc bacterial growth in both kimchi cabbage cultivars pretreated with MJ. dpi, days post-inoculation. Data points are the mean ± standard errors of four independent experiments. The means with different letter were significant at the 5% level as determined by Duncan’s multiple range test.
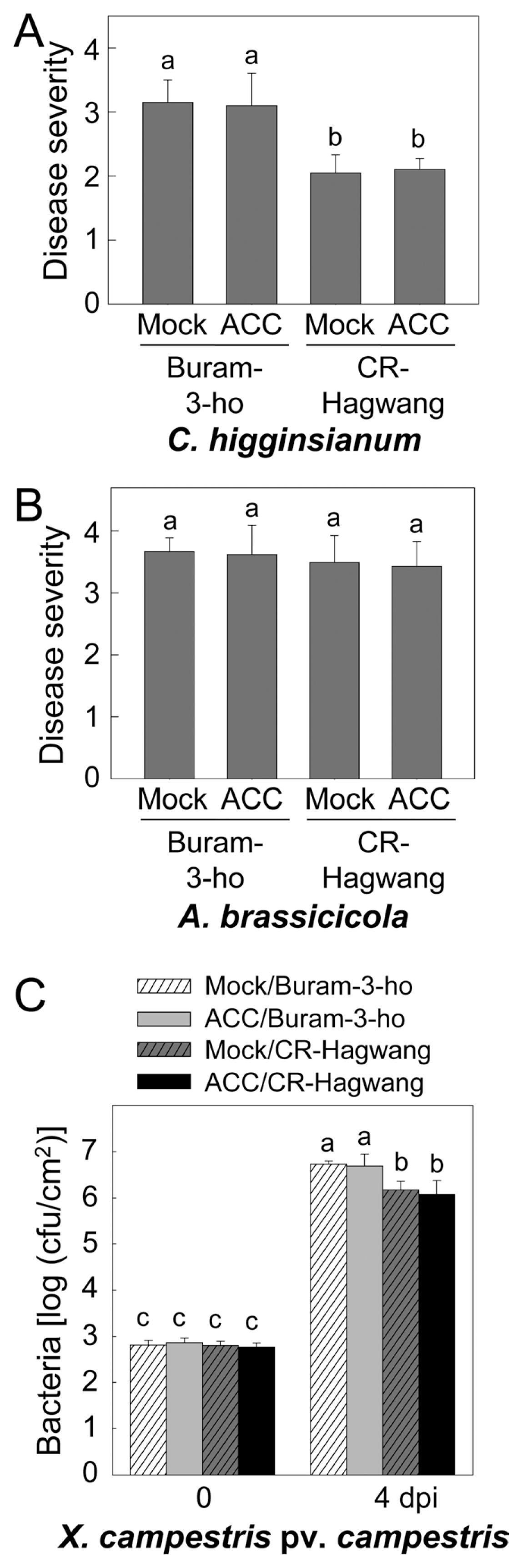
Altered disease resistance of kimchi cabbage seedlings to fungal and bacterial infections in susceptible (cv. Buram-3-ho) and resistant (cv. CR-Hagwang) plants pretreated with 0.1 mM of 1-aminocyclopropane-1-carboxylic acid (ACC). (A) Anthracnose disease severity of the inoculated seedlings pretreated with ACC was evaluated. (B) Black spot disease severity of both kimchi cabbage cultivars pretreated with ACC. (C) Xcc bacterial growth in both kimchi cabbage cultivars pretreated with ACC. dpi, days post-inoculation. Data points are the mean ± standard errors of four independent experiments. The means with different letter were significant at the 5% level as determined by Duncan’s multiple range test.
Disease resistance to Xcc was not altered by JA and ethylene treatment in both susceptible and resistant cultivars of kimchi cabbage seedlings. This result correlates with the facts that SA diminished JA-induced PDF1.2 gene expression but JA could not suppress SA-inducible PR1 gene expression (Leon-Reyes et al., 2009; Spoel et al., 2007).
In recent years, abscisic acid (ABA) and nitric oxide (NO) as novel plant defense regulators have been integrated into well-known defense signaling crosstalk associated with SA, JA and ethylene (Audenaert et al., 2002; de Torres Zabala et al., 2009; Leitner et al., 2009; Mur et al., 2013). Involvement of these small molecules in the disease resistance of kimchi cabbage seedlings remains to be elucidated.
In conclusion, defense hormones SA, JA and ethylene may alter innate immunity of susceptible and resistant cultivars of kimchi cabbage against pathogen infections with different invasion strategies. The current study will provide the molecular and genetic bases to gain more insight on defense signaling in disease susceptibility and resistance of kimchi cabbage.
Acknowledgments
We are grateful to Ms. Sharon Pike (University of Missouri, USA) for the helpful discussion and critical reviewing our manuscript. This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ008211)”, Rural Development Administration, Republic of Korea.