First Molecular Characterization of Colletotrichum sp. and Fusarium sp. Isolated from Mangrove in Mexico and the Antagonist Effect of Trichoderma harzianum as an Effective Biocontrol Agent
Article information
Abstract
The aim of this study was to characterize potential fungal species affecting mangrove species in Mexico. The phytopathogens were identified based on morphological and molecular characteristics using internal transcribed spacer (ITS1/ITS4) primers then sequenced and compared with the other related sequences in GenBank (NCBI). Three fungal species were identified as Colletotrichum queenslandicum (Weir and Johnst, 2012) from black mangrove (Avicennia germinans); Colletotrichum ti (Weir and Johnst, 2012) from white mangrove (Laguncularia racemosa) and buttonwood mangrove (Conocarpus erectus); Fusarium equiseti (Corda) from red mangrove (Rhizophora mangle). In addition, C. ti and F. equiseti were identified from mango Mangifera indica L. sampled close by the mangrove area. This study provides first evidence of anthracnose on four mangrove species caused by Colletotrichum and Fusarium species in the “Términos” coastal lagoon in Campeche State southern Mexico. This is the first time that C. queenslandicum and C. ti are reported in Mexico. F. equiseti has not been reported affecting M. indica and R. mangle until the present work. Little is known regarding fungal diseases affecting mangroves in Mexico. These ecosystems are protected by Mexican laws and may be threatened by these pathogenic fungus. This is the first report of the effect of Trichoderma harzianum TRICHO-SIN as an effective biological control against of Colletotrichum and Fusarium species.
In recent years, mangrove forests have received more attention from government due their ecological importance (Duke et al., 2007; Hector and Bagchi, 2007; Lee et al., 2014). According to Kristensen et al. (2008), mangrove wetlands are one of the most productive coastal ecosystems in tropical and subtropical regions and tolerate the effluents of aquaculture farms due to their enormous demand for nutrients (Bao et al., 2013; Kovacs et al., 2009). Particularly, these plants play a critical role in the removal and degradation of pollutants such as heavy metals, nitrogen, phosphate compounds, and pesticides (Adame and Lovelock 2011; Bayen, 2012). Their permanence and survival are threatened by anthropogenic activities mainly by aquaculture and agricultural pressures (Flores-de-Santiago et al., 2012; Osorio et al., 2014). For this reason, mangrove forests are considered as threatened vegetal species by the Mexican laws (NOM-059-SEMARNAT-2001), and worldwide, they have a special ecological endangered consideration (Food and Agriculture Organization of the United Nations, 2007). Unfortunately, among the threats, mangroves despite their importance, display evidence of disease in the form of anthracnose symptoms. Anthracnose has been defined in a number of different of ways, primarily as a fungal disease of plants characterized especially by necrotic dark lesions on the vegetal tissue (Weir et al., 2012) but with high divergence in morphological characters and infectiousness. Anthracnose is caused by Colletotrichum Corda, 1831 and Fusarium sp., among the most common and important genera of plant pathogenic fungi (Cai et al., 2009; Cannon et al., 2012; Weir et al., 2012).
Anthracnose exists in wide variety of agricultural plants, such as mango (Mangifera indica L.) and papaya (Carica papaya L.) The disease is seen in Mexico affecting several commercial orchards in Campeche and Chiapas in the southeast of Mexico (Rojo-Báez et al., 2017; Tapia-Tussell et al., 2016; Torres-Calzada et al., 2013), including areas adjacent to mangrove forests. Current research has found Fusarium sp. in different cane crops and in tomato (Solanum lycopersicum L.) (Isaac et al., 2018; Martínez-Fernández et al., 2015) in the same zone. There is agricultural developing nearby into the mangroves, potentially adding to parallel transmission diseases on this species. However, little is known about the pathogens that attack mangroves in Mexico. Therefore, the aim of this study was to identify the pathogen causing anthracnose symptoms in four species of mangrove forest in the Gulf of Mexico plus a nearby mango orchard and contribute to the current knowledge of infection, its spread and the possible ecological deficit e.g. effect on the photosynthesis caused by anthracnose due to agricultural practices.
The biological control of phytopathogens by antagonistic organisms is one of the main topics in agricultural research to minimize financial loss and better assure export products (Guigón-López et al., 2010). Trichoderma species (Ascomycetes) are being considered promising contenders for the biological control of plant pathogenic fungi (Benítez et al., 2004; Hermosa et al., 2013). This is the case of TRICHO-SIN a biological fungicide formulated based on the fungus (Trichoderma harzianum Rifai 1969), which produces antifungal metabolites and hydrolytic enzymes according with Agrobionsa (2020). Howell (2003) and Ali et al. (2012) report that the main antagonistic properties of Trichoderma species are based on various mechanisms including competition for nutrients and mycoparasitism (Gveroska and Ziberoski, 2011; Nunes, 2012).
Nevertheless, not much is known about phytoparasitic fungi in mangroves in the tropics. In Mexico, there is no information concerning elimination of fungal diseases affecting mangroves in the Gulf of Mexico or the Pacific coast. Therefore, we also carried out antagonism tests for potential use in the field with promising results.
Materials and Methods
Sample collection and fungal isolation
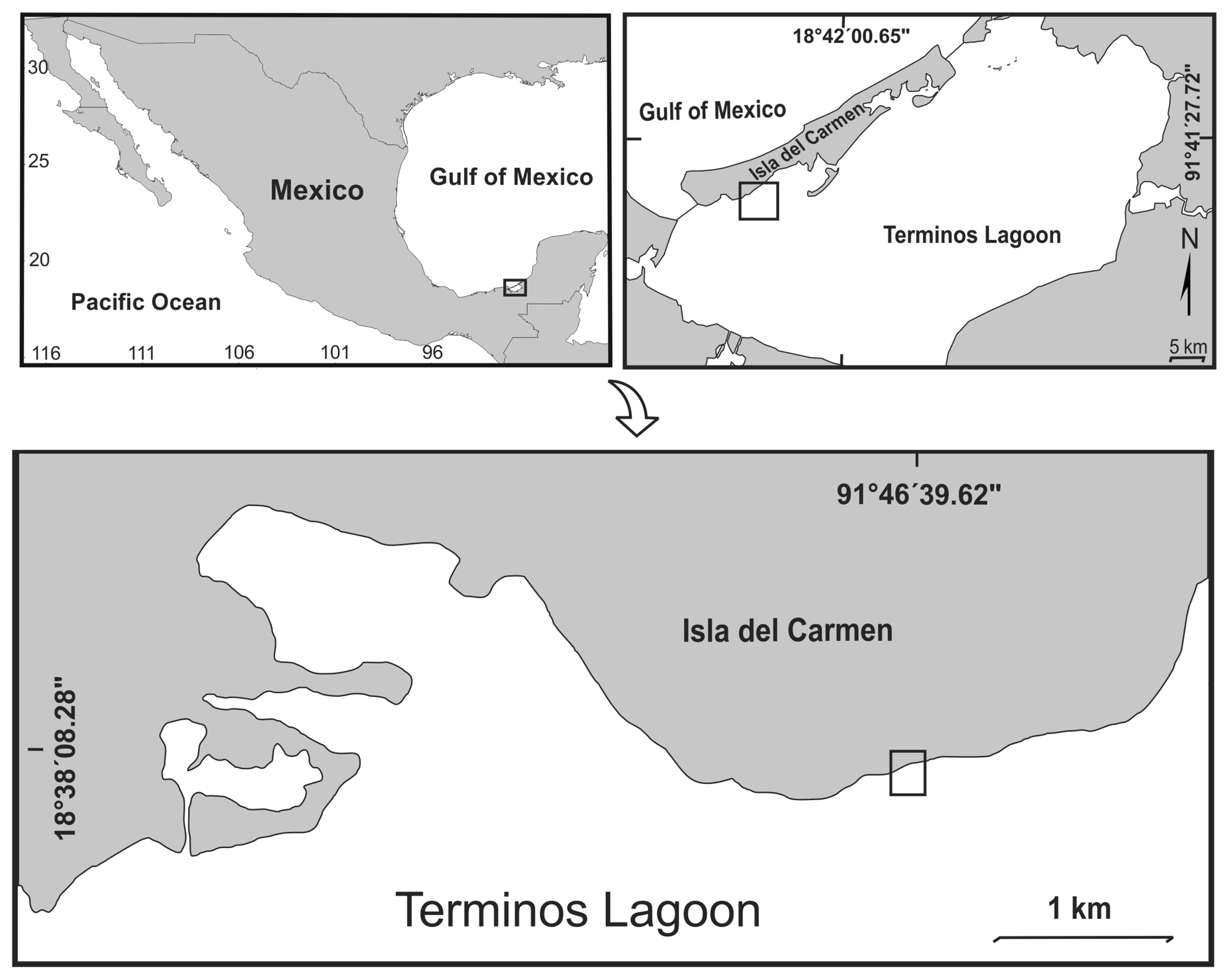
Diseased leaves with visible anthracnose symptoms (dark lesions, necrosis, and deformations) were collected from four mangrove species: black mangrove (Avicennia germinans), white mangrove (Laguncularia racemosa), button mangrove (Conocarpus erectus), red mangrove (Rhizophora mangle), and from mango (M. indica) from Terminos Coastal Lagoon (18°37′40″N, 91°47′02″W) (Fig. 1). The leaves were placed in species-labeled paper bags and transported in coolers to the ecophysiology laboratory at the Marine Science Faculty (FACIMAR-UAS) for examination. Leaves were individually washed according the protocol of Wanderley Costa et al. (2012) as follows: The leaves were surface-disinfested by being washed in cold running water for 1 min, then disinfected with 70% ethanol and then with 1% sodium hypochlorite for 30 s. A small square (1 cm2) from each leaf was dissected using a sterile dissecting scissors and four sub-samples were placed into potato dextrose agar (PDA, Sabouraud dextrose agar [SDA]) plates. They were exposed to sun light for 1min and incubated at 30°C in 12 h light/dark regimes for 5 days. For the next 4 weeks, the plates were examined daily for fungal colonies from the leaf fragments. Following observation, fungal species were isolated and identified according to morphological characteristics (Weir et al., 2012). Spore masses were collected from the agar culture (pink pale color) using a sterilized bacteriological needle. Each conidial sample was then mounted on a glass slide in a drop of sterile distilled water and stained in situ by the addition of a drop (~3 μl) of lactophenol blue solution for microscopy to the edge of the coverslip which was drawn under the coverslip by capillary action. The coverslip was then sealed with transparent nail vanish. The morphological descriptions were recorded using a compound microscope (Olympus BX51, Tokyo, Japan) at 40×, 100×/oil immersion magnification. Additionally, conidia were identified through specialized literature (Weir et al., 2012).
Extraction and quantification of DNA
DNA extraction was performed using 200 mg of fresh mycelium resulting from fungal cultures in PDA using the method described by Aljanabi and Martinez (1997). Furthermore, a treatment with RNase was carried out. Then, the RNA was precipitated twice with 40 μl of 3M sodium acetate solution, 11.5 M acetic acid and 220 μl of absolute ethanol. Subsequently, the DNA samples were quantified by spectrophotometry using 1 μl of sample in a nanodrop. At the same time, these samples were run on a 1.5% agarose gel to observe the integrity and quality of the DNA.
Phylogenetic analysis
In order to identify the fungi at the molecular level to detect at the species level of the isolated fungus belonged to each of the four mangrove and mango plants, rDNA internal transcribed spacers (ITSs) were sequenced. The sequences obtained were subjected to quality analysis and editing to improve their quality, using the Geneious Prime program (Kearse et al., 2012). One mango sample was discarded from the analysis, due to its general poor DNA quality. Each sample was subjected to BLAST analysis (Altschul et al., 1990), employing the database for ITS. Subsequently, the sequences with the highest percentage of identity and coverage of the BLAST analysis results were downloaded, and an outgroup was selected. Additionally, sequences of species were included belonging to Colletotrichum sp., which has been reported to cause anthracnose (Liu et al., 2018). The sequences were then aligned using Geneious Prime and ClustalW (Thompson et al.,1994). The resulting alignment was used to determine the appropriate phylogenetic model using the jModelTest version 2.1 program (Darriba et al., 2012). Later, the construction of the phylogenetic tree was carried out using the MEGA X program (Kumar et al., 2018) with the maximum likelihood (ML) statistical method, Jukes-Cantor model (Jukes and Cantor, 1969), and bootstrap analysis with 1,000 repetitions.
The antagonistic effect of T. harzianum against Colletotrichum and Fusarium species
The antagonistic activity of T. harzianum (TRICHO-SIN, Agrobionsa) was evaluated by triplicate on agar PDA medium and SDA culture medium. A 5 mm diameter mycelia disc was cut from the phytopathogens and placed on the one edge of a Petri dish. Another 5 mm mycelial disc from TRICHO-SIN was placed in the other edge on the same Petri plate, in parallel, at a distance of 6 cm from each other. Then, the plates were incubated at 30°C and plates containing only Colletotrichum and Fusarium spp. pathogens were used as controls. Photographs were taken of the Petri dishes at 7 and 15 days of incubation. The antagonism interface of both colonies was observed and described. In order to validate the fungus identity, DNA extraction and PCR amplification from each sample were also performed.
Results
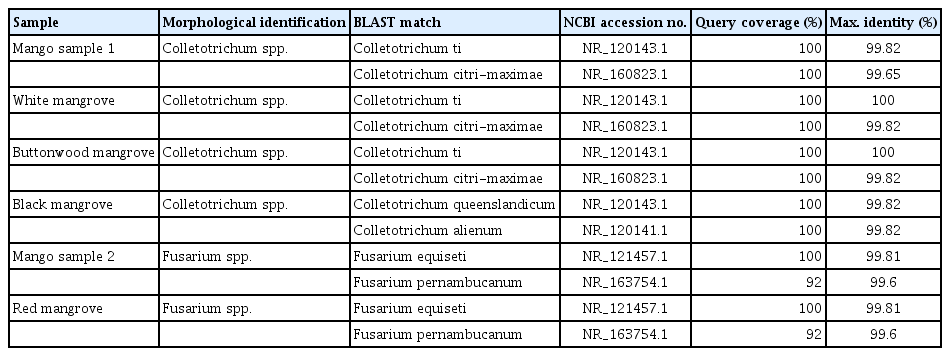
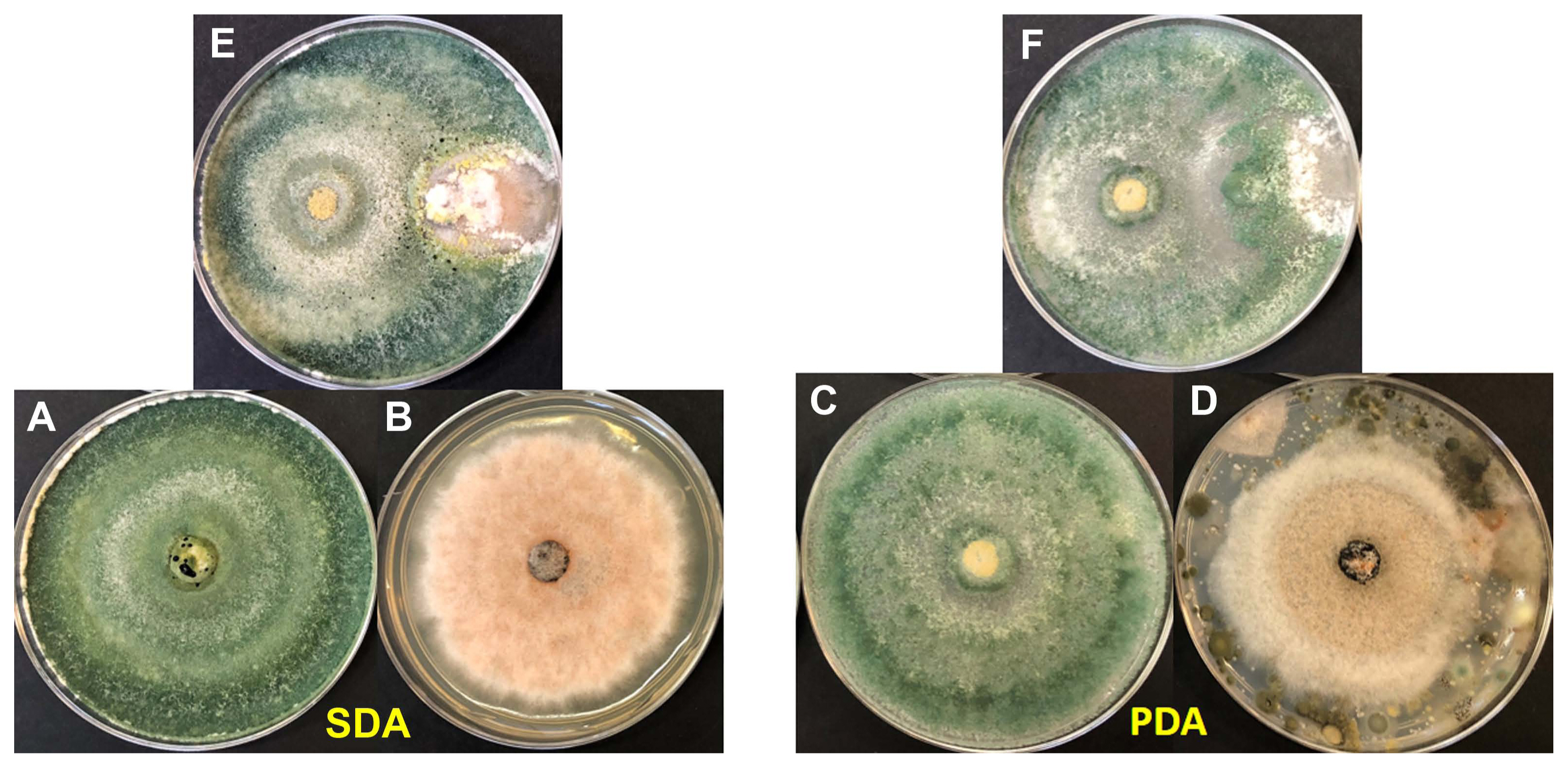
Two different genus of fungus were identified as: Colletotrichum sp. and Fusarium sp. from the diseased leaves of mangroves: (1) Colletotrichum queenslandicum was identified from black mangrove (A. germinans); (2) Colletotrichum ti was identified from white mangrove (L. racemosa) and in button mangrove (C. erectus); (3) F. equiseti was identified from red mangrove (R. mangle). In the mango samples, C. ti and F. equiseti were identified in the same sampling mangrove area. Colonies of Colletotrichum and Fusarium on PDA were gray to dark gray showing an aerial mycelium and orange or pale pink conidial masses distributed on surfaces of fungal mycelia (Fig. 2). Colletotrichum spp. conidia were hyaline, dikaryotic (showing two nuclei), ellipsoid with cylindrical with round at ends. Asci were clavate to cylindrical. Fusarium equiseti conidia produced one-celled, hyaline, falcate shape with acute apex (Fig. 2).

Morphotypes of isolates of Colletotrichum spp. and Fusarium spp. Plates in aerial view, 5 days grown on potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) at 30°C. (A–D) Colletotrichum queenslandicum isolated from Avicennia germinans (A), SDA (B) and PDA (C), conidia (D). (E–H) Fusarium equiseti isolated from Rhizophora mangle (E), SDA (F) and PDA (G), conidia (H). (I–L) Colletotrichum ti isolated from Laguncularia racemose (I), SDA (J) and PDA (K), conidia (L). (M–P) C. ti isolated from Mangifera indica L.: SDA (M, N) and PDA (O) at 30°C, conidia (P). (Q–T) C. ti isolated from Conocarpus erectus (L.): SDA (R) and PDA (S), conidia (T). The black arrow was to point the spores.
Phylogenetic analysis
Consensus sequences were edited and searched in the BLAST. The results obtained (Table 1) correspond with the morphological identification of the samples. The ML in the phylogeny analysis, obtained from the ITS was established with 24 taxa and 634 characters, showed that the morphologically categorized species of mangrove fungi is accurate. The generated phylogenetic tree (Fig. 3) shows that the Colletotrichum and Fusarium species are correctly differentiated starting from a common ancestor. Samples of fungus isolated from black, white, button mangrove, and mango belong to the genus Colletotrichum sp., with a high support value (bootstrap) (92%), which gives us high fidelity with accurate grouping. In the case of the isolated samples of red mangrove and mango morphologically identified and belongs to the genus Fusarium sp., they are included in a separate group with a support value of (87%), relating to the species of the genus Fusarium spp., F. equiseti and Fusarium pernambucanum.
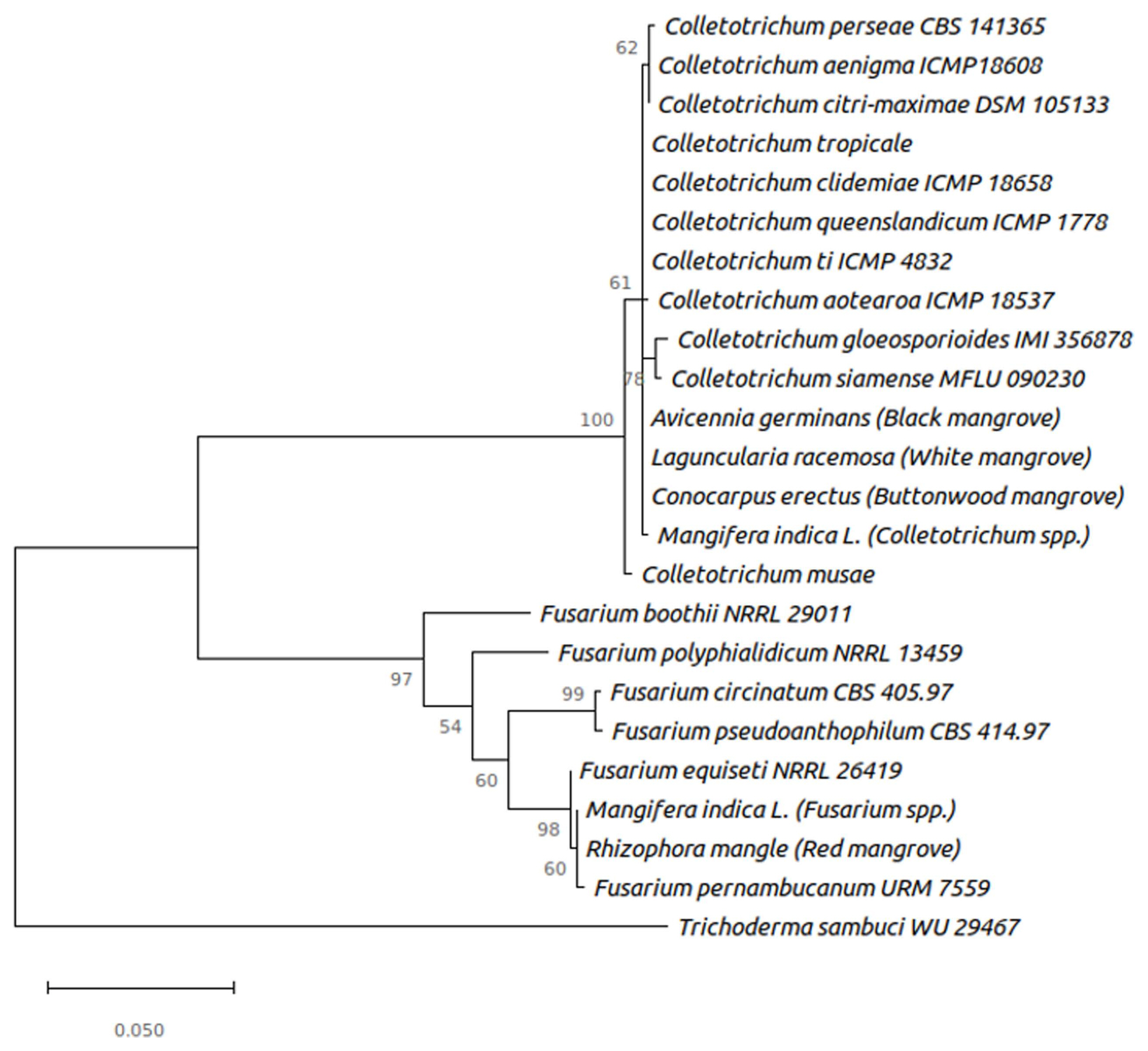
Phylogenetic tree built using the maximum likelihood Jukes-Cantor model. The tree is using Trichoderma sambuci as an outgroup.
Once aligned between R. mangle and M. indica sample sequences, a 100% similarity was observed between both ITSs, which suggests that they belong to the same species. Additionally, in the phylogenetic analysis, these were grouped very closely with the fungi Fusarium equiseti and F. pernambucanum (Table 1, Fig. 3).
Biocontrol effect of T. harzianum against Colletotrichum and Fusarium species
T. harzianum showed an inhibited mycelium grow, competition and space of all tested plates in both Colletotrichum and Fusarium species by least 70% (±10%) at 5 days at 30°C (Figs. 4–8).
(A, C) Trichoderma harzianum (TRICHO-SIN Agrobionsa) 5 days grown on potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) at 30°C. (B, D) Colletotrichum ti from Conocarpus erectus 5 days grown on PDA and SDA at 30°C. (E, F) Antagonic activity of T. harzianum against C. ti.

(A, C) Trichoderma harzianum (TRICHO-SIN Agrobionsa) 5 days grown on potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) at 30°C. (B, D) Colletotrichum queenslandicum from Avicennia germinans. (E, F) Antagonic activity of T. harzianum against C. queenslandicum.
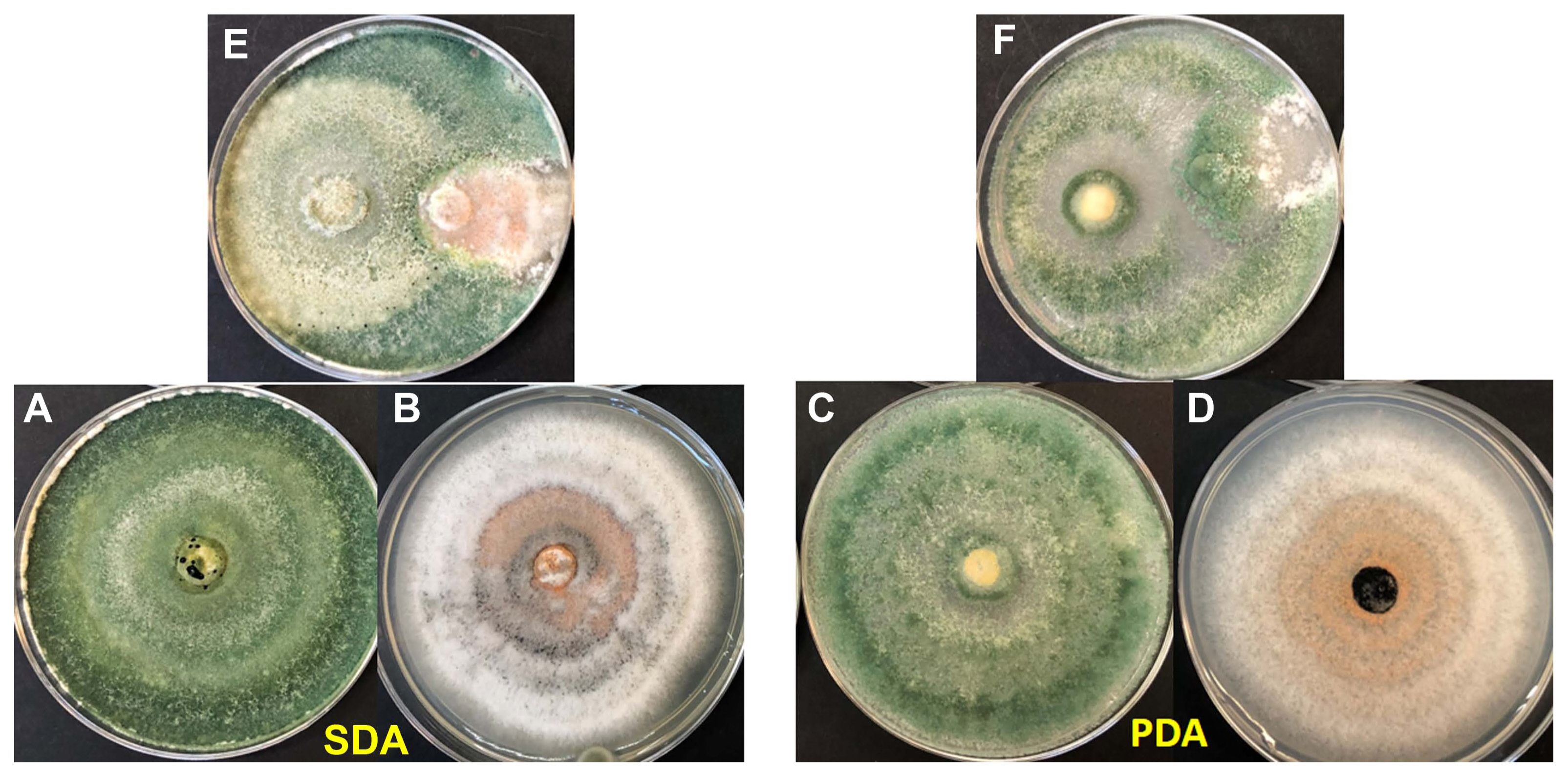
(A, C) Trichoderma harzianum (TRICHO-SIN Agrobionsa) 5 days grown on potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) at 30°C. (B, D) Colletotrichum ti from Laguncularia racemose. (E, F) Antagonic activity of T. harzianum against C. ti.

(A, C) Trichoderma harzianum (TRICHO-SIN Agrobionsa) 5 days grown on potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) at 30°C. (B, D) Fusarium equiseti isolated from Rhizophora mangle on PDA and SDA at 30°C. (E, F) Antagonic activity of T. harzianum against F. equiseti.
Discussion
The present study employed morphological and molecular analysis methods to identify fungi at the species level using rDNA ITS sequences comparison and analysis isolated from four species of mangrove, including mango in the Gulf of Mexico. So far, 82 Colletotrichum species have been recorded in Mexico (Rojo-Báez et al., 2017) and globally Colletotrichum species has been recorded on many hosts according to the extensive review presented by Weir et al. (2012). Based on the result of our study, we found that Colletotrichum spp. and Fusarium spp. were causing anthracnose in mangrove leaves which also has been reported in fruit crops in the same southeast Mexico region (Rojo-Báez et al., 2017; Silva-Rojas and Ávila-Quezada 2011; Tapia-Tussell et al. 2016). Mango plants have been affected by several Fusarium species identified as causal agents of anthracnose. For example in Malaysia, five species were identified affecting mango: F. proliferatum, F. semitectum, F. mangiferae, F. solani, and F. chlamydosporum (Omar et al., 2018). In Mexico, a new species, F. mexicanum was described causing malformation in mango orchards (Betancourt Resendes et al., 2012). Fusarium oxysporum and F. subglutinans were studied in mango in Michoacan state (Pacific coast) by Mora-Aguilera et al. (2003). However, F. equiseti affecting M. indica and R. mangle have not been reported previously until the present work.
The morphological analysis confirmed that Colletotrichum spp., and F. equiseti were the causal agents for anthracnose disease of mangrove forest in the Gulf of Mexico. Our molecular analysis demonstrated that the ML phylogenetic tree shows a correct separation between Colletotrichum spp. and Fusarium spp., as well as the outgroup used Trichoderma sambuci. In the species of the genus Fusarium, the isolated samples of R. mangle and M. indica which are clustered within the same clade, is to be expected taking into account the alignment and BLAST results, where the species were identical (Table 1, Fig. 3). These are related to the Colletotrichum genus such as C. tropicale, C. ti, C. gloeosporioides, which causes anthracnose in different plants.
In addition, the relationship of infection and grouping of the mango species fungus with those of mangroves could be related, due to its evolutionary proximity in the tree. These fungi are very closely grouped with the fungi F. equiseti and Fusarium pernambucanum, which could indicate a very close relationship between these species, having possible variations among them (Fig. 3). On the other hand, in the species of the genus Colletotrichum, the samples of A. germinans, L. racemosa, and C. erectus are grouped within the same clade, as well as that of M. indica, the latter having a slight separation from the others, in agreement with the results obtained in the alignment. The mangrove samples showed a similarity of between ~96–100% in the BLAST results with the species of C. ti, C. queenslandicum, and Colletotrichum tropicale. Additionally, in the phylogenetic tree, these are very close, which could indicate that the mangrove isolates are Collectotrichum species with slight variations. The samples are closely related to the species of Colletotrichum gloeosporioides and Colletotrichum siamense, which have been reported in the literature as two of the main causes of anthracnose disease in various plant species (Weir et al., 2012). Those authors conducted an extensive investigation concerning Colletotrichum spp. They reported that C. tropicale was identified affecting Japan, Panama, and Australia; C. siamense was impacting countries such as China and Vietman. The same authors, also identified C. queenslandicum (MycoBank MB563593) (Weir et al., 2012) in Australia affecting papaya Carica papaya, and C. ti (MycoBank MB563594) (Weir et al., 2012) in New Zealand affecting papaya. This is the first time that C. queenslandicum and C. ti are reported in Mexico and also affecting mangrove. We found that C. queenslandicum affected black mangrove while C. ti affected both white mangrove and buttonwood mangrove. Therefore M. indica species, despite being very close to the others in the clade, may not be completely related.
The finding of plant pathogens physically proximate mango and mangrove plants is an example of parallel transmission from agriculture crops to the endemic mangroves. This suggests that agricultural diseases caused by fungi generate potential risks such as anthracnose and may have an ecological impact on mangrove biodiversity. Further studies including foliar damage are needed to extend the current knowledge of these anthracnose diseases to protect mangrove forest.
In the present study, T. harzianum exhibited an evident antagonism in all samples against Colletotrichum and Fusarium species. The effective mechanism of Trichoderma includes lysis, mycoparasitism, antibiosis (Harman, 2006; Howell, 2003). While the assessments were carried out for T. harzianum isolated from the commercial product TRICHO-SIN Agrobionsa in Mexico, the findings in this study are e comparable to other related antifungal evaluations and could be applied successfully against Colletotrichum sp. and Fusarium sp. phytopathogens. Reports on biologic control of plant pathogens is still scarce in a developing country like Mexico where damage is occurring. Our promising results point to the potential efficacy of antagonism as a means to control phytopathogens in mangrove and commercial mango.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We are thankful to the Facultad de Ciencias Naturales, Universidad Autónoma de Ciudad del Carmen (UNACAR). Also thankful to the Laboratorio de Ecofisiología de Organismos Acuáticos de la Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa (UAS) and the Laboratorio de Inmunogenética y Biología Molecular de la Facultad de Química (UAS) for their support during this investigation.