 |
 |
| Plant Pathol J > Volume 31(4); 2015 > Article |
Abstract
Antifungal activities of crude extractum of Nanshancha Seed Cake (NSC), to inactivate postharvest pathogens were investigated. Highest inhibitory rate was found against C. musae, C. gloeosporioides and C. papaya P.Henn, which was much stronger than that by tea saponin. Compared to tea saponin, effects of NSC extractum was relatively weak and similar on C. gloeosporioides Penzig and P. italicum. In an in vivo study, best controlling effects by NSC extractum was found with banana anthracnose disease development, which showed no inhibitory effects by tea saponin. NSC extractum controlled in vitro C. musae growth through directly inhibiting germination rate and germ tube elongation, and causing distortation, rupture and indentation of C. musae mycelium. In banana fruit subject to C. musae inoculation, higher PAL, POD, GLU and CHT activity was observed in banana fruit treated with crude NSC extractum than that of water control fruits. Current study proved the best controlling effects of crude NSC extractum in C. musae in vitro and in vivo development, which through direct inhibition of C. musae growth and increasing defense system of the banana fruit.
Colletotrichum and Penicillium species are the main causal agents of tropical and subtropical fruit such as banana, mango, papaya, guava and citrus, which are highly perishable fruits that have short shelf-life and suffer severe losses because of these fungal diseases (Costa and Erabadupitiya, 2005; Finlay and Brown, 1993). Fungi may also cause the production of mycotoxins and off flavor formation in fruit (Steel et al., 2013). Chemical fungicides provide the primary means for controlling postharvest fungal decay of fruit and vegetables (Khan et al., 2001). However, the application of chemical preservatives as antimicrobial agents faces imminent problems and becoming increasingly restricted. One major problem is the increasing resistance to antibiotics and chemotherapeutics, the other problem is increasing health risk concerns (Meziane et al., 2006; Tripathi and Dubey, 2004; Zhang et al., 2006). Therefore, there is a growing need to develop alternative antifungal products (Gachango et al., 2012; Maqbool et al., 2010).
In recent years, many researchers have focused on the search for natural and biocontrol fungicides. Various plant products like plant extracts, essential oils, gum, resins… etc. were shown to exert biological activity in vitro and in vivo and are used as bio-fungicidal compounds (Ahmet et al., 2005; Kulakiotu et al., 2004; Yahyazadeh et al., 2008). Askar and Rashad (2010) reported that four medicinal plant extracts exhibited high antifungal activities against Rhizoctonia solani on pea root in the greenhouse pot experiment. Oil extracts of cinnamon completely controlled wound-inoculated disease on orange caused by three postharvest pathogens (Xing et al., 2010). The aqueous extract of Acacia nilotica showed pronounced antifungal activity against P. italicum and enhanced the shelf life of oranges for 6 days (Tripathi and Dubey, 2004). Moreover, the utilization of plant extracts could be used in combination as a novel approach to control postharvest diseases and may exert a synergistic action in the management of postharvest diseases. The incorporation of essential oils into applied wax or the packaging material was effective in controlling postharvest P. digitatum on citrus (Meepagala et al., 2006; Obagwu and Korsten, 2003; Palou et al., 2007; Sharma et al., 2009). However, nearly all the plants used before are rare valuable plants or herbs, their further development and practical applicability is restricted due to limited resources. Therefore, it is necessary to looking for low-cost and resource-rich plant material to extract antimicrobial agent.
Camellia semiserrata chi (Nanshancha) seed cake is the by-products of camellia oil pressed from the seeds of the plant, which is a common widely distributed source of the raw materials in south China mountain area. The preservative nature of the residua has been known for centuries and its antimicrobial activity against many kinds of microorganism has been traditionally used for disinfection purposes (Wang et al., 2008). Though the antimicrobial nature of Nanshancha seed cake has been known from practical disinfection use, its antifungal activities has not been studied against postharvest pathogens of fruits so far. It was reported that tea saponin is the main component of Nanshancha seed cake, and significant antimicrobial and antifungal activities against a variety of pathogens have been proved by tea saponin (Hao et al., 2010; Hu et al., 2005). In this study, antifungal performance of Nanshacha seed cake extractum was investigated, tea saponin with the purity of 90% as a parallely control to prove if tea saponin is also a main component of the extractum. In addition, in vivo antifungal activity and defense ability change was subsequently examined on the three and one kind of fruits respectively, to help to understand the antifungal activity and its possible inhibitory mechanism.
Fresh fruits of Camellia semiserrata Chi (Nanshancha) were harvested in August 2009 from the Zhaoqing mountain area in Guangdong Province. The stone seeds were harvested and squeezed using a mechanized oil pressing machine, then the Nanshancha seed cake (NSC) were obtained. The seed cake was completely sun-dried and saved at ambient temperature. For extraction, 1,000 g of dried seed cake were finely pulverized to get powder through a 100 mesh sieve. The fine powder were firstly dipping in distilled water overnight, then the filtrate was steam distilled using a self-built steam still fitted with a 15 kW boiler (Protherm®). Total 350 ml concentrated extractum was obtained and then drying under the temperature of 100-120°C. Finally, Total 150 g dried extractum was obtained from 1,000 g dry NSC. The extractum were dissolved in water to make different concentrations needed.
Tea saponin (purity of 90%), which was purchased from Jinhan Biological Technology Ltd. Changsha, China, was used as a control to compare the in vitro and in vivo activity of NSC extractum. Tea saponin was dissolved in water or supplemented with PDA media to make different concentrations needed.
Colletotrichum musae and Penicillium italicum, originally isolated from a naturally infected banana and Shatang mandarin fruit respectively, were purified, routinely cultured and maintained on nutrient PDA media. Test fungi (Colletotrichum papaya P. Henn., Colletotrichum gloeosporioides Penzig and Colletotrichum gloeosporioides from mango) was gratefully provided by Prof. Hu Meijiao, Chinese Academy of Tropical Agricultural Sciences and maintained in PDA media. All the cultures were incubated in a 28°C incubator and spores were harvested after one week and conidial suspension at a concentration of 106 conidia/ml were prepared for the in vivo inoculation experiment.
The extractum was dissolved in distilled water to make a stock solution, which was supplemented aseptically with PDA media to make different concentration needed respectively. Final concentrations of the test substances ranged from 1 mg/ml to 50 mg ml−1. Controls consisted of PDA plates without any supplementation. The agar was poured into 90 mm petri dishes and a 5 mm diameter agar plug, containing mycelia and spores of the four tested fungus, and aseptically collected from the edge of actively growing fungal colony, was placed onto the centre of each PDA plate after setting. All plates for in vitro were incubated in a 28°C incubator. After 7 days, mycelial diameter was measured with digital calipers, and results were expressed as percentage inhibition rate, which was calculated using the formula: %Inhibition=100 (Control − Treatment)/Control. The experiment was carried out with 10 replicates per test.
Freshly harvested banana, ‘shatang’ mandarin and mango fruit harvested from local orchard were used for inoculations. Fruits were surface sterilized by immersion in 10% commercial bleach for 5 min, rinsed with distilled water and air-dried at ambient temperature. Air-dried fruits were then wounded by inflicting two 5 mm deep wounds using a sterile needle, and inoculated with test fungi respectively by placing a 10 μl aliquot of a conidial suspension on each of the wound. Fruits were again kept at ambient for drying before dipping into NSC or tea saponin solution at a different corresponding concentration respectively. Each dip treatment consisted of 30 fruits, i.e., 60 inoculation replicates per treatment. Treated fruit were packed into fruit cartons, covered with PE bag and stored at 25°C and 95% relative humidity. The diameter of each observed lesion was measured using digital calipers at a corresponding storage time. Banana peel tissues surrounding the wounds of three fingers were sampled after 6 days for enzyme activity determination. The experiment was repeated three times.
C. musae was grown for 5 days on PDA plates and conidia were harvested by washing the plates with sterile distilled water. After dilution and counting under microscopy, a drop of 106 conidia/ml was spread on a microscope glass slide, germination was performed in an incubator with the temperature was 28°C and relative humidity was 95%. 10 h later, light microscopy was performed with a Zeiss Axioskp 2 epifluorescence microscope to count the germinated spores in five visual fields, and length of fifty germ tube in one visual field was measured. Inhibition rate of spore germination and germ tube elongation was the average number in NSC extractum or tea saponin treated samples divided by the number of control.
SEM was conducted, according to the methods described by Meng et al. (2012), to check the morphological changes of three-day-old cultures of C. musae caused by NSC extractum.
One gram of peel tissue was homogenized with corresponding buffer to extract crude enzyme, then the homogenate was centrifuged at 4°C for 15 min at 13,000×g and the supernatant was used for enzyme assay. PAL, POD, GLU and CHT activity were assayed and determined with the same method published by Meng et al. (2012) in our lab.
Percentage inhibition rate of NSC extractum, parallely compared with tea saponin, against tested fungi by the agar dilution method are presented in Table 1. Both the inhibition rate value of the aqueous NSC extractum and tea saponin against C. musae were 100%, but the concentration of tea saponin (5 mg/ml) is five folds than that of NSC extractum (1 mg/ml). The inhibition rate of the extractum under the same concentration of 5 mg/ml against mango and papaya C. gloeosporioides was 90.9% and 85.3% respectively, much strong inhibitory effect in comparison with that of tea saponin, which was 49.8% and 41.3% respectively. The inhibition rate of the 50 mg/ml NSC extractum and tea saponin against guava C. gloeosporioides was 58.7% and 100% respectively. However, NSC extractum and tea saponin showed same inhibitory level against P. italicum. Moreover, the antifungal activity of NSC extractum and tea saponin was both improved with increasing their concentration (data not shown).
Fig. 1 showed the in vivo controlling effects of postharvest decay in banana, mango and Shatang mandarin caused by C. musae, C. gloeosporioides and P. italicum, respectively, by crude NSC extractum as lesion diameter after different storage time under ambient temperature.
Results showed that the anthracnose lesion diameter of non-treated banana fruit (control, CK) gradually developed to 2.8 mm, 6.6 mm and 14.6 mm after 6th day, 9th day and 12th day respectively, tea saponin with the concentration of 5 mg ml−1 proved no inhibitory effects against anthracnose decay development. 5 mg/ml NSC extractum provided completely control of anthracnose decay development after 6 and 9 days of storage, and after 12 days of storage, the lesion diameter was reduced by 83% compared to the control (Fig. 1A).
For mango fruits treated with 10 mg/ml extractum and tea saponin, lesion diameter remained a similar level during the tested three storage time, but was reduced by 60-70% compared to that of non-treated control fruit (Fig. 1B). In Shatang mandarin inoculated by P. italicum, compared to non-treated control, 50 mg/ml NSC extractum treatment reduced the disease index by about 49% after 4 days of storage, but after 6 and 8 days of storage, NSC extractum was less effective against the P. italicum infection. Tea saponin provided 13% controlling effects of the mold decay development on Shatang mandarin at 4 days of storage, but less significant disease control reduction was observed after 6 and 8 days of storage (Fig. 1C).
As shown in Fig. 2, obvious inhibition in the germination rate of C. musae and germ tube elongation was observed in the plates with NSC extractum, in which germination rate and germ tube elongation was markedly inhibited by 88.7% and 68.4% respectively at 0.5 mg/ml, 100% completely inhibition was observed with NSC extractum above 1 mg/ml. Inhibition rate of spore germination under 0.5, 1, 2 and 5 mg/ml of tea saponin was 22.8%, 50.2%, 64.5% and 87.6% respectively, much reduced inhibitory effect in comparison with those of NSC extractum. Germ tube elongation as affected by NSC extractum and tea saponin had similar changes with that of spore germination.
The effect of NSC extractum on the morphological changes of C. musae was further examined by scanning electron micrograph (SEM) (Fig. 3). In the water control, normal growth of C. musae mycelia on M3S medium was observed, the hyphae were homogeneous with smooth cell walls and clear development of conidiosphore (Fig. 3A). In the treatment of NSC extractum at 1 mg/ml, a strongly distorted, indented, and ruptured mycelium was observed, and thinning of the hyphal cell wall and reduction of the hyphal diameter were also observed under NSC extractum (Fig. 3B).
NSC extractum showed best inhibitory effect on in vitro and in vivo C. musae development, so effects of extractum treatment on the induced resistance of banana fruit against C. musae infection was further investigated with respect to the activities of defense-related proteins including pathogenesis-related proteins or enzymes, which are known to have a role in plant defense. This included the PAL, the enzyme of the phenylpropanoid pathway involved, for example, in flavonoids and lignin biosynthesis, peroxidase, enzymes of oxidative stress responses, hydrolytic enzymes (β-1,3-glucanase, chitinase) able to cleave fungal cell walls. The activity of PAL was determined to decrease (from more than 100 unit to about 20 unit) upon inoculation with C. musae in both control and NSC extractum treated fruit. However, higher PAL activities were recorded in the treated fruit from 3rd up to 12th day of storage than the non-treated control fruit, where up to 1.5-fold activity was observed on the 12th day (Fig. 4A).
In NSC extractum-treated banana fruit, peroxidase (POD) activity was observed dramatically higher than that of control fruit during the whole incubating time, except that, on day 6, lower activity was detected in the treated fruit. An increase in POD activity by about 2-fold on day 3 and day 12 was recorded in treated fruit than in the control fruit (Fig. 4B).
A constant increase in β-1,3-glucanase (GLU) activity was observed in both NSC extractum treated and control banana fruit. However, the treated fruits displayed more marked enhancement in the activity, with 30%-40% higher activity on the 6th, 9th and 12th day, than untreated fruits, except on 3rd, where no GLU activity difference was found between treated and control fruit (Fig. 4C). Higher chitinase (CHT) activity at 3rd, 6th and 9th day of storage was observed in the NSC extractum treated fruit, when compared to the control. A strong increase in chitinase activity up to 2.4-fold was observed in the treated fruit on day 6 compared to the control. However, on day 12, CHT activity remained to the same level in both control and treated fruit (Fig. 4D).
In China, Nanshancha seed cake (NSC) has been used for traditional disinfection remedies, such as be used as natural substitutes of shampoo, throwing it into fishpond for providing a disinfected environment for fish (Wang et al., 2008). Together with other traditional uses show NSC’s antimicrobial activity. Crude NSC extractum were studied here in the search for naturally occurring biological control agents for postharvest disease of fruit. Mycelial growth of C. musae, C. gloeosporioides and P. italicum were significantly inhibited (Table 1). Banana anthracnose decay development could be completely controlled by 5 mg ml−1 NSC extractum. 10 mg/ml extractum could reduce mango anthracnose lesion diameter by 60-70%. While NSC extractum was less effective in controllingShatang mandarin mold disease development (Fig. 1). These results proved that crude NSC extractum had strong antifungal activities against tested fungi, firstly providing new evidence for the traditionally regarded antimicrobial activities. Moreover, the results also showed that the antifungal activity of NSC extractum is different because of different pathogen or different fruit. It has been recognized that some plant extracts, such as cinnamon and clove extracts, have different antimicrobial activities against individual strains of microorganisms (Deriu et al., 2008). According to Tzortzakis (2009), fungal spore production inhibited up to 63% at 25 ppm of cinnamon extracts concentration and fungal sporulation (except for B. cinerea) was completely retarded at the highest concentration (500 ppm) employed. The differences in antifungal activity may be because of the difference of the methods, environmental and quality conditions in different fruit. The antifungal activity of the Nanshancha crude extractum needs further investigation in different and more fruit cultivars.
Many kinds of plant extracts have been previously reported as biological control agents (Askar and Rashad, 2010; Yahyazadeh et al., 2008). This research, however, is the first report where a recycled by-products was used for antifungal activity evaluation on postharvest pathogen of tropical fruit. Moreover, NSC has advantage of low-cost and resource-rich, increasing the potential to commercially develop it to biological agents used in postharvest disease controlling.
Present study proved that crude NSC extractum have the best potential in controlling banana anthracnose disease development, followed by mango anthracnose, while the controlling effects on Shatang mandarin inoculated by P. italicum is less effective (Fig. 1). However, in all the tested cases, both in vivo and in vitro antifungal effects of NSC extractum is much better than that by tea saponin, which was largely supposed and described as the main fungicidal component of NSC by former research (Hao et al., 2010; Hu et al., 2005; Li et al., 2012). Results in this text indicates that other active antifungal components existing in the crude extractum other than tea saponin, and the antifungal activity of our NSC extractum is the synergistic effects of more than one compound. Further research is required to separate these active components and validate their antifungal activity.
NSC extractum completely inhibited C. musae mycelia growth, and delayed anthracnose development. In vitro results proved its direct inhibitory effect on the pathogen growth (Fig. 2 and Fig. 3). Biological treatments was also found to elicit defense responses in postharvest fruits. In tomato fruit, postharvest chitosan treatment induced a significant increase in the activities of polyphenoloxidase (PPO) and peroxidase (POD), and enhanced the content of phenolic compounds (Liu et al., 2007). Increase in the activities of chitinase (CHI), β-1,3-glucanase (GLU) and peroxidase (POD) was reported in oligochitosan-treated banana fruit (Meng et al., 2012). In our study, we also found that the activities of phenylalanine-ammo-nia-lyase (PAL), POD, GLU and CHT in crude extractum treated banana fruit increased significantly (Fig. 4), indicating that components in NSC extractum is also an effective agent for inducing defense responses. So, the control of banana anthracnose by NSC extractum was due to the direct inhibition of growth of C. musae and indirectly through enhanced activity of enzymes involved in the biosynthesis of phenolic compounds that impart disease resistance.
Acknowledgements
This research was supported by ‘Function analysis of CDA gene in C. gloeospoioides growth and pathogenicity’ program (No. 31301824) of National Natural Science Foundation of China, and program No. 2013B050800015, No. 2014A020208055 and No. 2013B020313007, which was funded by Guangdong Science and technology Department.
Fig. 1
Fruit lesion diameters on banana (A), mango (B) and shatang mandarin (C) fruit treated with aqueous crude extractum of Nanshancha seed cake or with tea saponin and inoculated with Colletotrichum musae (A), Colletotrichum gloeosporioides (B) and Penicillium italicum (C) respectively. Bars represent standard deviations of the means.
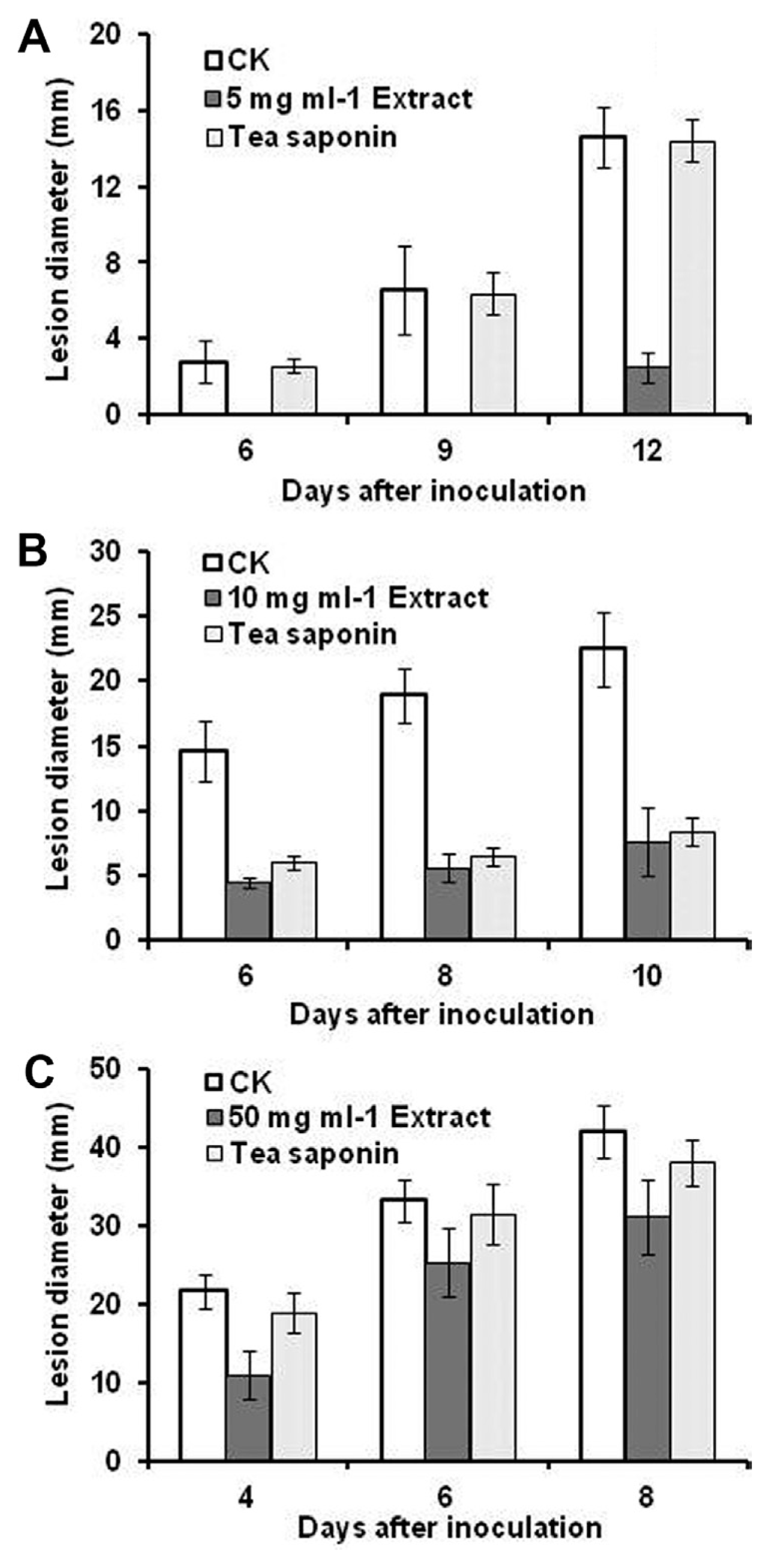
Fig. 2
Inhibition rate of C. musae spore germination (A) and germ tube elongation (B) as affected by NSC extractum and tea saponin. Bars represent standard deviations of the means.
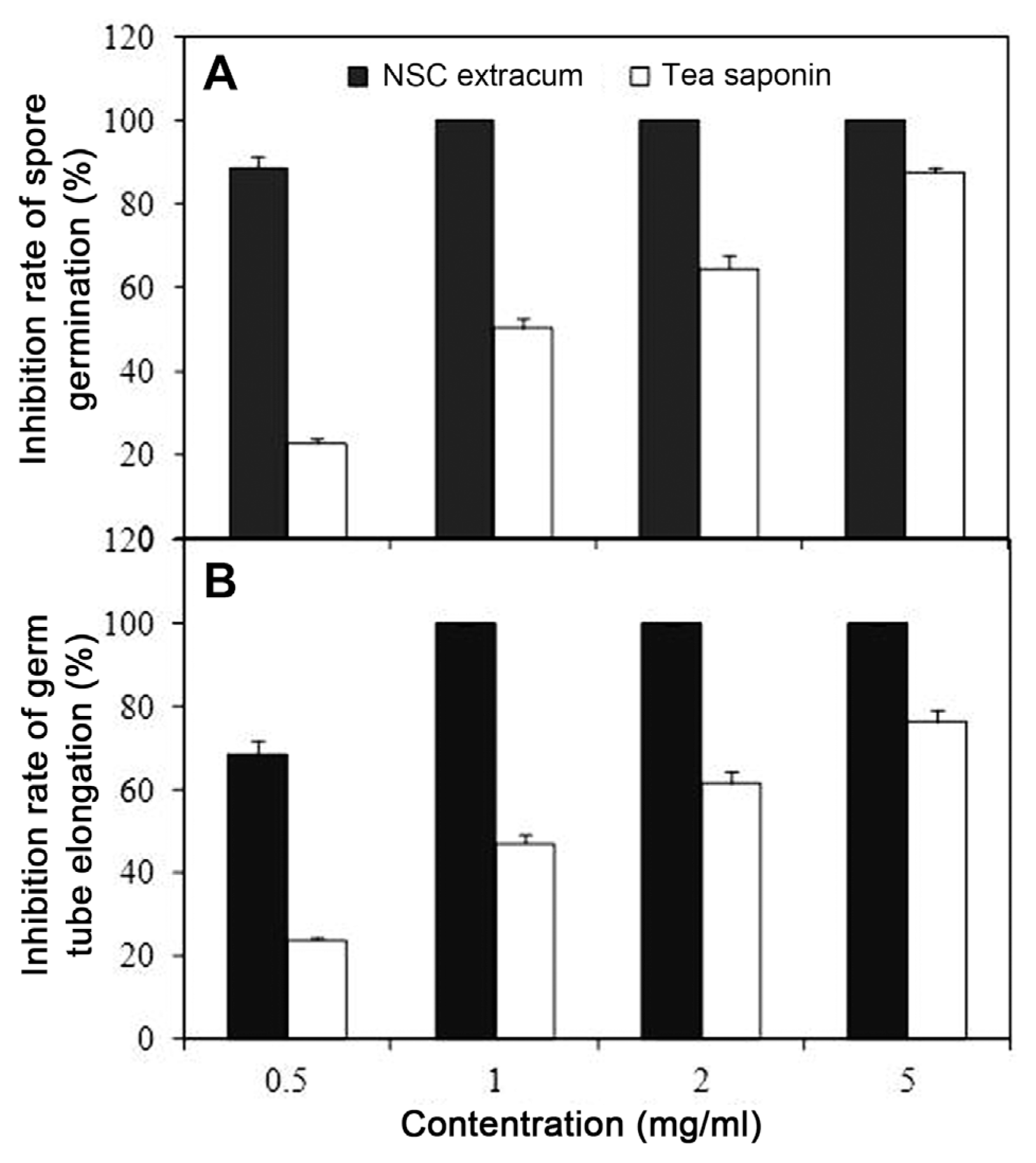
Fig. 3
Scanning electron micrograph (SEM) of Colletotrichum musae hyphae exposed to water control (A) and 1 mg/ml NSC extractum (B) for 3 d at 28°C. The samples were viewed in a Philips FEI-XL300 SEM operated at 20 kV at 4,000 × magnification. The black arrows in A show normal hyphal and sporulation, and the white arrows in B show distortion, indentation and thinning of the hyphal wall.
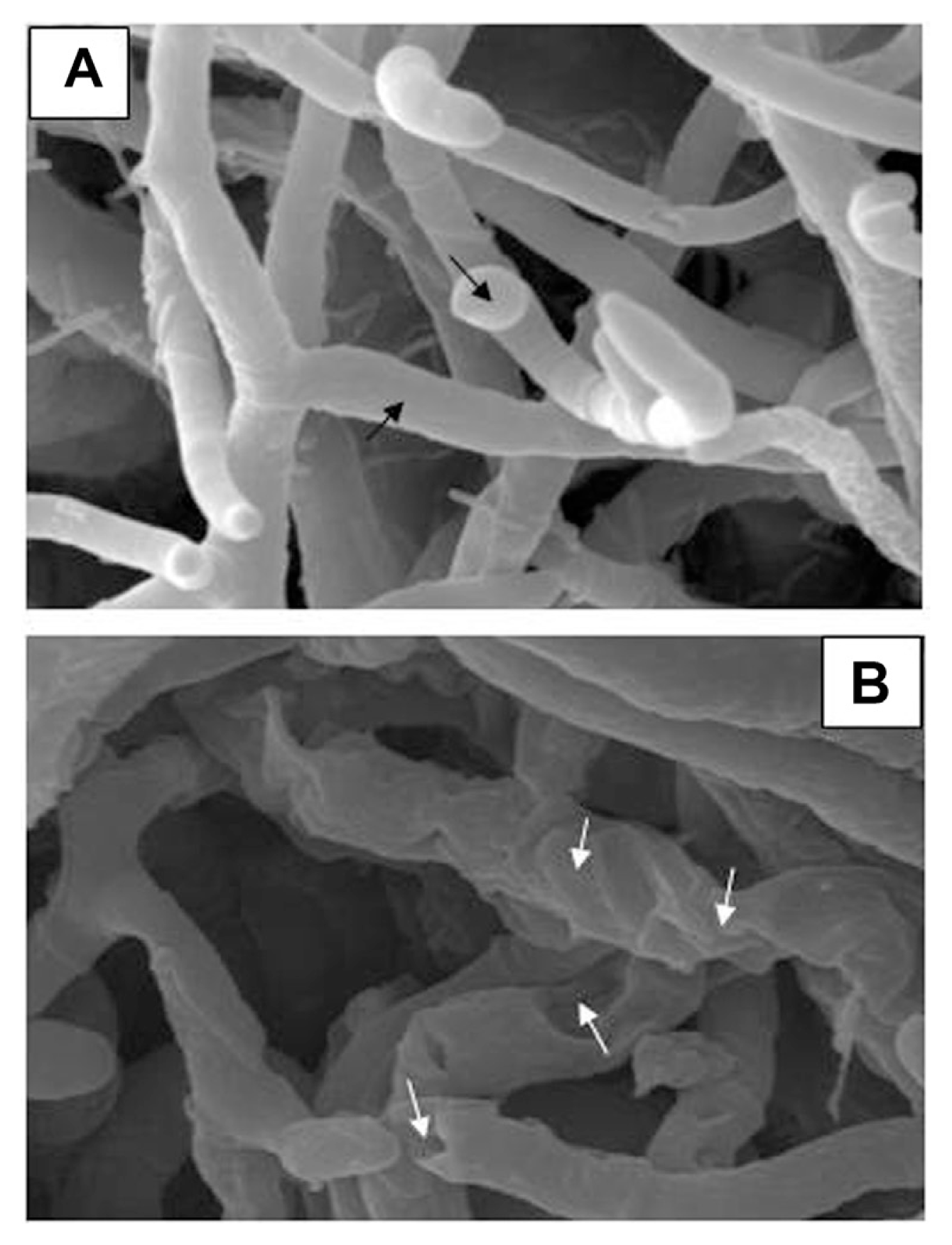
Fig. 4
Activities of phenylalanine ammonia-lyase (PAL), peroxidase (POD), β-1,3-glucanase (GLU) and chitinase (CHT) in banana pericarp treated with water (control, CK) and 5 mg/ml water extractum of seed cake. Bars represent standard deviations of the means.
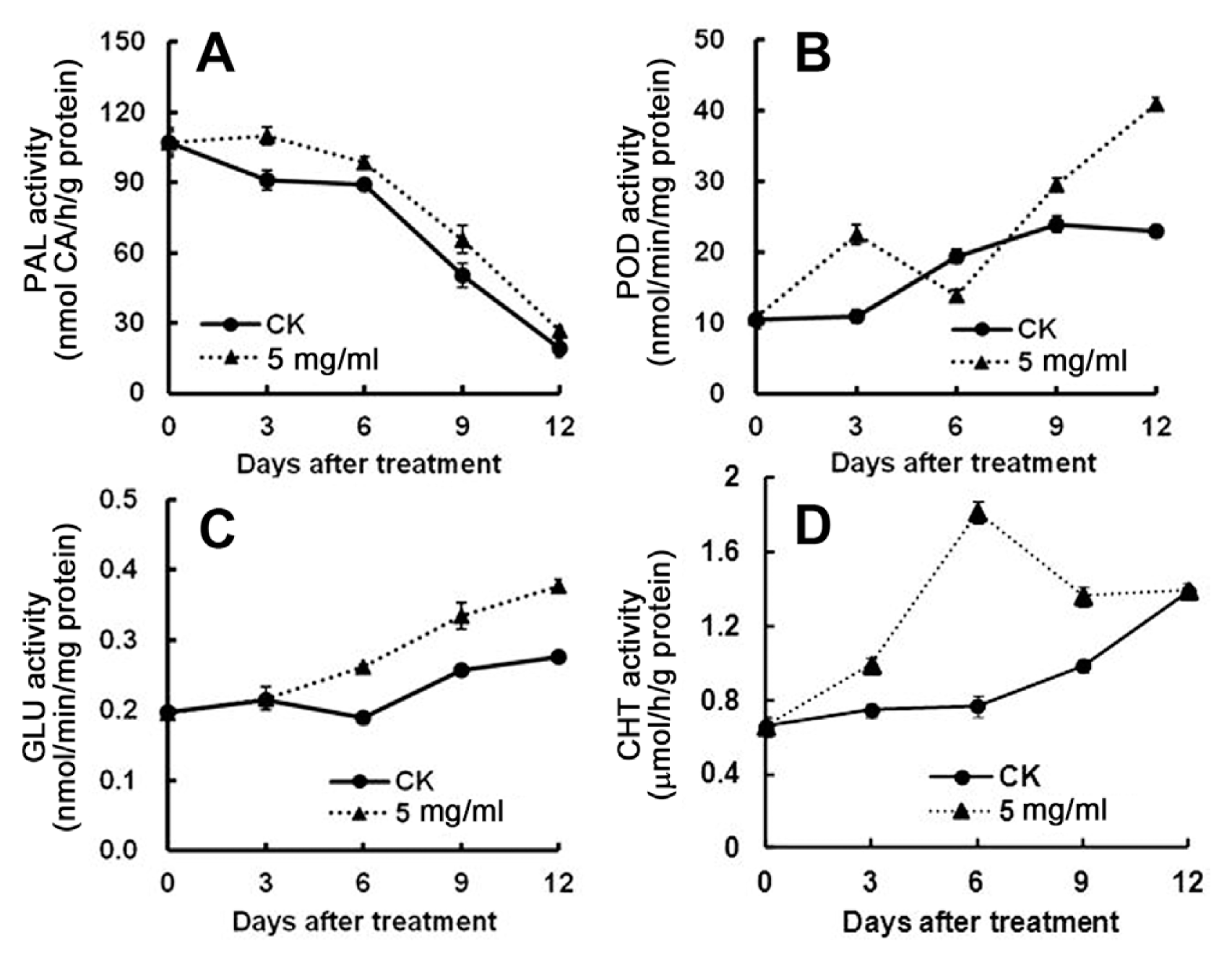
Table 1
In vitro effects of water extractum from Nanshancha seed cake on mycelial growth of postharvest pathogens expressed as percentage inhibition rate
Nanshancha and tea Saponin seed cake respectively. Mycelial growth inhibition was calculated after 7 days of incubation at 28°C in relation to the control without any supplementation. Data are the mean of 15 replications across three experiments. Data after ‘±’ represent standard deviations of the means.
References
Ahmet, C, Saban, K, Hamdullah, K and Ercan, K 2005. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem Syst Ecol. 33:245-256.

Askar, AA and Rashad, YM 2010. Efficiency of some plant extracts against Rhizoctonia solani on pea. J Plant Protec Res. 50:239-243.
Costa, DM and Erabadupitiya, HRUT 2005. An integrated method to control postharvest diseases of banana using a member of the Burkholderia cepacia complex. Postharvest Bio Tec. 36:31-39.

Deriu, A, Zanetti, S and Sechi, LA 2008. Antimicrobial activity of Inula helenium L. essential oil against Gram-positive and Gram-negative bacteria and Candida spp. Int J Antimicrobial Agents. 31:588-590.

Finlay, AR and Brown, AE 1993. The relative importance of Colletotrichum musae as a crown rot pathogen on Windward Island bananas. Plant Pathol. 42:67-74.

Gachango, E, Kirk, W, Schafer, R and Wharton, P 2012. Evaluation and comparison of biocontrol and conventional fungicides for control of postharvest potato tuber diseases. Biol Control. 63:15-120.

Hao, WN, Zhong, GH, Hu, MY, Luo, JJ, Weng, QF and Muhammad, R 2010. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochlorza. Postharvest Bio Tec. 56:39-43.
Hu, WL, Liu, JX and Ye, JA 2005. Effect of tea saponin on rumen fermentation in vitro. Anim Feed Sci Tech. 120:333-339.

Khan, SH, Aked, J and Magan, N 2001. Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathol. 50:601-608.


Kulakiotu, EK, Thanassoulopoulos, CC and Sfakiotakis, EM 2004. Biological control of Botrytis cinerea by volatiles of ‘Isabella’ Grapes. Phytopathology. 94:924-931.


Li, J, Zhang, A, Qi, Y, Meng, X and Zhang, Z 2012. Research progress in tea saponin from oil residue of Camellia semiserrata. China Food Science. 33:276-279.
Liu, J, Tian, SP, Meng, XH and Xu, Y 2007. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Bio Tec. 4:300-306.

Maqbool, M, Ali, A and Alderson, PG 2012. Effect of cinnamon oil on incidence of anthracnose disease and postharvest quality of bananas during storage. Int J Agri Biol. 12:516-520.
Meepagala, KM, Sturtz, G and Wedge, DE 2006. Antifungal constituents of the essential oil fraction of plants. Nat Protoc. 1:387-396.

Meng, X, Tang, Y, Zhang, A, Huang, X and Zhang, Z 2012. Effect of oligochitosan on development of Colletotrichum musae in vitro and in situ and its role in protection of banana fruits. Fruits. 67:147-155.

Meziane, H, Gavriel, S, Ismailov, Z, Chet, I, Chernin, L and Hofte, M 2006. Control of green and blue mould on orange fruit by Serratia plymuthica strains IC14 and IC1270 and putative modes of action. Postharvest Bio Tec. 39:125-133.

Obagwu, J and Korsten, L 2003. Integrated control of citrus green and blue mold using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Bio Tec. 28:187-194.
Palou, L, Crisosto, CH and Garner, D 2007. Combination of postharvest antifungal chemical treatments and controlled atmosphere storage to control gray mold and improve storability of ‘Wonderful’ pomegranates. Postharvest Bio Tec. 43:133-142.

Sharma, RR, Singh, D and Singh, R 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol Control. 50:205-221.

Steel, CC, Blackman, JW and Schmidtke, M 2013. Grapevine Bunch Rots: Impacts on wine composition, quatilty and potential procedures for the removal of wine faults. J Agr Food Chem. 61:5189-5206.
Tripathi, P and Dubey, NK 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruits and vegetables. Postharvest Bio Tec. 32:235-245.
Tzortzakis, NG 2009. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov Food Sci Emerg. 10:97-102.

Wang, X, Tian, C and Jiang, M 2008. Antioxidation ability and antibacterial effect of the extract from oil-tea camellia seed cake. China Oils Fats. 33:40-43.
Xing, Y, Li, X, Xu, Q, Yun, J and Lu, Y 2010. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int J Food Sci Tech. 45:1837-1842.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




