Physiological and Biochemical Changes in Lucerne (Medicago sativa) Plants Infected with ‘Candidatus Phytoplasma australasia’-Related Strain (16SrII-D Subgroup)
Article information
Abstract
Changes in physiological and biochemical patterns in lucerne plants caused by the presence of ‘Candidatus Phytoplasma australasia’, which is one of the significant pathogens causing yield losses in lucerne plants, were investigated. Significant differences were evident in total chlorophyll, chlorophyll a, chlorophyll b, and protein amounts between ‘Ca. Phytoplasma australasia’-positive and negative lucerne plants. Stress-related metabolites such as phenol, malondialdehyde, and proline accumulations in ‘Ca. Phytoplasma australasia’-positive plants were remarkably higher than those of phytoplasma-negative plants. As a response to disease attack, phytoplasma-positive plants exhibited higher antioxidant enzymes (peroxidase and catalase) and non-enzymatic metabolite responses such as jasmonic and salicylic acids. We state that partial disease responses were revealed for the first time to breed resistant lucerne lines infected by ‘Ca. Phytoplasma australasia’.
Lucerne also known as alfalfa (Medicago sativa) is one of the most cultivated forage crops worldwide. It belongs to the legumes (Fabaceae) family. Its strong and deep root structure makes many contributions to the soil. It allows the soil to be aerated, prevents the soil structure from being tight and allows the control of erosion. It also enriches the soil with organic matter and nitrogen (Özyazıcı et al., 2013). In addition to these contributions to the soil, high grass yield with high nutritional value make lucerne the most cultivated crop in many parts of the world when compared to those of other forage crops (Açıkbaş et al., 2017; Manga et al., 2003; Turan et al., 2017). Lucerne is also rich in protein content that plays a crucial role in animal nutrition (Alçiçek, 2002; Gawel and Grzelak, 2014).
Lucerne has been exposed to many kinds of abiotic and biotic stress agents that reduce the quality and quantity in crop production. In the last decade, phytoplasma diseases have resulted in serious yield losses in lucerne plants around the world. Phytoplasmas are plant pathogenic bacteria that belong to the class of Mollicutes. It has no cell wall. It is non-culturable and limited to the phloem with reduced genomic template. Since they are limited to phloem, it spreads systemically within the plant system and are transmitted from plant to another by sap-feeding vector insects and grafting (Bendix and Lewis 2018; Güldür et al., 2019; Sugio et al., 2011). It causes serious economic losses in many plants around the world and posses serious threats especially to fruit trees, shrub plants, forest trees, ornamental plants and most importantly perennial plants (Negro et al., 2020). We, previously, had observed symptoms such as small leaf formation, redness of the leaves, stunting and witches’ broom in lucerne cultivated areas. Samples had been taken from these plants and subjected to molecular tests. We then reported that these infected plants were associated with ‘Ca. Phytoplasma australasia’ (16SrII-D subgroup) in lucerne (Ayvacı et al., 2020). In this study, we investigated the biochemical modifications occurred in lucerne plants following infection by ‘Ca. Phytoplasma australasia’. It has been known that the pathogen affects the various changes in terms of physiological, biochemical, and metabolic pathways in mungbean (Vigna radiata) plants (Hameed et al., 2017). In recent years, quite a few studies have been carried out on physiological changes. For example, phytoplasma infections have been known to change some compounds as shown in lime plants (Zafari et al., 2012). One of the earliest reports was made by Chang (1977) who stated that a decrease in chlorophyll synthesis in Catharanthus roseus infected with phytoplasma was evident during plant development. Then, de Oliveira et al. (2002) stated that the protein content of phytoplasma-infected maize plants was lower when compared to those of healthy maize plants. It was also reported that peroxidase (POX) activity increased while protein content decreased in leaves of lemon (Citrus aurantifolia) plants infected with witches’ broom disease of lime caused by phytoplasma when compared to those of healthy lemon leaves (Zafari et al., 2012). Maize plants infected with maize bush stunt phytoplasma expressed higher phenolic compounds and lower chlorophyll contents as compared to healthy maize plants (Junqueira et al., 2004). However, a deeper understanding is needed in terms of chlorophyll, phenol, enzymatic activities, and protein levels as well as jasmonic acid (JA) and salicylic acid (SA), proline, and malondialdehyde (MDA) contents to control the disease and breed the resistant lucerne cultivars. In general, the salicylate-jasmonate interactions along with the activation of defense genes (Derksen et al., 2013) and local/systemic resistance are observed in plants (Dempsey and Klessig, 2012). However, little information is available about the defensive responses and the salicylate-jasmonate levels in phytoplasma-infected lucerne plants. For example, proline and MDA levels, which indicate the resistance levels of crop plants as well as defense enzymes were not elucidated in phytoplasma-infected lucerne plants. While proline and MDA accumulation occur in response to stresses resulted from abiotic stresses such as drought, salinity, freezing and biotic stress factors, it is important to determine the pathway in systemic reactions as occurred in phytoplasma-lucerne interactions.
In this study, various biochemical properties related to chlorophyll, phenol, POX, and catalase (CAT) activities, protein, JA, SA, proline, and MDA contents in lucerne plants infected with ‘Ca. Phytoplasma australasia’ were investigated for the physiological and biochemical responses of lucerne plants to breed phytoplasma-resistant lucerne cultivars.
Materials and Methods
Plant materials
Plant samples were collected from the experimental and research lucerne fields of the General Directorate of Agricultural Enterprises (TIGEM, Şanlıurfa, Turkey) in 2021. Leaf samples were taken from the symptom exhibiting lucerne plants. The samples were labeled with details such as place, date, and observed symptoms, etc. After labeling, the samples were brought to laboratory under appropriate aseptic and cold chain conditions (4–6°C) and were then kept at −20°C until use for both DNA extraction and biochemical studies.
DNA extraction and polymerase chain reaction
Previously characterized lucerne fields with phytoplasma in 2019 (Ayvacı et al., 2021b) were visited for the reinspection in 2021. Leaf samples were taken from the symptom exhibiting lucerne plants. The plant showing different symptoms described above were tested through polymerase chain reaction (PCR) to confirm if the symptoms were associated with ‘Ca. Phytoplasma australasia’ and to see if any genetic modifications took place on the pathogen. The leaf samples were labeled with details such as place, date, and observed symptoms, etc. After labeling, the samples were brought to the laboratory under appropriate aseptic and cold chain conditions (4–6°C) and were then kept at −20°C until use for both DNA extraction and the biochemical studies. We tested 10 different symptomatic lucerne plants along with the positively identified lucerne DNA sample which was obtained from our previous study (Ayvacı et al., 2020). In PCR analysis, we run asymptomatic and symptomatic lucerne samples along with the negative (ddH2O) and the positive control which was obtained from cactus plant that produced 1,400 bp which is specific for phytoplasma identification (Ayvacı et al., 2021). We performed PCR tests on symptomatic and healthy plants to confirm if the symptoms are associated with ‘Ca. Phytoplasma australasia’ and to see if any genetic modifications took place on the pathogen. DNA extraction was made following the method of Ahrens and Seemüller (1992). Four ml of CTAB buffer (2% w/v cetyltrimethylammonium bromide, 1.4 mol/l NaCl, 0.2% β-mercaptoethanol, 20 mmol/l EDTA, 100 mmol/l Tris-HCl, 2% polyvinylpyrrolidone, pH 8.0) was used for each sample. The obtained DNAs were stored in 100 μl of Tris EDTA (1×) at −80°C. PCR amplification was performed at two stages, direct and nested (Davis and Lee, 1993; Duduk et al., 2013). Universal primers R16F1/R16R0 (5′-AAGACGAGGATAACAGTGG-3′/5′-GGATACCTTGTTACGACTTAACCCC-3′) yielding 1,400 bp in direct-PCR, and R16F2n/R16R2 (5′-GAAACGACTGCTAAGACTGG-3′/5′-TGACGGGCGGTGTGTACAAACCCCG-3′) yielding 1,250 bp in nested-PCR primer pairs were used. PCR products were obtained via initial denaturation (1 cycle) at 94°C for 3 min; denaturation at 94°C for 1 min; primer bonding (35 cycles) at 50°C for 2 min; elongation at 72°C for 3 min; final elongation (1 cycle) at 72°C for 10 min. For the nested-PCR, initial denaturation (1 cycle) at 94°C for 3 min; denaturation at 94°C for 1 min; primer bonding (35 cycles) at 55°C for 2 min; elongation at 72°C for 3 min; final elongation (1 cycle) at 72°C for 10 min were performed. Amplification products were visualized at 1% agarose gel after electrophoresis in 1× TBE buffer (67 mmol/l Tris-HCl, 22 mmol/l boric acid, 10 mmol/l EDTA, pH 0.8) and stained with ethidium bromide (EtBr, 10 mg/ml) then visualized in a UV transilluminator. All our products positively identified PCR were identical followed by the DNA sequence analysis.
Biochemical analysis
Positively identified lucerne plants in terms of phytoplasma appearance were used for the biochemical analysis. Since all positively identified plants were identical in DNA sequence, therefore, one representative lucerne plant was chosen and the biochemical analysis was conducted on that plant to minimize the standard errors arised from different samples.
Determination of protein contents
In order to determine the protein amount, approximately 0.5 g leaf sample was taken and homogenized in 5 ml of 50 mmol/l sodium phosphate buffer, pH 7. Then, 5 ml of Coomassie Brilliant Blue G-250 was mixed with 100 μl of the plant extract and the solution was read at 595 nm. Bovine Serum Albumin Fraction V (Sigma, St. Louis, MO, USA) at different concentrations (10–100 μg/ml) was used for the protein standard curve.
Chlorophyll analysis
Chlorophyll contents of lucerne leaves were made following the method of Arnon (1949) with minor modifications (Karakas et al., 2020). Approximately 0.5 g leaf samples were taken from the plants and homogenized in 5 ml of acetone:water (80 v/v) mixture. Then, the liquid part was obtained by passing through the filter paper and transferred to light-proof tubes. The solution was then read in a spectrophotometer (Epoch-BioTek, BioTek Instruments, Winooski, VT, USA) against 80% acetone control at 663.5 nm for chlorophyll a and 645 nm for chlorophyll b. Chlorophyll contents of the leaves were calculated with the following formula then expressed as mg/g fresh g of leaves.
Determination of phenol contents
The protocol prepared by Shetty et al. (1995) was employed to determine the phenol content with minor modifications. Approximately 0.5 g of leaves from lucerne plants were sampled and homogenized in 80% methanol, incubated at 95°C for 30 min, and then centrifuged at 10,000 ×g for 10 min. Then, in order to determine the total amount of phenol, 300 μl of the plant extract was taken and 1.5 ml of Folin-Ciocalteau (diluted with 1:10 water) was added to it. After 5 min, 1.2 ml of 20% Na2CO3 was added and mixed and kept in the dark at 40°C for 30 min. The color change was measured at 760 nm in a spectrophotometer (Epoch-BioTek). Leaf phenol contents were calculated via using gallic acid standard curve prepared at different concentrations.
Determination of proline contents
Proline determination was made according to Bates et al. (1973) with some modifications (Karakas et al., 2021). The compound consisting of an acid-ninhydrin mixture was employed as a color reagent in the analysis. A reaction mixture was prepared by dissolving 1.25 g of ninhydrin in 30 ml of glacial acetic acid and 20 ml of 6 mol/l phosphoric acid (stable for 24 h at 4°C). A sample of 0.5 g was taken from the fresh leaf, homogenized in liquid nitrogen, and solubilized by adding 10 ml of 3% sulfosalicylic acid. The resulting extract was then passed through filter paper Whatman No. 1 and 2 ml of the mixture was added to 2 ml of acid-ninhydrin solution, and the mixture was boiled at 100°C for 1 h. The reaction was then terminated in ice. Then 5 ml of toluene was added to the reaction mixture and vortexed for 30 s. Then, it was kept until the formation of 2 phases and the upper phase was taken with the help of a micropipette and measured by reading against pure toluene blank in a spectrophotometer (Epoch-BioTek) at 515 nm. The standard curve was prepared at different concentrations of L-proline.
Determination of MDA contents
MDA, also known as lipid peroxidation, was measured following the method of Heath and Packer (1968) with some modifications (Karakas et al., 2021). Approximately 0.5 g of fresh leaf sample was taken and homogenized in 10 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 10,000 ×g for 5 min. After centrifugation, 4 ml of 20% TCA containing 5% thiobarbituric acid was added to 1 ml of extract. The resulting mixture was incubated at 95°C for 30 min. It was then rapidly cooled down with the aid of an ice bath. After centrifugation at 10,000 ×g for 10 min, 300 μl of supernatant was then taken, and the absorbance readings were made in a spectrophotometer (Epoch-BioTek) at 532 and 600 nm. MDA content was calculated according to the formula given.
POX (E.C.1.11.1.7) activity
POX measurement was performed following the protocol prepared by Cvikorov et al. (1994) with some modifications (Karakas et al., 2019). Approximately 0.5 leaf samples were taken from the plants and homogenized 50 mmol/l in phosphate buffer, pH 7.0. Then, 100 μl of the obtained leaf extract was taken and 3 ml of reaction mixture (13 mmol/l guaiacol, 5 mmol/l H2O2 and 50 mmol/l sodium phosphate, pH 6.5) was added. The reaction was started with the addition of H2O2 and read 3 times at 25C at 470 nm with 1-min interval. One unit of POX activity was determined as 0.1 absorbance/min at ΔA470 nm. Results are expressed as unit/mg protein.
CAT (E.C.1.11.1.6) activity
The protocol prepared by Milosevic and Slusarenko (1996) was used to measure CAT activity with same modifications (Karakas, 2013). Approximately 0.5 g of leaf samples were taken from the plants and homogenized with the phosphate buffer, pH 7.0. Then, 50 ml of the obtained supernatant was taken and 2.95 ml of the reaction mixture (10 mmol/l H2O2, 50 mmol/l sodium phosphate buffer, pH 7.0 and 4 mmol/l Na2EDTA) was added to it, then it was read in the spectrophotometer at 25°C at 240 nm for 30 s. One unit CAT enzyme is expressed as the amount of enzyme that breaks down 1 μmol H2O2 in 1 min (Wang and Han, 2009) and stated as unit/mg protein.
Determination of JA content
JA determination was performed according to Annigeri et al. (2011). First, 1 g of leaf sample was taken and kept in 10 ml of ethanol for 12 h at room temperature in the dark. After 12 h, the mixture was filtered through No. 1 Whatman paper and 1 ml of the homogenate was read at 323 nm in a spectrophotometer (Epoch-BioTek). Calculation of JA concentration was made from the standard curve prepared from various concentration of JA solubilized in absolute ethanol.
Determination of SA content
SA content was determined after the method of Rainsford (2004) with minor modifications. According to the protocol; 1 g of leaf sample was kept in 10 ml of ethanol for 12 h at room temperature in the dark. The solution was then centrifuged at 10,000 ×g for 10 min. From the supernatant, 100 μl was taken and mixed with 1% freshly prepared ferric chloride (FeCl3). Total volume of the mixture was then made up to 3 ml with reaction mixture. The complex formed between Fe3+ ion and SA, violet in color, was measured at 540 nm in a spectrophotometer (Epoch-BioTek). SA content was calculated according to SA standard curve ranging from 0–100 ppm SA acid concentration prepared in ethanol. It is important to note that standard solutions of SA is able to react with almost all solvents and has a high solubility.
Statistical analysis
Phytoplasma-infected and healthy plants were tested in terms of statistical differences after biochemical analyses via the use of Minitab for Windows version 20.1 software (Minitab Inc., State College, PA, USA) (Minitab Inc., 2020). All data are means of triplicates ± standard error (n = 3).
Results and Discussion
Plant symptoms and molecular detection of the pathological agent
When the cultivated lands of lucerne plant were examined, the plants exhibiting symptoms such as small leaf formation, stunting, witches’ broom, and chlorosis were sampled and subjected to PCR analysis for the molecular confirmation on the basis of 16S rRNA if Ca. Phytoplasma was responsible for the above symptoms. Since Koch’s postulates are not appropriate for the confirmation of phytoplasmas, we used the molecular tools for the confirmation of phytoplasma (Fig. 1) (Dermastia et al., 2017). Lucerne plants with no symptoms were also tested for the phytoplasma detection.

Symptoms characterized with ‘Candidatus Phytoplasma australasia’. (A) Small leaf. (B) Healthy and infected leaf. (C) Appearances of healthy and infected plants exhibiting small leaf, yellowing and witches’ broom in field conditions. (D) Appearance of small leaf, witches’ broom and yellowing on infected plant.
Our findings showed that the symptomatic lucerne plants gave positive results in terms of PCR results. The causative agent was confirmed as ‘Ca. Phytoplasma australasia’. Healthy or symptom-free plants gave negative results (Fig. 2A and B).
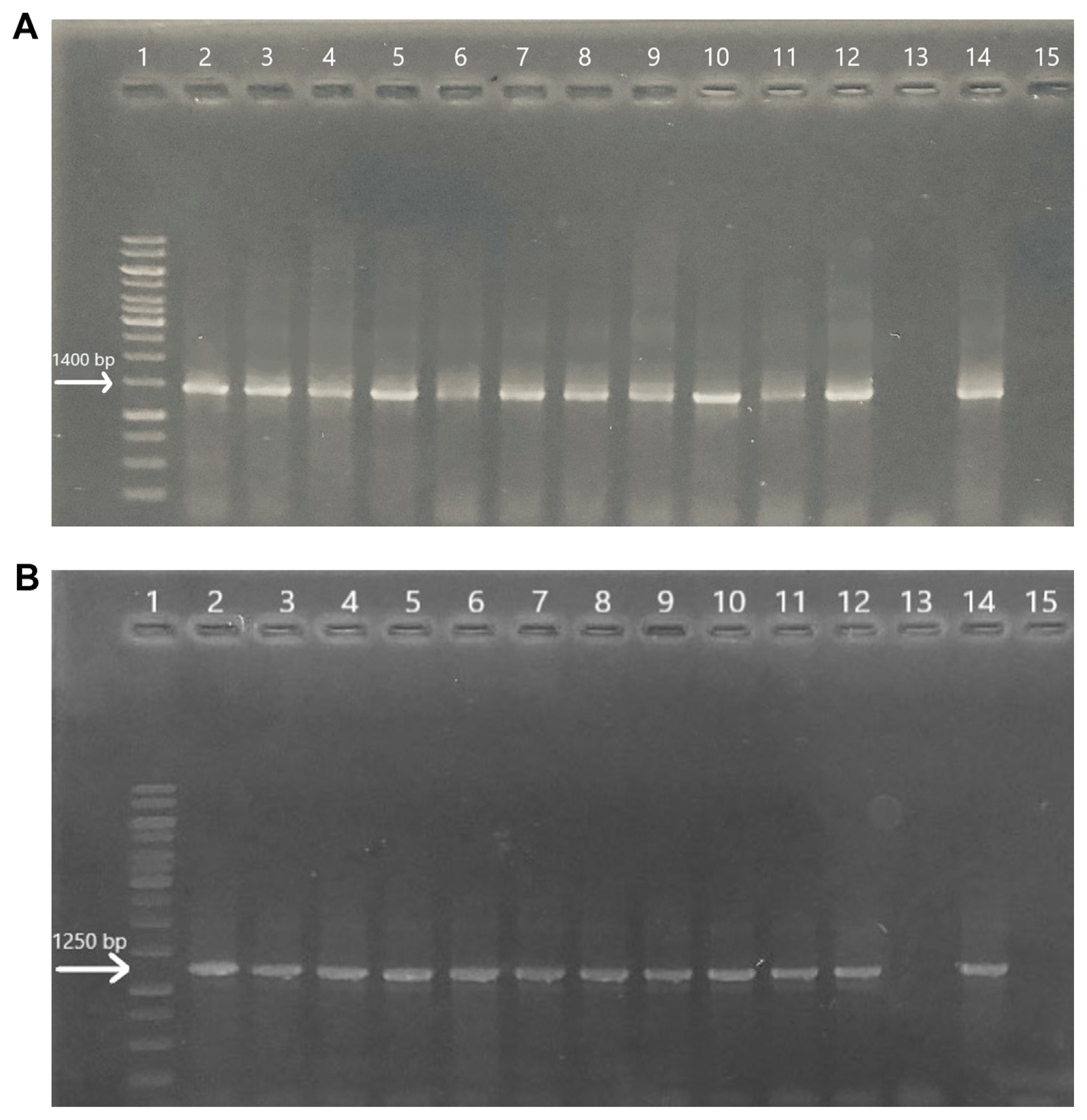
Gel electrophoresis images of lucerne plants following direct-polymerase chain reaction amplification (A) and nested-PCR amplification (B): lane 1, marker; lanes 2–11, infected plants; lane 12, positively identified lucerne DNA sample which was obtained from our previous study; lane 13, healthy plant (asymptomatic lucerne); lane 14, positive control (cactus that produces 1,400 bp which is specific for phytoplasma identification) and lane 15, negative control (ddH2O) (1% agarose gel).
Biochemical analyses
Biochemical changes in healthy and infected plants associated with the ‘Ca. Phytoplasma australasia’ (16SRII-D subgroup) group phytoplasma were investigated followed by the confirmation through PCR analysis.
Chlorophyll and protein contents
We determined that the chlorophyll contents of lucerne leaves were significantly lower when compared to those of healthy leaves, total chlorophyll, chlorophyll a and b values were remarkably lower in ‘Ca. Phytoplasma australasia’ infected lucerne plants (Fig. 3A–C). Similarly, protein contents were negatively affected and found remarkably lower when compared to those of healthy lucerne plants (Fig. 3D).
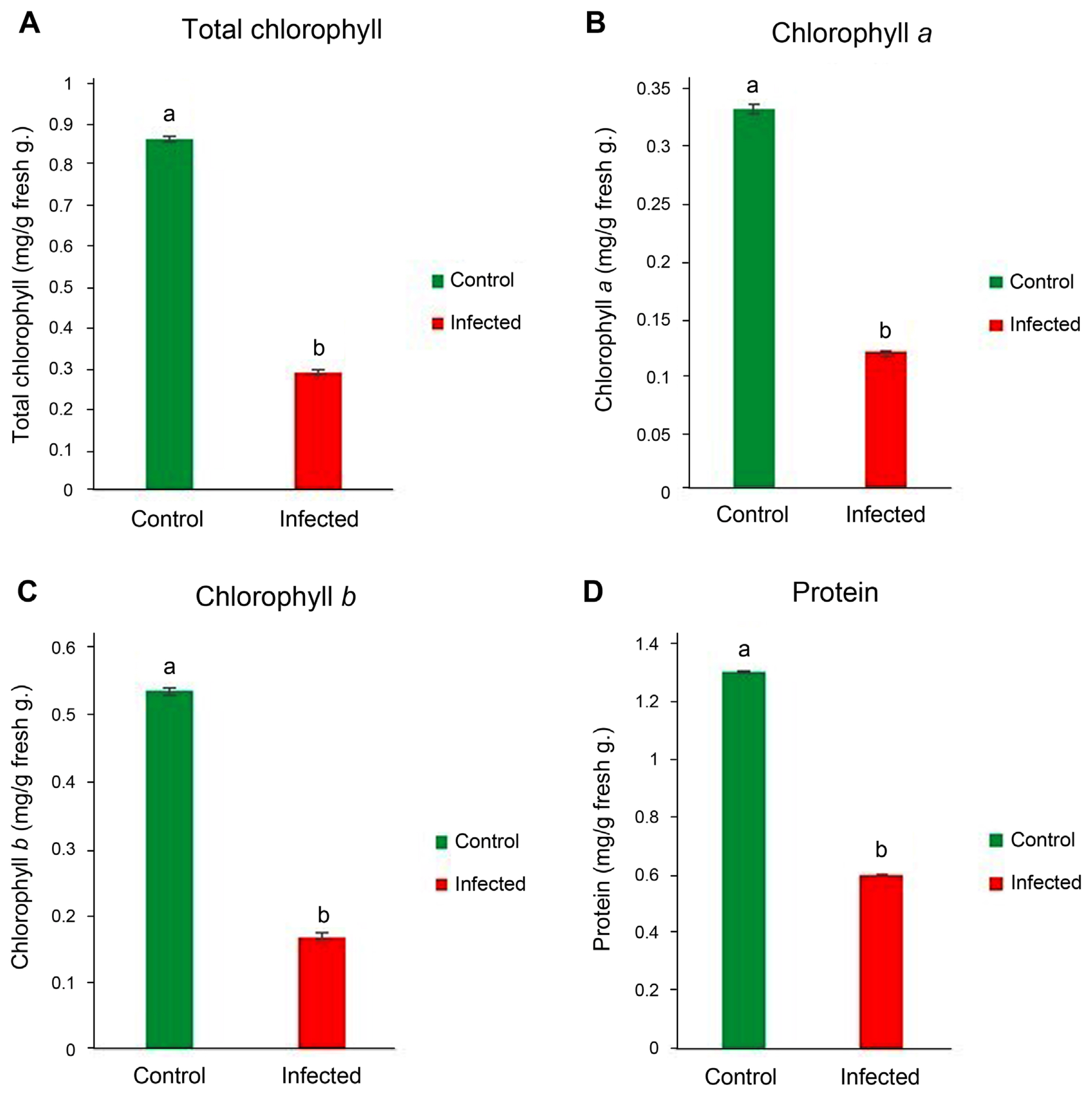
Total chlorophyll (A), chlorophyll a (B), chlorophyll b (C), and protein contents (D) of lucerne leaves infected with ‘Candidatus Phytoplasma australasia’. Different letters on bars show statistical differences according to the least significant difference test based on P ≤ 0.05 value (n = 3).
It is clear that chlorophyll and protein synthesis is disrupted following infection of ‘Ca. Phytoplasma australasia’. One of the main mechanisms behind this could be attributed to infection capacity of the causative agent that is most probably interfering with the chlorophyll pathway such as blockage of Mg2+ and Fe2+ and photosystem I and II (Buoso et al., 2019; Raiesi and Golmohammadi, 2020; Xue et al., 2018). Decreased photosynthetic activity corresponded to a decrease in protein content in infected plants (Bertamini et al., 2004). We also noted that protein contents of leaves in infected lucerne plants significantly decreased following ‘Ca. Phytoplasma australasia’ (Fig. 3D). It is quite clear that when photosynthetic activity and photosynthesis are negatively affected and decreased, this could be reflected in protein synthesis (Boex-Fontvieille et al., 2013). Since protein synthesis depends on the energy gradient, which means energy is needed for the synthesis of protein and its subunits, the reduced amount of chlorophyll (total, a and b) was quite visible and reflected on the protein content of infected leaves. For example, Bertamini et al. (2003) reported that phytoplasma infection caused a significant infection on photosystem 2 activity. The decrease in photosystem 2 was possibly due to the decrease in the content of chlorophyll and light-harvesting chlorophyll-protein 2 complexes (Arce-Leal et al., 2020).
Decreases in the protein expression level in plants exposed to different stresses is fairly common as a response to disease attack (Janmohammadi et al., 2015). Decreases in protein levels could be very important for the plants if their leaves are edible and consumed by human and/or animals. Lucerne plants, in this respect, are crucially important since their leaves are used for animal feed. For this reason, it is important to note that phytoplasma reduces the protein synthesis and is able to reduce crop production where lucerne field is under the threat of phytoplasma diseases.
Determination of stress-related metabolites (phenol, MDA, and proline)
Phenolic contents are very important as they lead to some structural polymers in plant defense systems and play significant roles as signaling molecules to trigger plant defense genes (Jayaraj et al., 2010; Singh et al., 2014; Tuladhar et al., 2021). The phenol content of ‘Ca. Phytoplasma australasia’-positive and ‘Ca. Phytoplasma australasia’-negative plants were measured to determine the amount of phenol in the infected and control plants. A remarkable increase in phenol was evident in infected plants along with the other metabolites such as MDA and proline (Fig. 4A–C). Fig. 4A, B, and C. Increases in stress metabolites indicated that ‘Ca. Phytoplasma australasia’ was a significant threat to lucerne plant metabolism. These stress-related metabolites play remarkable roles in defense strategies via regulating osmotic potential and increasing antimicrobial capacities of the defending plants. For example, high amount of phenols and phenolic compounds increases the plant resistance by playing antimicrobial properties, decreases the dissemination of the attacking pathogen and reduces the propagation rate in the plant tissue (Kubalt, 2016; Rasouli et al., 2016). A similar finding was made by Lopes et al. (2021) on halophytes plants. They stated that high antioxidant and antimicrobial activities of halophytes turned phenolics into bioactive molecules that can be used in different areas of biosynthesis of defense-related molecules (Lopes et al., 2021).
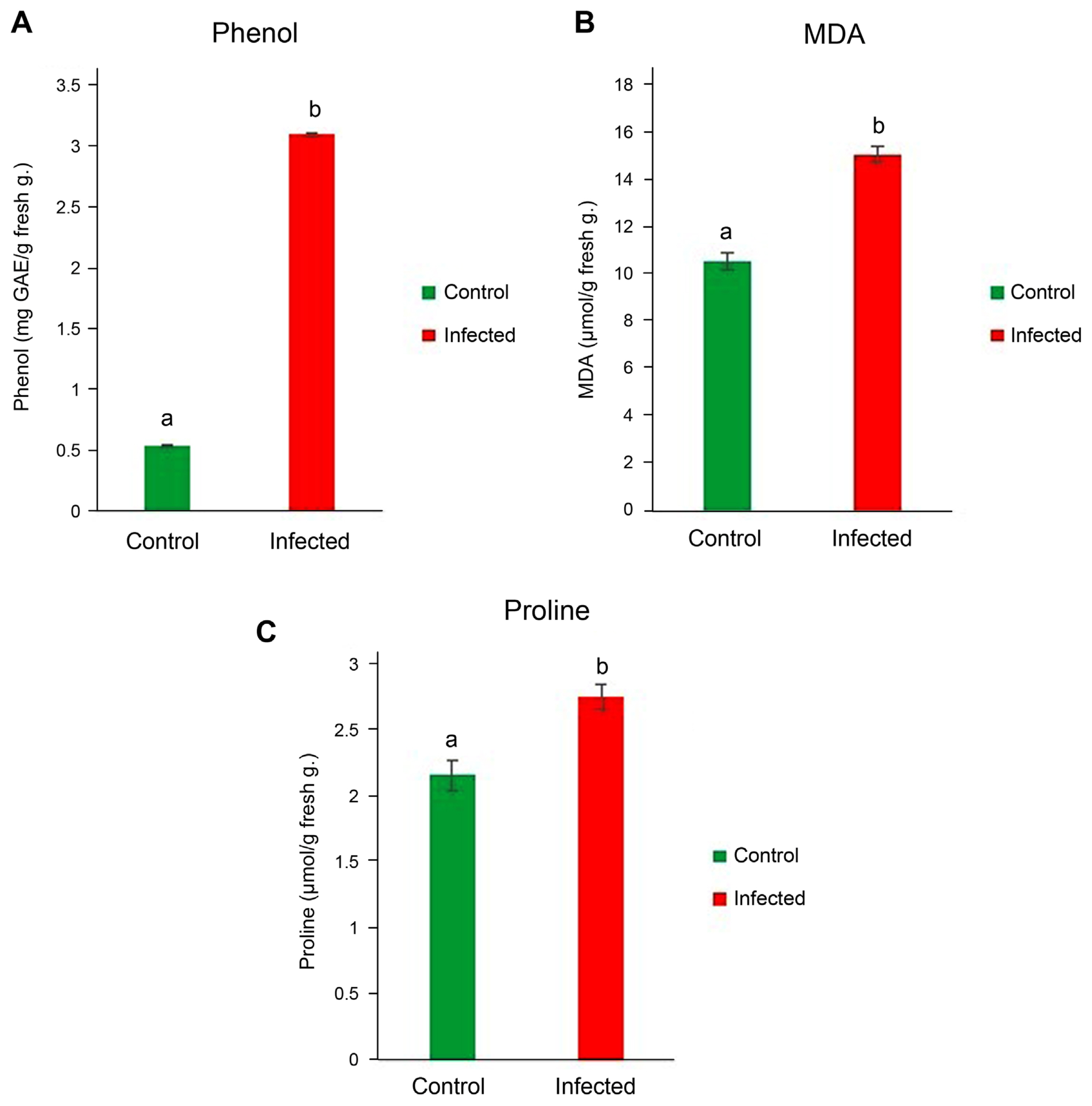
Phenol (A), malondialdehyde (MDA) (B), and proline (C) contents of lucerne leaves infected with ‘Candidatus Phytoplasma australasia’. Different letters on bars show statistical differences according to the least significant difference test based on P ≤ 0.05 value (n = 3).
Accumulation of proline, a water-soluble amino acid, under stress conditions acts as an indicator of the plant’s endurance ability and at high concentrations does not damage cell structures but lowers cell osmotic potential (Naliwayski and Sklodowska, 2021). Thanks to this ability, the amount of proline present in infected and healthy plants was investigated in this study. We determined that the amount of proline in the infected plant was remarkably high and contributed to plant defense mechanism.
We measured lipid peroxidation level which was significantly higher ‘Ca. Phytoplasma australasia’-positive plants as compared to those of healthy plants (Fig. 4B). Accumulation of MDA is a good marker to detect if plants are under stress. The level of it indicates the severity of stress as well as resistance of the plants to the stress (Yasmin et al., 2021). For example, He et al. (2018) reported that membrane damage, reactive oxygen species (ROS) damage, protein denaturation and protein oxidation as well as osmotic stress (primarily dehydration) were in line with the increase of MDA following abiotic stress. In our study, we collected leaf samples from the field upon observation of distinct symptoms related to pyhtoplasmas. This meant that the disease in lucerne plants was already firmly established in the vascular system. Firmly established phytoplasmas in the vascular system could create ionic imbalance due to intracellular and extracellular osmotic mismatch in plants under stress (Bernardo et al., 2019; Ghoulam et al., 2002). This imbalance could change the level of enzymes involved in the synthesis of lipids and proteins (Hong et al., 2016; Rawat et al., 2020). As a result, permeability and protein oxidation increase (Niu and Xiang, 2018).
Determination of antioxidant enzyme levels
The CAT enzyme is one of the antioxidant enzymes that reduces or breaks down H2O2 in cells (Ahmad et al., 2021). It has been stated that the increase in CAT activity increases the resistance of the plant against the stress caused by the pathogen so that the plant can maintain low concentrations of H2O2 (Kim et al., 2020; Magbanua et al., 2007; Pan et al., 2020). CAT activities of ‘Ca. Phytoplasma australasia’-positive plants were higher than those of negative plants. Similar findings were also found with that of POX activity. The POX enzyme is also one of the crucially antioxidant enzymes that provides rapid defense against pathogens by participating in various defense mechanisms (Salari et al., 2012; Siddique et al., 2014). POX activity is involved in the production of ROS that limit the spread of the pathogen by increasing lignification and cross-linking of the plant cell wall (Patel et al., 2011). In addition, POX activity has an important role in suberization or wound healing (Yang et al., 2020). It was clearly shown that the increased enzyme activities corresponded to the increased defense metabolites and compounds as illustrated in Fig. 5 for stress metabolites and in Fig. 6 for the increased antimicrobial compounds such as JA and SA.

Catalase (CAT) (A) and peroxidase (POX) (B) contents of lucerne leaves infected with ‘Candidatus Phytoplasma australasia’. Different letters on bars show statistical differences according to the least significant difference test based on P ≤ 0.05 value (n = 3).

Jasmonic acid (JA) (A) and salicylic acid (SA) (B) contents of lucerne leaves infected with ‘Candidatus Phytoplasma australasia’. Different letters on bars show statistical differences according to the least significant difference test based on P ≤ 0.05 value (n = 3).
Plant hormones such as JA and its derivatives (JAs) and SA have been accepted as key regulators for the plant defense against stresses such as pathogens and abiotic stresses (Pieterce et al., 2012; Raza et al., 2021; Sharma and Gayen, 2021). JA is an important signaling molecule for triggering of defense responses to injury, herbivorous, and pathogen attacks (Costarelli et al., 2020; Rosahl and Feussner, 2004). The amount of JA in the plant provides us with important information about the activity of the plant’s defense mechanism against pathogens. In this study, the amount of JA between infected and healthy plants was compared. The JA content of ‘Ca. Phytoplasma australasia’-positive plants were significantly higher than those of the control plants (P ≤ 0.05) (Fig. 6A). On the other hand, SA was determined and measured in lucerne plants. SA is a phenolic phytohormone that plays a significant role in photosynthesis, transpiration, ion uptake, and transport (de Freitas et al., 2019; Matilla-Vazquez and Matilla, 2014). It regulates leaf anatomy and chloroplast structure (Poór et al., 2019; Warrier et al., 2013). Our findings showed that SA content was higher in the infected plants; however, this was not statistically significant (Fig. 6B). This also proved that the defense response of lucerne plants was active during infection.
Correlations among parameters were also performed to reveal the mechanism of disease pathway in infected lucerne plants. According to Table 1, we correlated parameters with each other in infected lucerne plant.
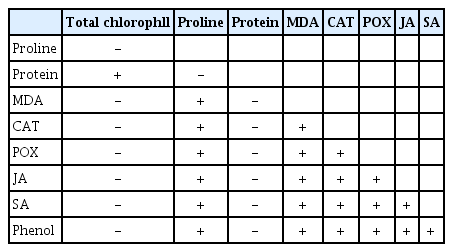
Relationship between biochemical parameters (total chlorophyll, proline, protein, MDA, CAT, POX, JA, SA, and phenol) measured in the infected plant (the LSD test based on P ≤ 0.05)
We briefly symbolized the relations with +/− signs to simply show the relations in clear. We found that stress-related metabolites such as proline, MDA, JA, SA, POX, CAT, phenol synthesized after the establishment of the disease was positively correlated with each other while they were negatively correlated with those of total chlorophyll, protein. We could establish that lucerne plants use their proteins to keep the cell structure in unity and reduce the symptoms down to a remarkable level to carry out their vegetative and generative stages (Staswick, 1994). It is clear that all stress-related metabolites use a common pathway, therefore, improving photosynthetic efficacy and protein levels would help us with the generation of new resistant lucerne lines. However, we should keep in mind that abiotic stress factors, which mainly use other stress pathways as significantly different from those of biotic stress factors would restrict and limit the success of breeding in plants inoculated with biotic agents (Dikilitas et al., 2020). Therefore, screening genes for multiple resistance and other metabolic pathways should be considered.
To the best of our knowledge, this is the first study on biochemical changes in lucerne plants infected with ‘Ca. Phytoplasma australasia’. ‘Ca. Phytoplasma australasia’ infection on lucerne plants showed that the metabolism of lucerne plants in field conditions was significantly disrupted. Chlorophyll and protein contents were lowered while stress metabolites such as MDA, proline, phenol increased. It is clear that ‘Ca. Phytoplasma australasia’ interfered with the energy metabolism of the plant by lowering photosynthesis activity and protein synthesis, on the other hand, lucerne plants produced metabolites to cope with the negative effects of the phytoplasmic agent. Increases in phenols, proline and MDA could be considered in this perspective. Increased activities of antioxidant enzymes showed that the plant defense system was active throughout the vegetation period. Since we sampled the leaves when the symptoms were visible, the disease was already established in the plant system. Therefore, we suggest that defense mechanism is continuously active throughout the season. We also noticed that plant JA were active and significantly higher than those of healthy lucerne plants. Although SA was high, however, this increase was not that significant.
In summary, lucerne plants after phytoplasma infection showed remarkably high defensive responses. However, due to climate changes and environmental pollution, more abiotic stress factors such as high temperature, drought, salinity, water stress, freezing temperatures along with the other biotic stress agents such as virus, bacteria or fungi, especially wilt fungi, could interact with the phytoplasma and cause severe stresses. Therefore, breeding tolerant to abiotic stresses and/or resistant to biotic stress agents are utmost important. Thus monitoring lucerne fields for new races of phytoplasmic agents or determine if phytoplasmas have been modified, which could play significant roles for the breeding studies.
Our new research program is on the agenda to determine the resistance gene/genes to monitor the resistance of lucerne plants for ‘Ca. Phytoplasma australasia’ and its possible subgroups and possible interactions.
Acknowledgments
The first author expresses her gratitude the their education YÖK (Phd 100–2000) and Harran University for support a bursary.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
