Effect of Salicylic Acid Formulations on Induced Plant Defense against Cassava Anthracnose Disease
Article information
Abstract
This study was to investigate defense mechanisms on cassava induced by salicylic acid formulation (SA) against anthracnose disease. Our results indicated that the SA could reduce anthracnose severity in cassava plants up to 33.3% under the greenhouse condition. The β-1,3-glucanase and chitinase enzyme activities were significantly increased at 24 hours after inoculation (HAI) and decrease at 48 HAI after Colletotrichum gloeosporioides challenge inoculation, respectively, for cassava treated with SA formulation. Synchrotron radiation–based Fourier-transform infrared microspectroscopy spectra revealed changes of the C=H stretching vibration (3,000–2,800 cm−1), pectin (1,740–1,700 cm−1), amide I protein (1,700–1,600 cm−1), amide II protein (1,600–1,500 cm−1), lignin (1,515 cm−1) as well as mainly C–O–C of polysaccharides (1,300–1,100 cm−1) in the leaf epidermal and mesophyll tissues treated with SA formulations, compared to those treated with fungicide carbendazim and distilled water after the challenged inoculation with C. gloeosporioides. The results indicate that biochemical changes in cassava leaf treated with SA played an important role in the enhancement of structural and chemical defense mechanisms leading to reduced anthracnose severity.
Anthracnose disease is widespread in cassava plants grown in various countries including Nigeria, Tanzania, Africa, India, and Thailand (William et al., 2012). The susceptibility varieties of cassava to cassava anthracnose disease (CAD) can affect the difference in disease severity. The damage caused by the disease has been reported in Congo, with severe destruction of up to 90% of cassava production. The disease is caused by Colletotrichum graminicola, C. gloeosporioides, and C. gloeosporioides f. sp. manihotis (Liu et al., 2019). In Thailand, anthracnose outbreaks have occurred in the northeastern region, it causes the loss of up to 80% of cassava products (Sangpueak et al., 2018). The physical symptoms of CAD are wilt, defoliation, necrotic lesions on leaves, bases of leaf petioles and stems and dieback (Liu et al., 2019; Sangpueak et al., 2018).
The new way to control CAD was salicylic acid (SA). SA has an essential role in the induction of defense response to stress responses (War et al., 2011). Furthermore, it has a role in regulating the components of the signaling pathway. Moreover, that can be contributed to cross-talk with many different paths act as intermediary plant resistance (Lu, 2009; Lu et al., 2016; War et al., 2011). The supporting data from induced resistance research, many commercial activator enhancers have been listed and used in crop production, e.g., Bion and Actigard (Syngenta), Elexa (chitosan; Safe Science), etc. The work of Gogbeu et al. (2015) in using SA, phosphoric acid, jasmonic acid, vitamin C, and ascorbic acid as effective elicitors to control cassava diseases seem to be the latest development in this field. At present, there are several techniques used in conjunction with traditional and molecular techniques that can be used to investigate the structural and functional changes of plant defense response to plant diseases (Alonso-Simón et al., 2011). So, this study was to investigate the biochemical and behavior changes of cassava after being induced by SA formulations using the SR-FTIR technique together with plant defense enzyme activities.
Materials and Methods
SA preparation and fungal growth and inoculum
Six percent SA for the stock solution, dissolved in 70% ethanol, then dilute the final concentration of the stock solution with sterile water and adjusted to the final concentration of 200 mg/l before use.
C. gloeosporioides was cultured on potato dextrose agar incubated 25°C at 14 days before harvested. Spore suspensions of respective fungal isolates were prepared with approximately 1 × 106 conidia/ml concentrations before sprayed onto three healthy cassava leaf until runoff.
Efficacy of SA to inducing resistance to anthracnose disease in cassava under a greenhouse condition
By design the randomized complete block design with four replications. Cassava stalks cv. Rayong 72 washed with 1% sodium hypochlorite solution for 2 min, followed by washing with sterile water three times, after that drying for 5 min at room temperature. Subsequently, the cassava stalks were soaked for 5 min in the 200 mg/l of SA before planting. Soaking the stalks in distilled water and 10 ml/20 l carbendazim 50% WP (Bentus, Sotus International Co., Ltd., Nonthaburi, Thailand) are negative and positive controls. After planting, the 200 mg/l SA, water, and 10 ml/20 l carbendazim were sprayed onto the plants, two more times at 1 and 3 months. At 24 h after the last foliar spray, spore suspensions of C. gloeosporioides at 1 × 106 conidia/ml concentration was sprayed onto the healthy cassava leaf until runoff. After the inoculation, the leaves were put in plastic bags within 48 h under the green-house. Disease severity scoring of CAD was collected at 14 days after the inoculation as follows by Sangpueak et al. (2018). After the scoring, the leaves were collected to detect biochemical changes associated with plant defense using SR-FTIR microspectroscopy.
Differential level of β-1,3-glucanases and chitinases activities
Protein concentration
Protein extract method was described by Nair and Umamaheswaran (2016). Cassava leaf was kept at 0, 24, and 48 hours after infection (HAI). Grind 1 g of tissue in liquid nitrogen and 5 ml extraction buffer that contained 0.1 M sodium phosphate buffer (pH 6.5) and polyvinyl pyrrolidone was added. The sample protein content was examined using the method from Bradford (1976), using bovine serum albumin to be a standard.
β-1,3-glucanases activity
The crude protein extract (62.5 μl) was mixed with 1% (w/v) laminarin (Sigma-Aldrich, St. Louis, MO, USA) in 0.05 M sodium acetate buffer (pH 5.0) after that incubated in 10 min at 40°C (Żur et al., 2013). Stop reaction by the increase of 375 μl (1% dinitro salicylic acid) and boiling 5 min in water. After that, cooling at 28°C (room temperature) and diluted 1:20. The reaction of enzyme was detected by measured at 500 nm followed by Prakongkha et al. (2013) and Żur et al. (2013). Enzyme activity is expressed in μg glucose released/min/g fresh weight/mg protein.
Chitinase activity
Chitinase activity was assayed by using crude protein extract (400 μl) mixed with 0.1% (w/v) colloidal chitin in 0.05 M sodium acetate buffer pH 5.0 at ratio 1:1 (v/v). Then, incubation mixture at 37°C for 2 h. Detection of N-acetyl glucosamine (GlcNAc) by absorbance at 585 nm followed by Prakongkha et al. (2013). For standard curve can be calculated using a standard curve prepared from GlcNAc.
Statistical analyses
The results of β-1,3-glucanases activity and chitinase activity were dissolved one-way analysis of variance (ANOVA) using SPSS version 14 (SPSS Inc., Chicago, IL, USA). The new Duncan’s multiple range test (DMRT) was used to differentiate treatment at P ≤ 0.05.
Biochemical changes analysis using SR-FTIR microspectroscopy
Sample preparation for SR-FTIR microspectroscopy
Cassava leaf samples were collected only the 5th top leaves at 7 days after treatment with SA treatment and inoculation with the pathogen for SR-FTIR microspectroscopic analysis. Leaves at the same position from that of the negative and positive controls were also included for the comparison. The analysis proceeded in a completely randomized design within three replications. The samples were subsequently cut to 1 × 1 cm size and embedded in the optimal cutting temperature compound, followed by Quick-freezing in liquid nitrogen. Then, the samples were stored at a −80°C as long as to do cryo-sectioning process. Subsequently, each cassava leaf sample was cut crosswise to 10 μm using a microtome of cryostat (Leica 3050 S, Jena, Germany) and stick on BaF2 window size 13 × 2 mm for SR-FTIR microspectroscopy.
Data measurement of SR-FTIR microspectroscopy
The determination was operated with a mapping by using a size of aperture at 10 × 10 μm with a resolution of 4 cm−1, with 64 scans. By used spectra was received with FTIR spectrometer (Vertex 70 from Bruker Optics, Ettlingen, Germany) together with infrared (IR) microscope (Hyperion series 2000, Bruker) with MCT detector refrigerate in liquid nitrogen through the determination range from 3,000 to 800 cm−1. Spectral equipment control was carried on by OPUS 7.2 software (Bruker Optics Ltd.) at the Synchrotron Light Research Institute.
Cassava image analysis
IR imaging of cassava tissues was created and analyzed using Cytospec 1.3.4 (Cytospec Inc., Croton-On-Hudson, NY, USA). The peak was transferred to the second derivative by using 13 smoothing points and vector normalized for the differing sample. The image structuring can be using a univariate model that generates up to peak intensity and peak area typically have meanings as chemical group maps. Hierarchical cluster analysis (HCA) was operated to separate the different biochemical components sample overranges of (3,000–2,800 and 1,800–900 cm−1). The 2D cluster maps are saved as figure files with a particular color to define a cluster. The spectra of cassava tissue from treated and non-treated plants were assays by principal cluster analysis (PCA) to differentiate the different biochemical composition of the tissue.
PCA analysis
Spectra from each cluster were assayed using PCA for differentiating biochemical elemental of tissue. The process was using 2nd derivative and vector normalized (Savitzky-Golay method, 3rd polynomial, 9 smoothing points) from the Unscrambler software version 9.7 (CAMO Software AS, Oslo, Norway).
Results
Efficacy of SA formulation in inducing resistance to anthracnose disease
At 14 days after the last foliar spray with SA at 200 mg/l, anthracnose severity on cassava treated with SA was among the lowest at 33.3%, comparable to that treated with carbendazim. The severity observed on the negative control, where the cassava was treated with water, was among the highest at 77.7% (Table 1).
β-1,3-glucanases activity
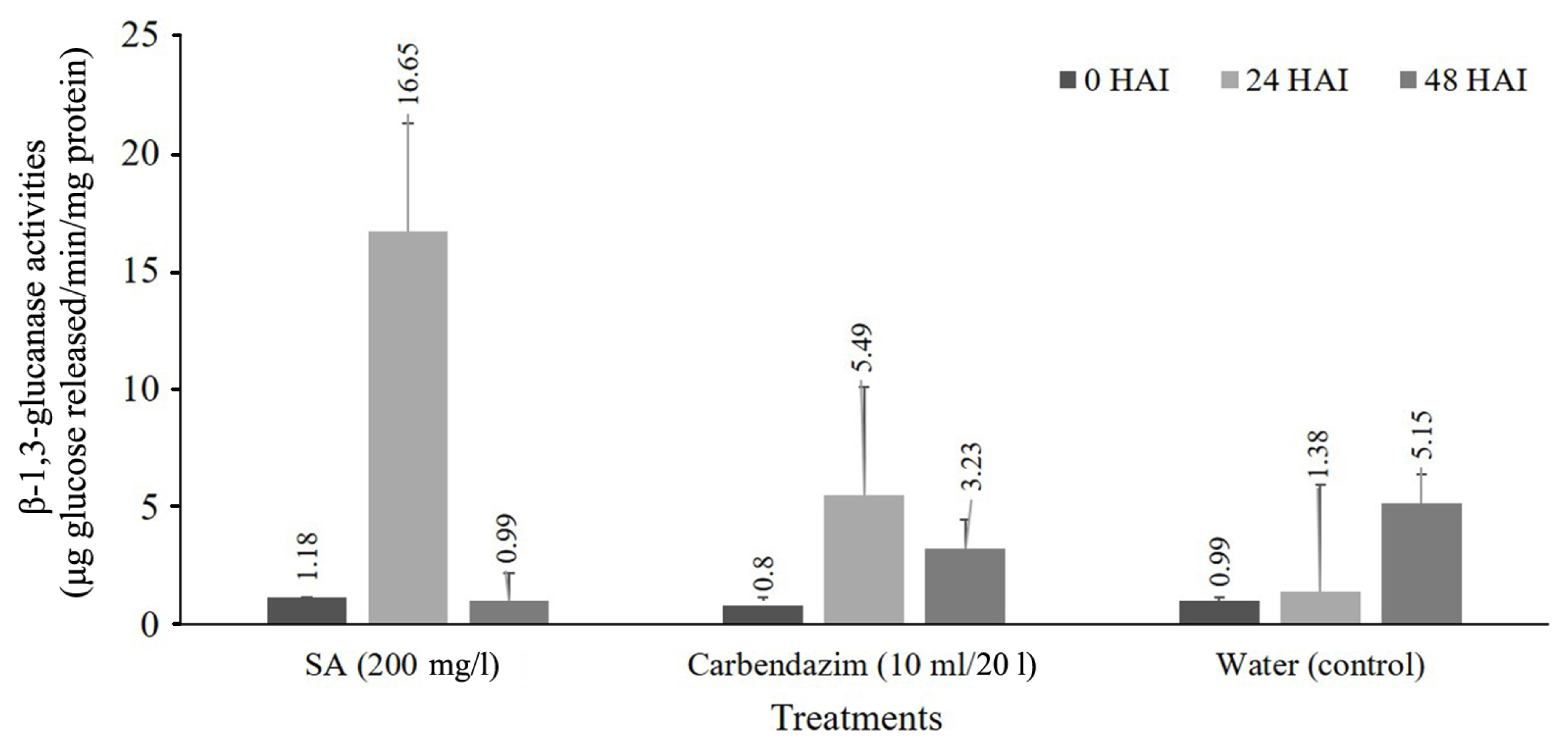
Induction of β-1,3-glucanase by treated with SA formulation and inoculation with pathogen showed the increased response of β-1,3-glucanase level of SA. It has increased in 24 HAI at 16.65 μg glucose released/min/mg protein was significantly higher compared with non-treated plant and decreased at 48 HAI significantly different when compared with control (Fig. 1).
Chitinase activity
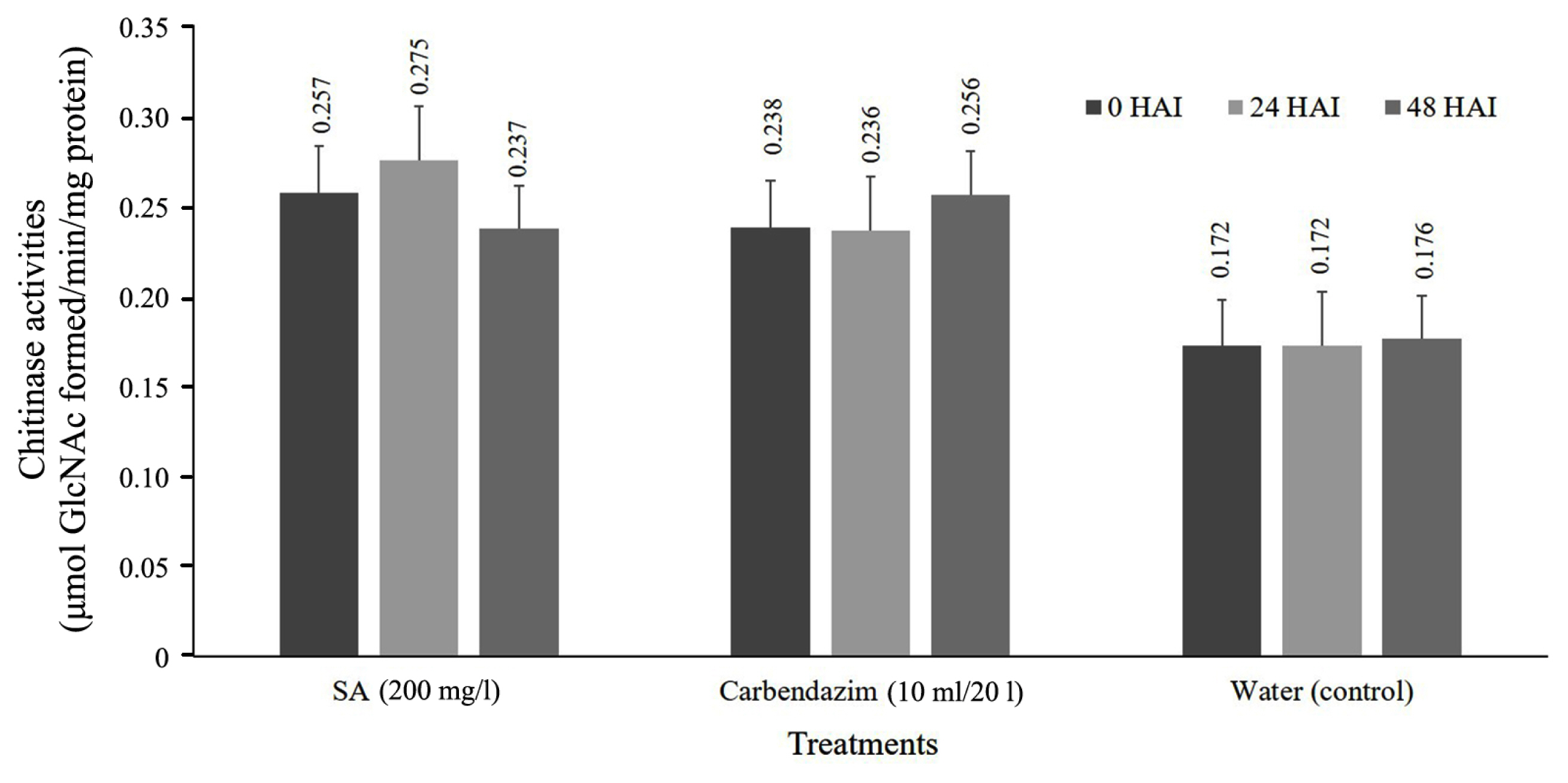
Chitinase activity of cassava leaf after treated with SA formulation and inoculation with fungal pathogen. The results show that chitinase activity was observed in all of the treatments of SA increased rapidly at 24 HAI and decrease at 48 HAI significantly different when compared with non-treated plant (control). SA can be induced by the accumulation of chitinase has the highest level at 0.275 μmol GlcNAc formed/min/mg protein (Fig. 2).
Biochemical changes analysis using SR-FTIR microspectroscopy
The purpose of this experiment to study the effect of SA formulations compared to that of the fungicide carbendazim of cassava physiological defense response. The biochemical and cell configuration of cassava leaf in epidermal and mesophyll tissues by using SR-FTIR. Biochemical changes were compared in the treated and nontreated cassava leaf (Fig. 3A–C). The IR images indicated absorbance intenseness of imaging were relative to color change: blue (show the lowest of chemical imaging) < green < red (show the highest of chemical imaging) (Fig. 3J–L). HCA was applicable to separate spectra depending on 5 clusters in the range of 3,000–900 cm−1, and changed the color of different groups. The chemical imaging of microstructures of cassava leaf is shown in Fig. 3D–I. Leaf tissues of the negative and positive controls were also observed to correlate the changes of spectral initiate in the proteins, lipids, and polysaccharides to that of the elicitortreated tissues.
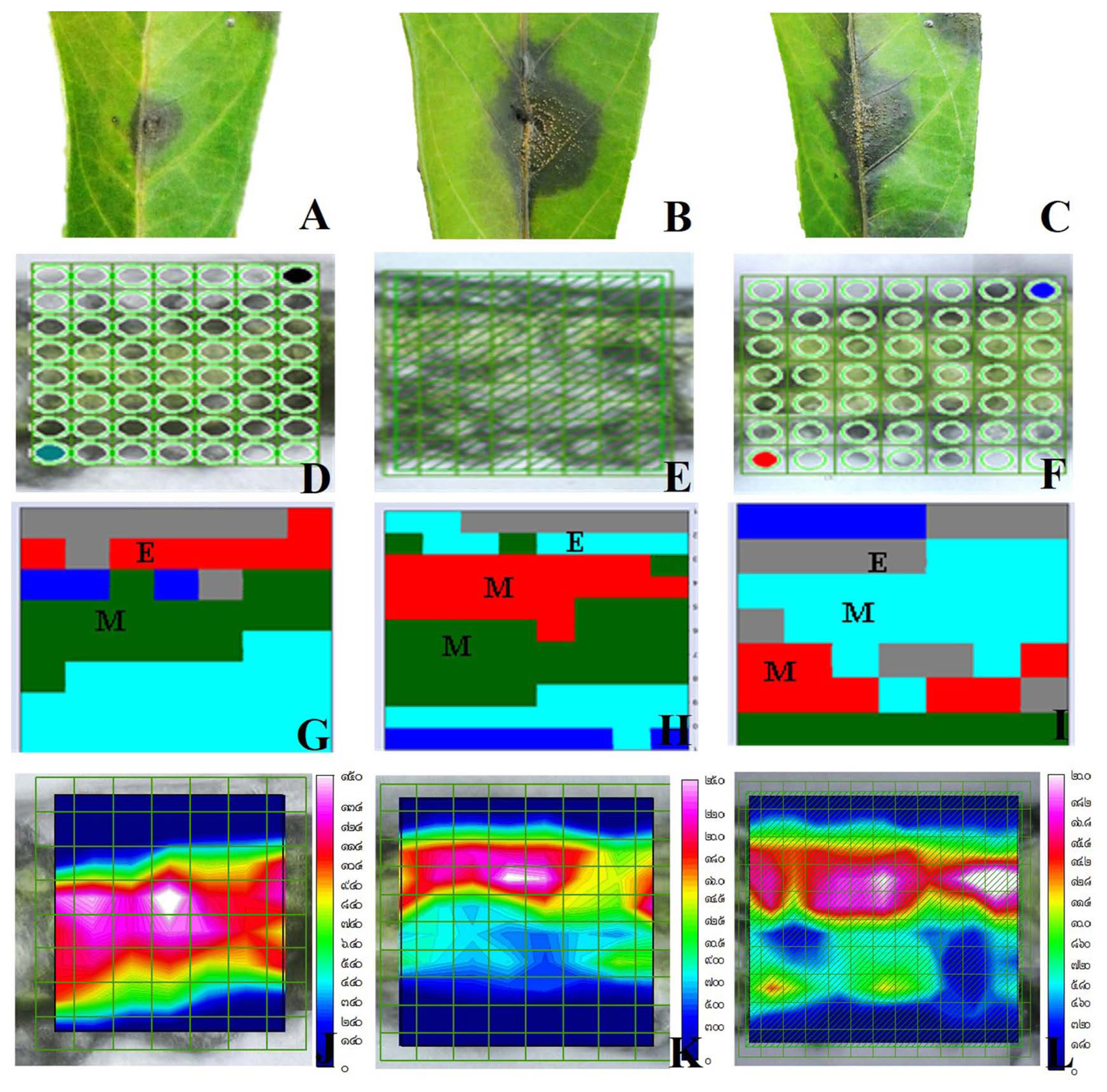
Biochemical changes of cassava after treated with treatment and fungal inoculation used synchrotron radiation–based Fourier-transform infrared microspectroscopy. (A) Cassava treated with salicylic acid. (B) Cassava treated with carbendazim. (C) Cassava treated with water (the control). (D–I) Map and hierarchical cluster analysis map of cassava treated with treatment. (J–L) Chemical mapping was proportional to color change: blue (the lowest) < green < red (the highest).
Spectra and secondary derivative average spectrum in the phase of 3,000–2,800 and 1,800–900 cm−1 were differences of the epidermal and mesophyll tissues (Fig. 4). Amide II protein (1,600–1,500 cm−1), no significant difference was found in the cassava epidermis of those treated with the SA formulation compared to those of the positive and negative controls (Table 2). The differences C=H stretching vibration (3,000–2,800 cm−1), pectin (1,740–1,700 cm−1), amide I protein (1,700–1,600 cm−1), lignin (1,515 cm−1), mainly C–O–C of polysaccharides (1,300–1,100 cm−1) were found at some vibrational peaks of (Fig. 4A and B). Mesophyll tissue of cassava leaf treated with SA also had a significant difference in the cassava epidermis of those treated with the SA formulation compared to those of the positive and negative controls. At the vibrational peaks of pectin, amide I protein, amide II protein, lignin, mainly C–O–C of polysaccharides while spectrum of C-H stretching was no significant difference in mesophyll tissue were calculated in Table 3 (Fig. 4C and D).

Representative original average spectra in the range of 3,000–2,810 cm−1 and 1,800–900 cm−1. (A) Original average spectra in epidermis tissues of cassava leaf. (B) Second derivative in epidermis tissues of cassava leaf. (C) Original average spectra in mesophyll tissues of cassava leaf. (D) Second derivative in epidermis tissues of cassava leaf mesophyll tissues. SA, salicylic acid.

The integral area of average spectra from epidermis of cassava leaf treated with salicylic acid and challenge inoculation with Colletotrichum gloeosporioides

The integral area of average spectra from mesophyll of cassava leaf treated with salicylic acid and challenge inoculation with Colletotrichum gloeosporioides
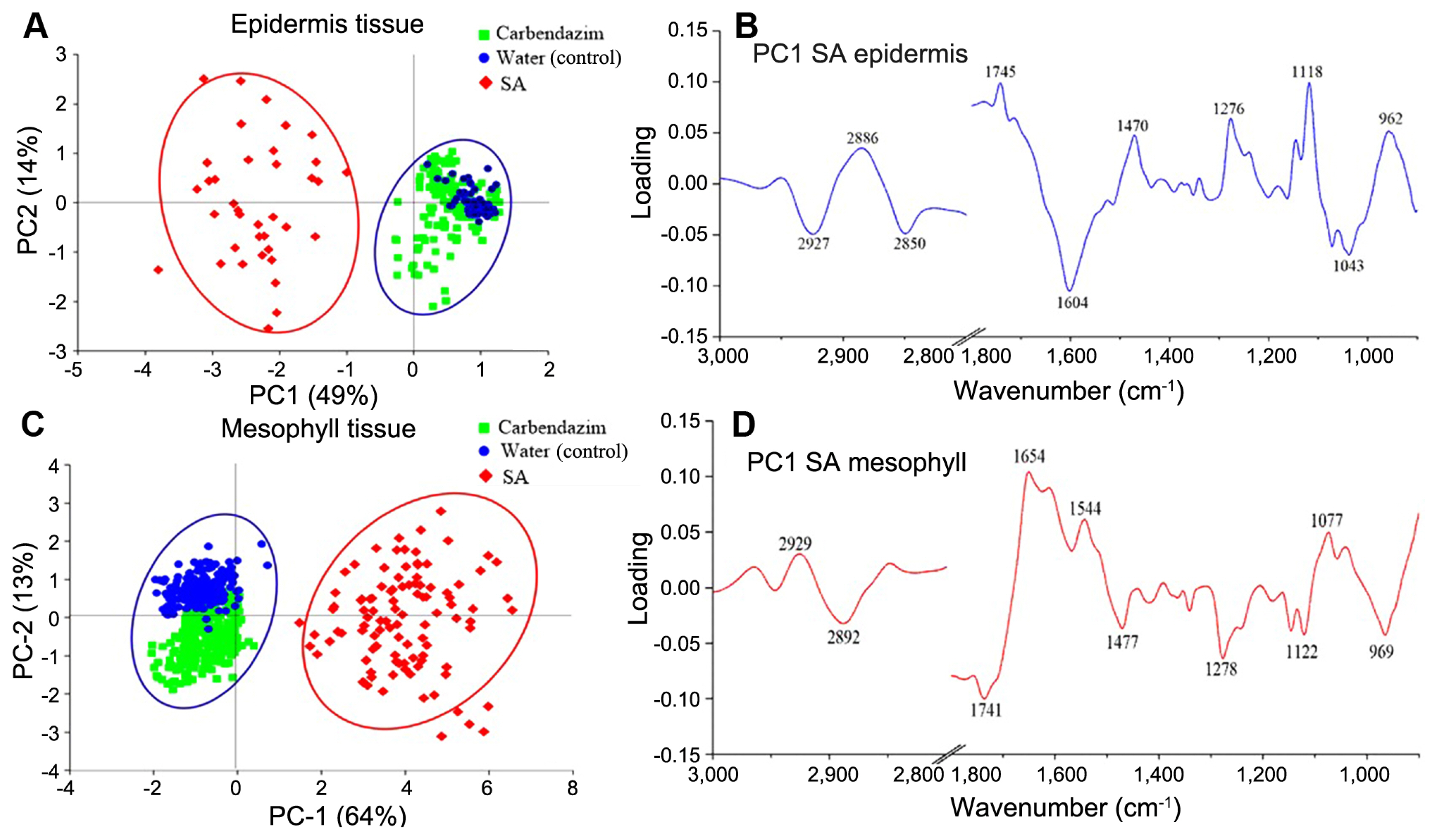
This information represents course changes of biochemical in cassava after being treated of SA formulation, elucidating the response to defense mechanisms against anthracnose disease. To used PCA technique was applied to analyze biochemical changes. This technique is useful in analyses of bio spectroscopic data by making it visible of similar spectra in scores and loading plots (data not showed) between treated and non-treated leaves. In addition, the data analysis represents that the PC1 and PC2 regularly are the most different clustering two or three groups. The red points representing SA treatment could be easily distinguished from the blue and green points of the water control and carbendazim. The PC1 and PC2 loading of SA treatment were shown that separation between PC1 and PC2 corresponded to total variance of 49% from PC1 and 14% in epidermis tissue (Fig. 5A and C). For mesophyll tissue found that the separation between PC1 and PC2 corresponded to total variance of 64% from PC1 and 13% (Fig. 5B and D). The results show structural and physiological changes associated with the defense mechanism in cassava.
Discussion
In this present study, the most efficacy for induction was obtained at concentrations at 200 ppm of SA and used to compare with fungicide and water control. SA can reduce disease severity by 33.3% compared with the control. Similar results were observed to previous studies, In hydroponic tomato plants, with the treatment of SA at 200 μM showed to control 9% of leaf yellowing of Fusarium wilt, compared with the control which exhibited disease incidence reaching 70%, at two weeks after inoculation (Jendoubi et al., 2015). Exogenous application of 50 μM SA at 15 days before inoculation reduced the severity of leaf curl virus in tomato (Ong and Cruz, 2016). And Koo et al. (2020) reported that SA effects enhanced disease resistance upon exogenous SA application in plants against the different species of pathogens, including Fusarium oxysporum, Alternaria alternata, Magnaporthe grisea, Colletotrichum gloeosporides, Xanthomonas spp., viruses and etc.
Also, SA can be induced, accumulate methodically are linked to systemic acquired response to biotic and abiotic stresses in different plants (Gao et al., 2015; Koo et al., 2020). These results show that the change of β-1,3- glucanase and chitinase at 24 HAI after last sprayed with SA formulation and inoculation with fungal pathogen that had been related to plant defense mechanisms against pathogen attack and can destroy the constituents of cell walls of C. gloeosporioides in the attacked cassava tissue (Gupta et al., 2012; Santos et al., 2004). These enzymes an essential role in induced cassava resistance to pathogens (Thakur and Sohal, 2013). It can be substantiated that elicitor can enhance the resistance of pathogenesis-related (PR)- proteins proteins and also act as increase plants resistance to different pathogens (El-kereamy et al., 2011; Jayaraj et al., 2004; Koo et al., 2020).
In addition, plants defense depends on the contribution of the cell wall to against an infection to pathogens. Because of the synchrotron light that gives the intensity and source brighter than conventional sources, it helps to reduce the analysis time compared to using conventional IR Source (Baker et al., 2014; Wang et al., 2016). The SR-FTIR mapping displays the integrated area of proteins, lipids, and polysaccharides at absorption between 3,000–2,800 and 1,800–900 cm−1 (Wang et al., 2016). The peak variation between treatments indicated that the changes in the intensity of lignin, lipids, amine I of protein, and amine II of lignin in epidermis and mesophyll tissues. The epidermis and mesophyll tissues treated by SA formulations showed spectra higher than those of the control significantly at the vibrational peaks of C=H stretching vibration, pectin, amide I protein, lignin, mainly C–O–C of polysaccharides that can help to improve cassava cell wall structure. Consistent research of Thanh et al. (2017), the present study, demonstrates that the higher ratios of pectin and lignin were observed in plants sprayed with SA. SA treated on rice plants shown higher amide I, β-sheet structure, and lipids. This physical defense may include the amplification of cell wall thickenings such as the blockage of plant vessels (Jendoubi et al., 2015). The results show physiological and chemical changes with the defense mechanism in cassava against anthracnose disease.
Conclusion
Cassava treated with SA formulation could be reduced anthracnose disease caused by C. gloeosporioides and can be the induction of β-1,3-glucanase and chitinase activity. By treatment with SA is correlated by the increased resistance of the cassava tissues against infection by the C. gloeosporioides pathogen. Moreover, SRFTIR microspectroscopy could be used as equipment for detecting biochemical changes at the most resolution. It’s showed the structural and biochemical of different cassava tissues that were treated with elicitors. This research offers that the changes of structure in cassava tissue have interaction with plant defense responses after stimulated with the SA formulation. Besides, one solution of products containing SA, especially SA would be to introduce an alternative product to prevent yield loss of cassava-related infections from anthracnose disease pathogen.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors wish to express grateful the research assistant of Plant Pathology and Biopesticide Laboratory, Suranaree University of Technology, for the technical assistance, and Synchrotron Light Research Institute (Public Organization) for providing the SR-FTIR instruments and beam times. This work was supported by the Research and Researcher for Industries (RRi), Thailand Research Funds [grant number PHD59I0084] to Miss Rungthip Sangpueak.