Citrus melanose disease caused by Diaporthe citri is one of the major citrus diseases (Hyun et al., 2013). Melanose does not affect the pulp quality of fruit but damages the surface of the rind, reducing fruit quality and marketability. Melanose on citrus tree infects all tissues, including leaves, twigs, and fruits, and most citrus species are susceptible to it (Gopal et al., 2014).
Inoculums of D. citri were produced from dead twigs, and fungal spore production with pathogenic Ī± type and non-pathogenic Ī² type were affected by environmental conditions such as the temperature and rainfall conditions in the orchard. To prevent damage caused by melanose, mancozeb is sprayed in most citrus orchards from mid-June to late August or mid-September (Yi et al., 2014).
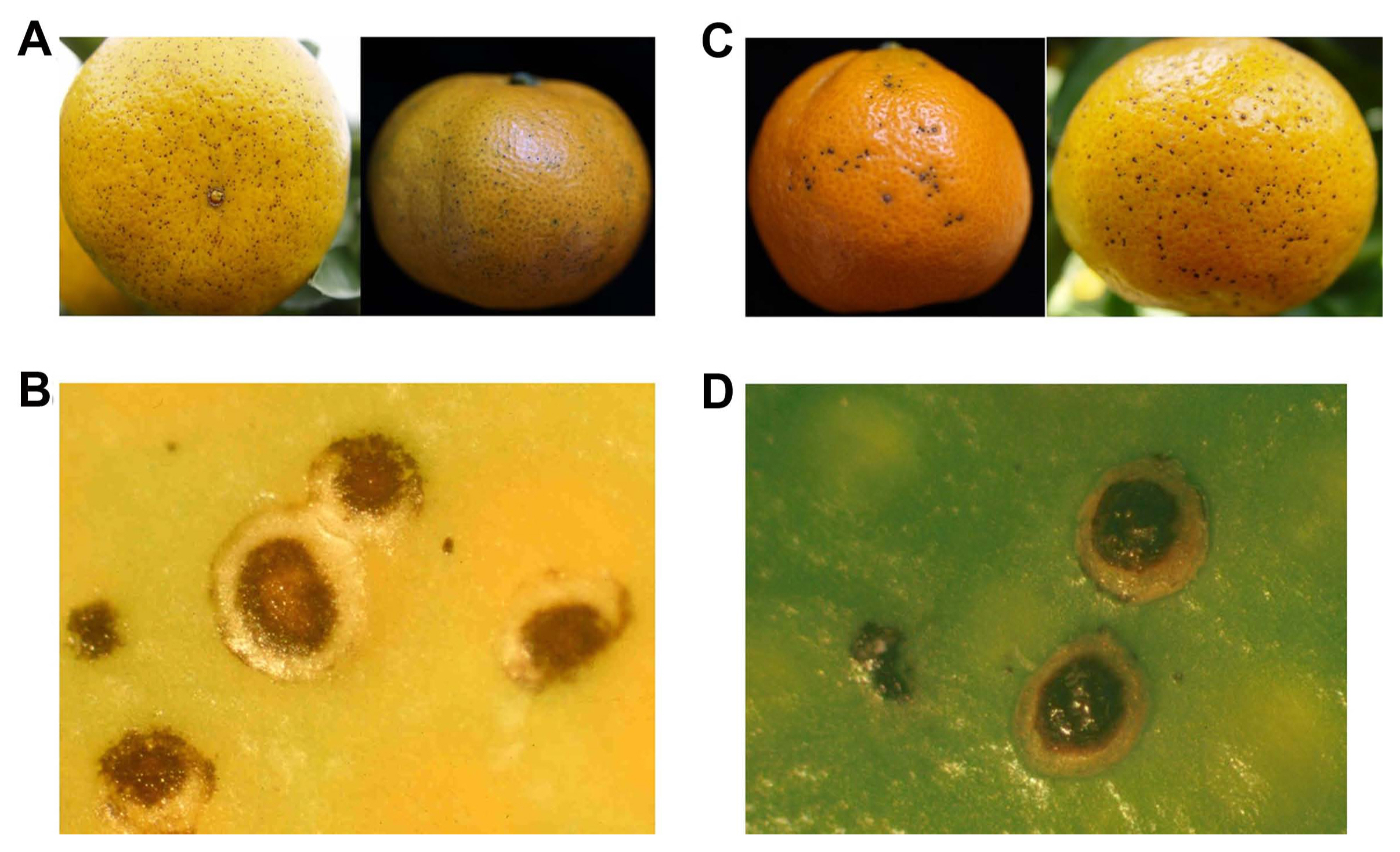
Melanose symptoms vary depending on severity. In mild conditions, the symptoms were scattered black spots; each spot in the center was depressed and surrounded by yellow areas. Under severe conditions, the symptoms spread widely on the surface of fruits with flowing water and appear as solid patches of blemish, forming a tear-drop or mudcake-like pattern (Whiteside and Timmer, 2000).
It is sometimes difficult to distinguish melanose and melanoses-like symptoms with the naked eye because they appear similar. Copper and zinc injuries on fruits resembled initial melanose symptoms. Copper sprays, such as bordeaux, are protectant fungicides and are used to control citrus diseases, such as melanose, mainly citrus canker caused by Xanthomonas citri subsp. citri, and scab disease caused by Elsinoe fawcettii, before disease infection. When spraying copper fungicide at higher concentrations (Albrigo and Grosser et al., 1996) or spraying in hot weather, the risk of copper damage can increase on leaves and fruits (Hyun et al., 2005). Zinc is a micro-element applied via spraying when citrus shows signs of zinc deficiency. When more than 0.3% zinc sulfate is sprayed only, the leaves or fruits might be damaged. Copper and zinc damage symptoms are stippling of leaves and fruits owing to spray, and are similar to the scattered black spots of melanose symptoms caused by D. citri.
Some other injuries on fruits are similar to the late-stage symptoms of melanose. Yellow tea thrips, Scirtothrips dorsalis hood, and pink citrus rust mite, Aculops pelekassi keifer, are also serious insects in citrus orchards. Once these insects feed on the rind of fruits, their surfaces become dead, rusty, and black (Childers and Achor, 1999; Hyun et al., 2012). Sunscald injury on fruits is caused by high temperature or high intensive solar radiation. Symptoms on fruits vary from yellow to brownish superficial blemishes with necrotic dark spots (Barber and Sharpe, 1971).
The melanose-symptomatic fruits of haryejosaeng, citrus unshiu, and melanose-like symptoms caused by copper spray were observed with the naked eye and a stereo microscope using Zeiss SteREO Discovery. V20 (Zeiss, Gƶttingen, Germany). To induce copper-injury on fruit, 4 g per liter of copper sulfate (CuSO4Ā·H2O) mixed with paraffin oil, twice the concentration of the standard protocol, was sprayed on the fruits of the haryejosaeng in the open research field. After 3 days, copper-injury on fruits was observed and collected. In melanose-symptomatic fruits, typical symptoms show scattered black spots, depressed in the center of lesions, and surrounded by yellow areas (Fig. 1A and B). Similar symptoms of melanose were also observed in copper injury on fruits (Fig. 1C and D).
We observed late-stage symptoms of melanose on fruits (Fig. 2A-C), late-stage symptoms on fruits damaged by yellow tea thrips (Fig. 2D-F) and pink rust mite (Fig. 2G-I) from environmentally friendly orchards. The symptoms on fruits caused by sunscald (Fig. 2J and K) were observed in the research greenhouse of the Citrus Research Institute (CRI).
To design specific marker for D. citri, the internal transcribed spacer region (ITS) sequence of D. citri was cloned and then the specific primers were determined by comparison with the ITS of other Diaporthe spp., such as Diaporthe cytosporella and Diaporthe foeniculina (Udayanga et al., 2014), Colletotrichum gloeosporioides, Botrytis cinerea, Altanaria citri, and Fusarium spp. found on citrus fruits. D. citri was isolated in CRI as described previously (Kwon et al., 2003).
To extract genomic DNA from these isolates, D. citri grown on oatmeal agar (60.0 g of oatmeal and 12.5 g of agar in 1 liter of distilled water) and C. gloeosporioides, B. cinerea, A. citri, and Fusarium spp. on potato dextrose agar (potato dextrose agar; 12.0 g of oatmeal and 15 g of agar in 1 liter of distilled water) were harvested at 7 days and then ground with sterilized mortars and pestles in liquid nitrogen. All homogenized samples were stored at ā20Ā°C until genomic DNA extraction. Total genomic DNA was extracted using the Qiagen DNeasy mini kit standard protocol (Qiagen, Germantown, MD, USA) following the standard protocol. Finally, genomic DNA samples were eluted in 100 Ī¼l of elution buffer and stored at ā20Ā°C. The ITS sequence of D. citri was cloned and sequenced. The ITS primers (Table 1) amplified a product of 604 bp from D. citri and then, the product was eluted from a 1% agarose gel using a QIAquick Gel Extraction Kit (Qiagen), cloned, and transformed using the Topo TA Cloning Kit (Invitrogen, Carlsbad, CA, USA). The plasmid was extracted using the QIAprep Spin Miniprep Kit (Qiagen) and sequenced. In addition, the ITS sequences of D. cytosporella (MN899309.1), D. foeniculina (MW020272.1), C. gloeosporioides (AY376534), B. cinerea (MN589849), A. citri (DQ339104.1), and F. oxysporum (MK002869.1) were obtained from the National Center for Biotechnology Information (NCBI). Five isolate sequences, including ITS of D. citri, were aligned using Bio-Edit software and then, Dcitri primers were designed with an area of 388 bp (Table 1, Fig. 3A).
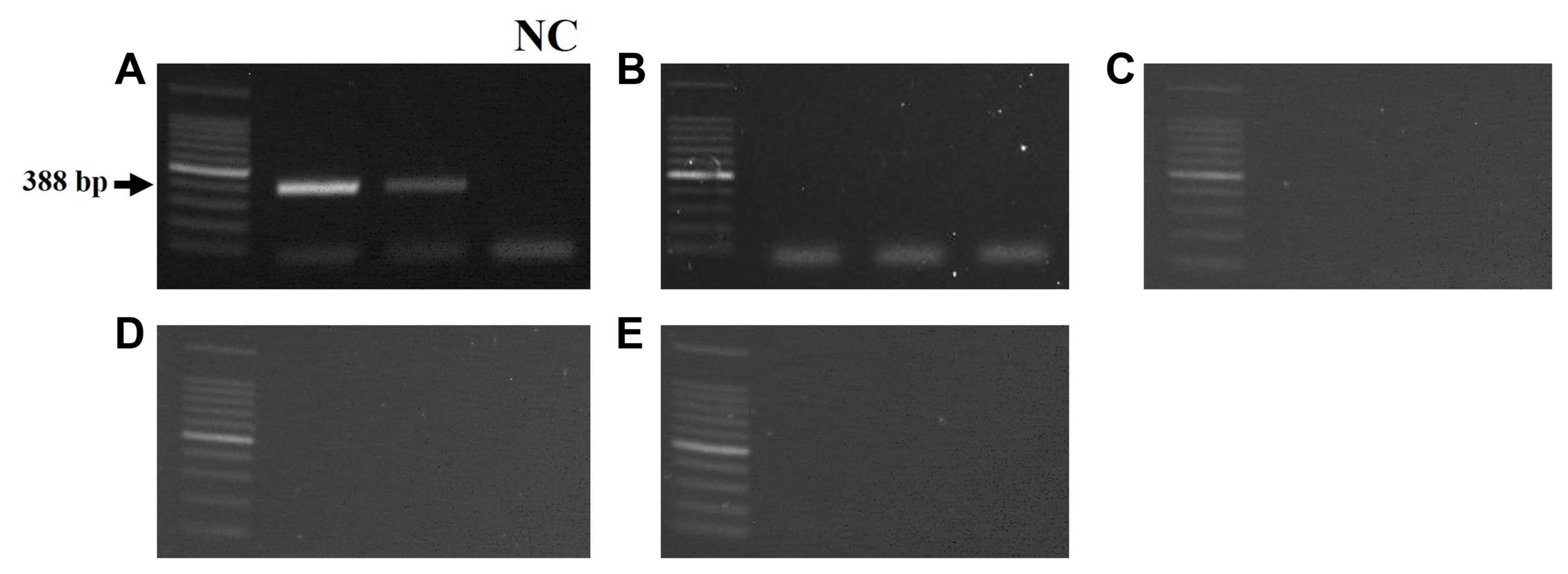
To evaluate the specificity of the Dcitri primers developed in this study, a PCR assay was performed. Total genomic DNA of five isolates, D. citri, C. gloeosporioides, B. cinerea, A. citri, and F. oxysporum were also used as templates. PCR was performed in a final volume of 20 Ī¼l of reaction mixture using the Accupower Hotstart PCR pre-mix (catalog no. K-5051, Bioneer, Daejeon, Korea). The PCR reactions were carried out as follows: pre-denaturation for 3 min at 95Ā°C, 40 cycles of denaturation for 30 s at 95Ā°C, annealing for 30 s at 63Ā°C, extension for 30 s at 72Ā°C, and final extension for 10 min at 72Ā°C. The PCR products were electrophoresed on a 1.5% agarose gel, stained with GelRed nucleic acid Ć10,000 in water (Biotium, Fremont, CA, USA), and then visualized under UV light using a Davinch-Gel Gel Imaging System (Davinch-K, Seoul, Korea). The PCR assay confirmed that Dcitri primers amplified a specific product of 388 bp in D. citri and not in other isolates, C. gloeosporioides, B. cinerea, A. citri, and F. oxysporum. The ITS primers, as an internal control, amplified a product of 604 bp from five isolates (Fig. 3B).
To confirm whether specific products were amplified only in melanose symptoms caused by D. citri, PCR reactions were performed using Dcitri primers. Fruits with melanose symptoms and melanose-like symptoms, including copper spray, sunscald, and damage owing to yellow tea thrips and pink citrus rust mite, were collected and then, the lesion of each symptom on fruit rind was disinfected for 1 min in 75% ethanol and then 1 min in 1% sodium hypochlorite, followed by rinsing in sterile water and cutting with scalpel blades. Total genomic DNA was extracted as described previously. Specific amplified bands were observed in melanose-diseased fruits (Fig. 4A), whereas no products were amplified in melanose-like symptom fruits caused by copper-injury, sunscald, and damage owing to yellow tea thrips and pink citrus rust mite (Fig. 4B-E).
In addition, the lesions of melanose-like blemish known to be caused by D. medusasea on fruits of very early satsuma mandarin, which is smaller lesion size than that of melanose, were sampled from the open field of CRI (Supplementary Fig. 1A and B) and then analyzed using PCR. Similar to the result of melanose, specific products were observed in DNA extracted from melanose-like lesions (Supplementary Fig. 1C). This result is believed to be because D. medusaea, which causes disease in citrus, was reclassified into the same strain as D. citri. And, the ITS region for D. cytosporella (MN899309.1) and D. foeniculina (MW020272.1), which are reported to be found in citrus but do not cause disease in citrus in the Europe and California (USA), were collected from NCBI and compared with Dcitri primer. It is thought that the same products will be amplified from D. cytosporella (MN899309.1) and D. foeniculina (MW020272.1). However, since these strains have not yet been reported to be found in Korean citrus and do not directly cause disease, it is judged that these strains had not significant effect on distinguishing the melanose induced by D. citri from melanose-like symptoms induced by other factors. In conclusion, we developed Dcitri primers in this study and confirmed using PCR assay that the specific products were amplified in D. citri isolate and melanose symptoms caused by D. citri. It is expected that Dcitri marker will be useful in accurately diagnosing melanose caused by D. citri and distinguishing it from melanose-like symptoms.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print






