 |
 |
| Plant Pathol J > Volume 30(4); 2014 > Article |
Abstract
Fig. 1
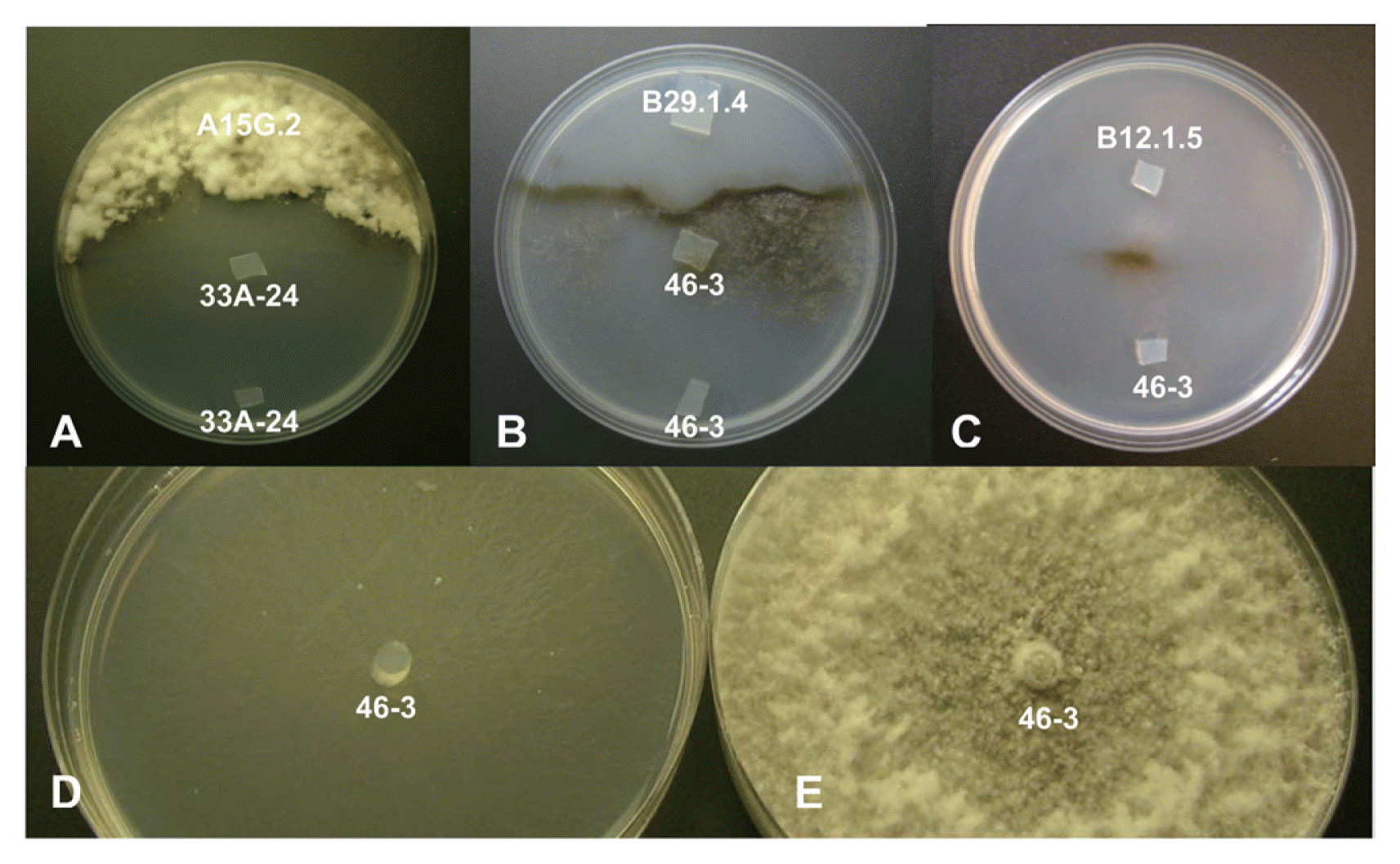
Fig. 2
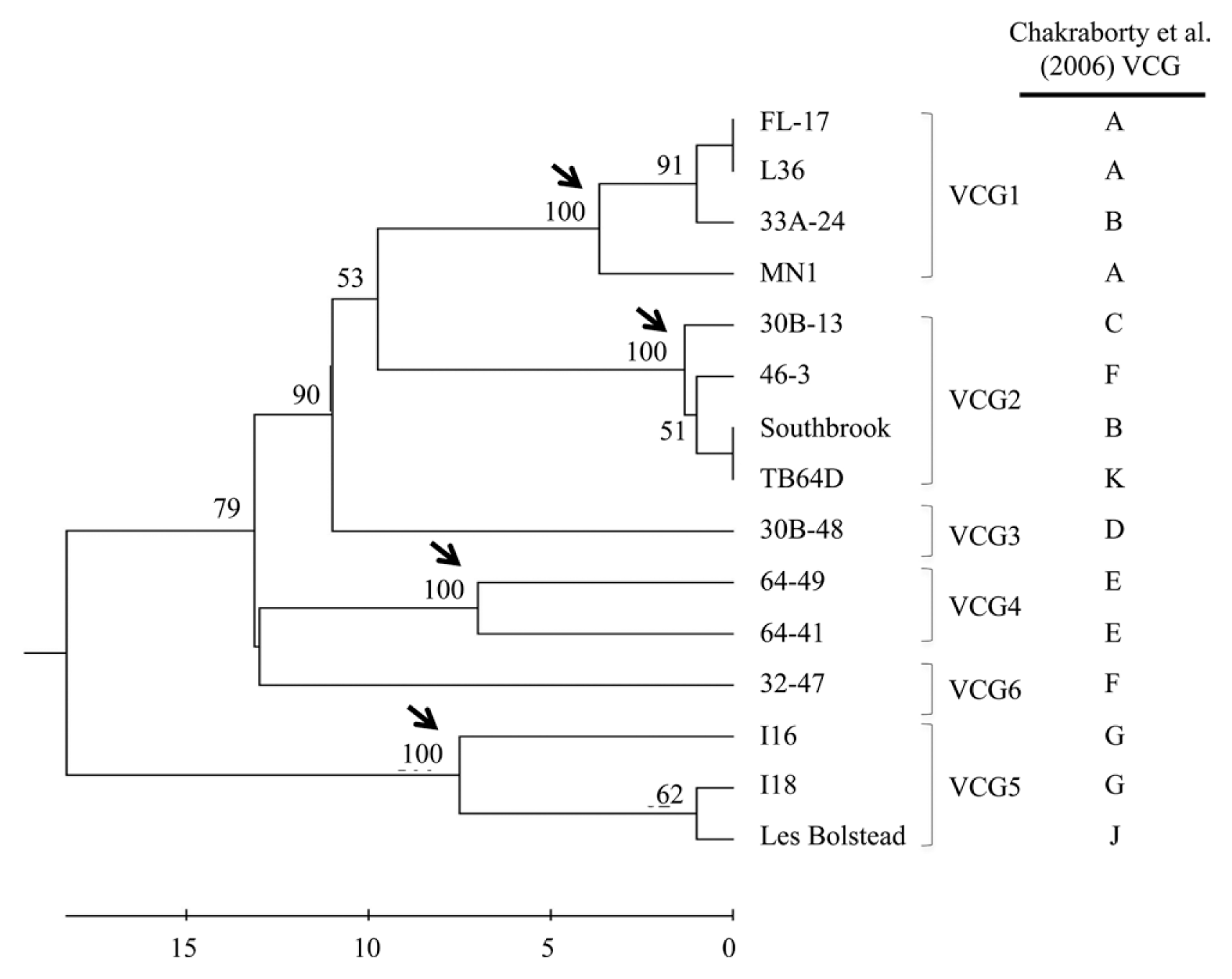
Table 1
| Isolate | Site of collection | Chakraborty et al. (2006)a | Jo et al. (2008)b | This study |
|---|---|---|---|---|
| FL17 | Unknown golf course, FL | A | Group 1 | VCG 1 |
| L36 | Unknown golf course, IL | A | Group 1 | VCG 1 |
| MN1 | Unknown golf course, MN | A | Group 1 | VCG 1 |
| 33A-24 | Hancock Turf Research Center, East Lansing, MI | B | - | VCG 1 |
| ARK | Unknown golf course, AR | B | Group 1 | - |
| Southbrook | Southbrook, MN | B | Group 1 | VCG 2 |
| A7 | Southbrook, MN | H | Group 1 | - |
| TB64D | Unknown | K | Group 1 | VCG 2 |
| 30B-13 | Lakewood Shores, MI | C | Group 1 | VCG 2 |
| 30B-24 | Lakewood Shores, MI | C | Group 1 | - |
| 46-3 | Forest Akers Golf, East Lansing, MI | F | Group 1 | VCG 2 |
| 30B-48 | Lakewood Shores, MI | D | Group 1 | VCG 3 |
| 48-54 | North Shore County Club, Chicago, IL | D | Group 1 | - |
| 64-41 | Evergreen Golf, Hudson, MI | E | Group 1 | VCG 4 |
| 64-49 | Evergreen Golf, Hudson, MI | E | Group 1 | VCG 4 |
| I16 | Les Bolstead, St. Paul, MN | G | Group 2 | VCG 5 |
| I18 | Les Bolstead, St. Paul, MN | G | Group 2 | VCG 5 |
| Les Bolstead | Les Bolstead, St. Paul, MN | J | Group 2 | VCG 5 |
| BRS | Unknown | L | Group 2 | - |
| 32-47 | Evergreen Golf, Hudson, MI | F | Group 3 | VCG 6 |
| 33A-9 | Hancock Turf Research Center, East Lansing, MI | C | Group 4 | - |
| 64-14 | Evergreen Golf, Hudson, MI | E | - | - |
a Vegetative compatibility groups (VCGs) determined by barrage formation at pairings of wild type isolates on potato dextrose agar medium using the method of Powell and Vargas (2001).
Table 2
| Functiona | Mutant designation | Growth on nitrogen sourcesb | Nitrite excretionc | |
|---|---|---|---|---|
|
|
||||
| Nitrite | Hypoxanthine | |||
| Structural gene for nitrate reductase | nit1 | + | + | NT |
| Major nitrogen regulatory gene | nit2 | − | − | NT |
| Pathway-specific regulatory gene | nit3 | − | + | − |
| Genes controlling production of a molybdenum-containing cofactor | NitM | + | − | NT |
| None | Wild type | + | + | + |
a Compiled from Correll et al. (1987), Marzluf (1981), and Tomsett and Garrett (1980) on the basis of mutant phenotypes used for Fusarium oxysporum, Aspergillus nidulans, and Neurospora crassa, respectively.
b Growth on Czapek solution agar (CDA) medium amended with two nitrogen sources. + = typical wild-type growth and − = thin growth with no aerial mycelium.
c Nitrite excretion test as described by Cove (1976). + = nitrite excretion, − = no nitrite excretion, and NT = not tested.
Table 3
| Sequence | Repeat motif a | Size (bp) | Left Primer | Right Primer | No. alleles |
|---|---|---|---|---|---|
| SSR02063 | (CTCAC)12 | 243 | CCTTGGCAGCCTCTGATTAT | TGAGGGTTCATGGAATAGCA | 2 |
| SSR05848 | (GTATGA)12 | 232 | TTGGTGTAGGTGGAGGCTAGA | CTCACGTTCACTCACGCACT | 2 |
| SSR06235 | (TG)17 | 197 | TTCTTCCTTTCGGGTGACAG | CTTTTTCGTCTGCCTTGTGG | 3 |
| SSR08045 | (AC)13 | 157 | GTCGTGGAGAGGAGAGGTGA | AACGCGAGCCAACACTATCT | 4 |
| SSR08400 | (ATCT)12 | 202 | CTATTCTCGCGCATCCTCAT | TCGACGGTATCCTAGCAAGTG | 3 |
| SSR08569 | (TATGGGA)13 | 227 | CCTCTCGTTCCTGGTTTCAC | CAATATCCATCCATCCATCCA | 3 |
| SSR09987 | (CA)12 | 233 | CTCCAACCAATCCTCCTTGA | TGGGCTACCGAGTACTTTGC | 2 |
| SSR15983 | (TAG)13 | 150 | TATAGCTCGCGGATGATGTG | AGACGGACTTACGCAATGCT | 4 |
| SSR17616 | (AC)16 | 172 | TCCGCACTACCGTTACACAC | GTGCGATGGAGATGGAGTCT | 2 |
| SSR20589 | (TCA)13 | 243 | ATCGACCCAAGAATCACCAA | AGGCTGGGTGCCTTAGTTTT | 2 |
| SSR20624 | (TTCA)16 | 184 | AGTTGGGCGAACGAATAAGA | GGCTGAAAGGGAGAAAGAACA | 3 |
| SSR21791 | (GAA)12 | 215 | CCATTCGTTCTATGGGTTCG | GGGACTTCTCCTTCCCATTC | 2 |
| SSR22804 | (AT)15 | 232 | CGGCTAGTTCGTCAATCAGG | AGCGGACGAGGAGGTAAACT | 2 |
| SSR23039 | (TC)16 | 197 | TCTTGCTCTGCTCTGCTCTG | TGGCCTTTTGCTTGCTTACT | 3 |
| SSR25827 | (AATC)15 | 188 | CCTTCCTTTCCAGCCTATCC | CCCGCTTTTTGGTTTTTGT | 5 |
| SSR27998 | (GTTAT)12 | 165 | ATTGATGGGCATCGGTTG | TCCTCTCCTCTCCTCCTCTGTA | 4 |
| SSR30326 | (TTGAC)14 | 167 | GCAATGAGTGAGCGTCTTGT | TCATATCATCAAACGCATCCA | 4 |
| SSR30530 | (TC)12 | 156 | AATCGAGCACAGTCCAGTCC | TCTGTCTACTTGTCCGTCGATTT | 3 |
| SSR30647 | (AC)14 | 170 | GCTGTGGCCCATAATACGAT | GGCTGGATGTGCTGGATAAA | 2 |
Table 4
| Isolate | No. of colonies (×105 cfu/ml)a | No. of mutantsb | Mutation rate (×105)c | nit mutant class d | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| nit1 | nit2 | nit3 | NitM | ||||
| A7 | 2.8 ± 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| ARK | 1.4 ± 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| BRS | 5.2 ± 2.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| FL17 | 4.9 ± 0.8 | 5 | 2.5 ± 0.3 | 4 | 0 | 0 | 1 |
| I16 | 3.4 ± 3.0 | 4 | 4.8 ± 4.2 | 1 | 0 | 0 | 3 |
| I18 | 3.0 ± 0.7 | 6 | 5.1 ± 1.2 | 4 | 0 | 0 | 2 |
| L36 | 3.4 ± 0.5 | 9 | 7.5 ± 10.6 | 9 | 0 | 0 | 0 |
| Les Bolstead | 4.4 ± 0.8 | 4 | 2.3 ± 0.4 | 1 | 0 | 0 | 3 |
| MN1 | 3.3 ± 0.7 | 1 | 0.9 ± 1.3 | 1 | 0 | 0 | 0 |
| Southbrook | 0.9 ± 0.5 | 1 | 5.0 ± 7.1 | 1 | 0 | 0 | 0 |
| TB64D | 1.8 ± 1.3 | 3 | 5.0 ± 1.8 | 0 | 3 | 0 | 0 |
| 30B-13 | 1.3 ± 0.8 | 5 | 11.3 ± 4.2 | 2 | 0 | 1 | 2 |
| 30B-24 | 2.2 ± 1.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30B-48 | 2.7 ± 1.2 | 6 | 8.3 ± 11.8 | 6 | 0 | 0 | 0 |
| 32-47 | 2.6 ± 0.4 | 1 | 1.1 ± 1.6 | 1 | 0 | 0 | 0 |
| 33A-9 | 1.4 ± 0.1 | 1 | 1.9 ± 2.7 | 1 | 0 | 0 | 0 |
| 33A-24 | 2.1 ± 0.6 | 42 | 51.9 ± 14.0 | 22 | 0 | 0 | 20 |
| 46-3 | 1.7 ± 0.9 | 5 | 6.8 ± 2.6 | 3 | 0 | 1 | 1 |
| 48-54 | 6.4 ± 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 64-14 | 1.6 ± 0.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 64-41 | 2.4 ± 0.8 | 3 | 3.6 ± 2.7 | 2 | 0 | 0 | 1 |
| 64-49 | 2.0 ± 0.7 | 5 | 7.0 ± 4.2 | 3 | 0 | 0 | 2 |
|
|
|||||||
| Total | 101 | 61 | 3 | 2 | 35 | ||
a CFU: colony-forming unit, number of colony produced from the shredded mycelial suspension on PDA (Potato Dextrose Agar).
c Mutation rate was determined as the number of nit mutants isolated on water agar medium amended with chlorate divided by the total number of CFUs on PDA per the same amount of mycelial suspension.
d Nit mutant phenotypes determined according to growth on CDA amended with different nitrogen sources.
nit1: mutation in a nitrate reductase structural locus, nit2: mutation in major nitrogen regulatory locus, nit3: mutation in a nitrate assimilation pathway-specific regulatory locus, NitM: mutation in one of five loci that affect the assembly of a molybdenum-containing cofactor necessary for nitrate reductase activity.
Table 5
| nit mutant phenotypea | nit1 | nit2 | nit3 | NitM |
|---|---|---|---|---|
| nit1 | − | + | − | + |
| nit2 | − | + | + or −b | |
| nit3 | − | + | ||
| NitM | + or − |
a nit1: mutation in a nitrate reductase structural locus, nit2: mutation in major nitrogen regulatory locus, nit3: mutation in a nitrate assimilation pathway-specific regulatory locus, and NitM: mutation in one of five loci that affect the assembly of a molybdenum-containing cofactor necessary for nitrate reductase activity.
Table 6
| VCG a | Average % genetic distance within VCGb | Number of isolates | Chakraborty et al. (2006) VCGs nested within |
|---|---|---|---|
| 1 | 5.3 | 3 | A, B |
| 2 | 2 | 4 | B, C, F, K |
| 3 | - | 1 | D |
| 4 | 14 | 2 | E |
| 5 | 10.7 | 3 | G, J |
| 6 | - | 1 | F |
| J1 | 18.7 | 10 | A, B, C, D, E, K |
| J2 | 10.7 | 3 | G, J |
| J3 | - | 1 | F |
| A | 5.3 | 3 | - |
| B | - | 1 | - |
| C | - | 1 | - |
| D | - | 1 | - |
| E | 14 | 2 | - |
| F | 21 | 2 | - |
| G | 15 | 2 | - |
| J | - | 1 | - |
| K | - | 1 | - |
a For the current nit mutant VCG assay (1-6), pairings among mutants of eighteen isolates recovered in Table 2 were made between two different nit mutants in all possible combinations [nit1 (or nit3) and nit2 (or NitM)].
Table 7
| Source of variation | df | Sum of squares | Percentage of variation | φPT | P value | |
|---|---|---|---|---|---|---|
| This study | Among nit VCG | 3 | 120.68 | 81.6 | 0.795 | <0.001 |
| Within nit VCGs | 9 | 27.17 | 18.4 | 0.795 | <0.001 | |
| Total | 12 | 147.85 | ||||
| Jo et al. (2008) | Among nit VCG | 1 | 41.32 | 19.0 | 0.299 | <0.006 |
| Within nit VCGs | 16 | 180.68 | 81.0 | 0.299 | <0.006 | |
| Total | 17 | 222.00 | ||||
| Chakraborty et al. (2006) | Among nit VCG | 6 | 145.83 | 69.7 | 0.572 | <0.001 |
| Within nit VCGs | 12 | 63.33 | 30.3 | 0.572 | <0.001 | |
| Total | 18 | 209.16 |
Table 8
| VCG1 | VCG2 | VCG4 | VCG5 | |
|---|---|---|---|---|
| VCG1 | 0.000 | 0.028 | 0.066 | 0.029 |
| VCG2 | 0.838 | 0.000 | 0.069 | 0.029 |
| VCG4 | 0.768 | 0.760 | 0.000 | 0.092 |
| VCG5 | 0.810 | 0.851 | 0.689 | 0.000 |
References
- TOOLS
-
METRICS

- Related articles
-
Triazole Fungicides Sensitivity of
Sclerotinia homoeocarpa in Korean Golf Courses2017 December;33(6)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



