Rapid and Visual Detection of Barley Yellow Dwarf Virus by Reverse Transcription Recombinase Polymerase Amplification with Lateral Flow Strips
Article information
Abstract
Barley yellow dwarf virus (BYDV) has been a major viral pathogen causing significant losses of cereal crops including oats worldwide. It spreads naturally through aphids, and a rapid, specific, and reliable diagnostic method is imperative for disease monitoring and management. Here, we established a rapid and reliable method for isothermal reverse transcription recombinase polymerase amplification (RT-RPA) combined with a lateral flow strips (LFS) assay for the detection of BYDV-infected oat samples based on the conserved sequences of the BYDV coat protein gene. Specific primers and a probe for RT-RPA reacted and optimally incubated at 42°C for 10 min, and the end-labeled amplification products were visualized on LFS within 10 min. The RT-RPA-LFS assay showed no cross-reactivity with other major cereal viruses, including barley mild mosaic virus, barley yellow mosaic virus, and rice black streaked dwarf virus, indicating high specificity of the assay. The sensitivity of the RT-RPA-LFS assay was similar to that of reverse transcription polymerase chain reaction, and it was successfully validated to detect BYDV in oat samples from six different regions and in individual aphids. These results confirm the outstanding potential of the RT-RPA-LFS assay for rapid detection of BYDV.
Barley yellow dwarf virus (BYDV) is a member of the genus Luteovirus, belonging to the family Luteovoriridae, and is among the most widespread and destructive viruses of cereal crops in the world (Choudhury et al., 2018). It infects a wide range of Poaceae grass species, including barley, wheat, maize, ryegrasses, and oat (McKirdy et al., 2002). The major symptoms of BYDV infection are stunting and leaf discoloration e.g., reddening in oat and yellowing in barley (Choudhury et al., 2018). BYDV is exclusively transmitted via over 25 species of aphids, like bird-cherry aphids (Rhopalosiphum padi) and grain aphids (Sitobion avenae), but not by mechanical or seed transmission; moreover, that to the host plant’s phloem tissue is limited (Ng and Perry, 2004). There are several BYDV strains or serotypes (PAV, MAV, and PAS), categorized based on vector transmission efficiency and serological and molecular characteristics (Ng and Perry, 2004). Among these, BYDV-PAV is the predominant and most destructive type worldwide. Particular environmental conditions, such as a humidity and mild temperature favor the development and reproduction of aphids, thereby strongly increasing the incidence and severity of disease (Fabre et al., 2005). The occurrence of BYDV has been a major problem for the production of wheat, barley, and oat due to an increased inflow of aphids in Korea due to global warming (Jo et al., 2018). Continuous spreading and economic losses due to BYDV infection are concerning, and research efforts are required to develop a method for the rapid detection of BYDV in host plants and insect vectors to facilitate effective control measures.
Several methods have been proven to be efficient for detecting BYDV infection, such as enzyme-linked immunosorbent assays (Chéour et al., 1993), reverse transcription polymerase chain reaction (RT-PCR) (Canning et al., 1996), and real-time TaqMan RT-PCR (Balaji et al., 2003). To overcome limitations associated with time, labor, expenses, and trained staff requirement, loop-mediated isothermal amplification assays (LAMP) were proposed as an alternative means of BYDV detection (Zhao et al., 2010). LAMP assays are reliable; however, they have some limitations that may impede their applicability, such as restrictions with respect to primer design (4–6 primers are required), inapplicability for cloning, limited multiplexing potential, and low specificity (Wong et al., 2018).
Recombinase polymerase amplification (RPA) is an isothermal nucleic acid amplification method, which has been used for rapid molecular detection of a wide variety of organisms (Li et al., 2019). It relies on three major enzymes, including a recombinase, a single-stranded DNA-binding protein, and a recombinase loading factor to achieve amplification of target DNA sequences at 37–42°C, thus facilitating DNA amplification in less than 20 min (Li et al., 2019). With remarkable advantages, including simplicity, high sensitivity, and simple operability, RPA assays have been used to detect several plant viruses/viroids, such as plum pox virus, tomato yellow leaf curl virus, apple stem grooving virus, and peach latent mosaic viroid (Babu et al., 2018). In general, RPA products can be analyzed using agarose gel electrophoresis, real-time systems with probe-based fluorescence, or lateral flow strips (LFS). Recently, an RPA assay was developed to detect BYDV in oat using an agarose gel electrophoresis system (Kim et al., 2020). RPA system combined with LFS (termed RPA-LFS) requires a set of primers, a 5′-biotin-labeled primer and its complementary sequence and a probe that is typically labeled with a FAM (carboxyfluorescein) antigen at the 5′ terminus, a tetradrofuran (THF) spacer at the center, and a C3 spacer at the 3′ terminus. The resulting amplicon containing FAM and biotin can thus be visualized using a nitrocellulose membrane coated with anti-biotin antibody and anti-FAM gold conjugates (Fig. 1). Thus, RPA-LFS assays can be performed under non-laboratory conditions after isolating nucleic acids. In this study, we developed and optimized an RT-RPA-LFS assay for the rapid detection of BYDV in oat plants. Moreover, we evaluated and compared the assay with conventional RT-PCR with respect to sensitivity and specificity. This assay was validated for the detection of BYDV in field-collected oat and viruliferous vector aphids.

Schematic representation of the RPA-LFS principle for the detection of BYDV. (A) Principle of the recombinase polymerase amplification. (B) Schematic diagram of the RPA-LFS. RPA-LFS, recombinase polymerase amplification combined with lateral flow strip; BYDV, barley yellow dwarf virus; FAM, 6-carboxyfluoreescein; THF, tetrahydrofuran.
BYDV-infected oat leaf samples exhibiting severe reddening of the leaf tips were collected from oat fields in Yeoncheon, Suwon, Jeongeup, Kimje, Iksan, Gangjin, and Haenam, Korea, in May 2021 and stored in a deep freezer at −80°C before experimentation. Total RNA was extracted from BYDV-infected and non-infected (healthy) oat leaves using the Clear-S Total RNA Extraction Kit (InVirus Tech Co., Gwangju, Korea), according to the manufacturer’s instructions. RNA quality and concentration were determined using a BioDrop spectrophotometer (Biochrom Ltd, Cambridge, UK), and the sample extracts were stored at −20°C. Isolated RNA was first tested for the presence of BYDV using RT-PCR with previously established diagnostic primers (Malmstrom and Shu, 2004). The RT-PCR assay was performed using 10 μl of 2× SuPrimeScript RT-PCR Premix (GeNet Bio, Daejeon, Korea), 125 nM forward primer, 125 nM reverse primer, 1 μl total RNA, and 7 μl DEPC-treated water, with a total reaction volume of 20 μl. The reactions were subjected to reverse transcription at 50°C for 30 min, followed by denaturation at 95°C for 5 min, 35 cycles at 94°C for 20 s, 55°C for 30 s, and 72°C for 1 min, and a final extension step at 72°C for 10 min. Total RNA extracted from the BYDV-infected samples was used as a template for RT-RPA-LFS assays; RNA from healthy leaves was used as negative control. RT-PCR products (approximately 831 bp) corresponding to the complete BYDV coat protein (CP) gene were separated using 1.5% agarose gel electrophoresis and stained using RedSafe nucleic acid staining solution (iNtRON Biotechnology Inc., Seongnam, Korea) (data not shown).
To design primers and a probe for the RT-RPA-LFS assay, the complete CP sequences of 22 BYDV isolates (LC530629, LC550011, LC550014, LC567411, LC567412, LC567413, LC567417, LC567418, LC567421, LC567425, EF521840, EF521843, MK962883, X07653, KT252978, KT252976, EU332333, AY855920, MK012661, AJ810418, KC571999, and D85783) originating from several countries were obtained from the GenBank database, which were then aligned using BioEdit version 7.0.5.3 to identify highly conserved sequences (Supplementary Fig. 1). The RT-RPA-LFR forward primer (5′-CCAATCGAGCAGGACCCAGACGACGAAATGG-3′), reverse primer (5′-BIOTIN-CTCAATAAAGATAGCGCCTGCCGTAGTGGCGG-3′), and near-field optical (nfo) probe (5′-FAM-ATACT-CAAGTCCTACCATCGTTACAAGATC-THF-CAAGTATCCGAGTTG-C3 spacer-3′) were designed according to the TwistDx RPA instruction manual and the Primed-RPA program (TwistDX, Ltd., Cambridge, UK) (Higgins et al., 2019) (Supplementary Table 1). RT-RPA-LFS was carried out using commercially available lateral flow (Laney et al., 2018) strips (GenLine HybriDetect MGHD1, Milenia Biotec, Giessen, Germany) and TwistAmp nfo Kit (TwistDX, Ltd.). The total volume of the reaction mix for the RT-RPA-LFS assay was 50 μl, comprising 1 μl total RNA isolated from BYDV-infected oat tissue, 29.5 μl rehydration buffer, 2.1 μl forward primer (10 mM), 2.1 μl reverse primer (10 mM), 0.3 μl nfo probe (2.5 mM), 1 μl M-MLV RTase (Promega, Madison, WI, USA), 1 μl recombinant RNase inhibitor (5,000 U; TaKaRa, Shiga, Japan), 10.5 μl DEPC-water, and 2.5 μl magnesium acetate (280 mM). Subsequently, 10 μl of the RPA products was added directly to the sample pad of the LFS, which was then immersed in 100 μl running buffer and incubated at room temperature (20–22°C) for 3–5 min. A purple line at the control and test bands indicated a positive result, and a purple line displayed only at the control band indicated a negative result.
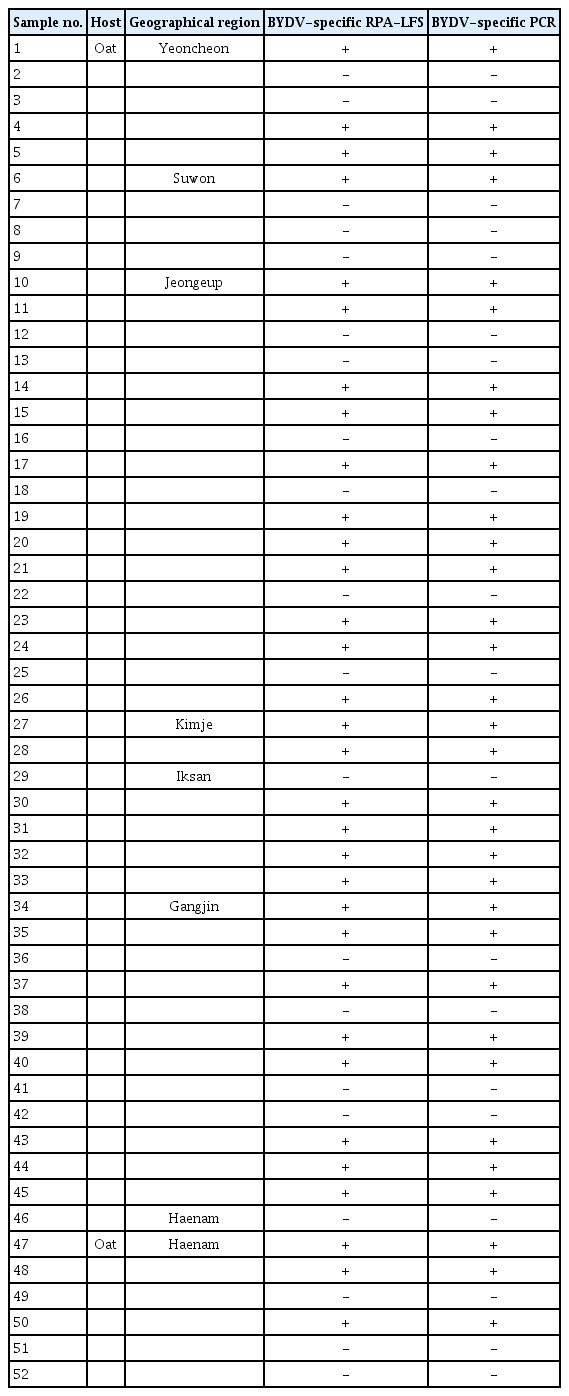
To establish the optimal operation conditions of the RT-RPA-LFS assay for detection of BYDV, different reaction temperatures (30°C, 33°C, 37°C, 42°C, and 46°C) and incubation durations (1, 5, 10, 15, and 20 min) were tested. Clear test bands were observed in reactions performed at 42°C (Fig. 2A). In addition, visual colored lines were detected after incubation for 10 min or more (Fig. 2B). Thus, the assays were subsequently performed at 42°C and 10 min incubation time.
Optimization of RT-RPA-LFS assay for BYDV detection. (A) Optimization of the reaction temperature of the assay. RT-RPALFS assays were performed on BYDV-infected oat leaves at 30, 33, 37, 42, and 46°C (lanes 1–5). Lane 6, healthy oat leaves. (B) Optimization of reaction time of the assay. RT-RPA-LFS assays were performed on BYDV-infected oat leaves for 1, 5, 10, 15, and 20 min (lanes 1–5). Lane 6, healthy oat leaves. The top line on the LFS is the control line, and the other line is the test line. RT-RPA-LFS, reverse transcription recombinase polymerase amplification combined with a lateral flow strips; BYDV, barley yellow dwarf virus.
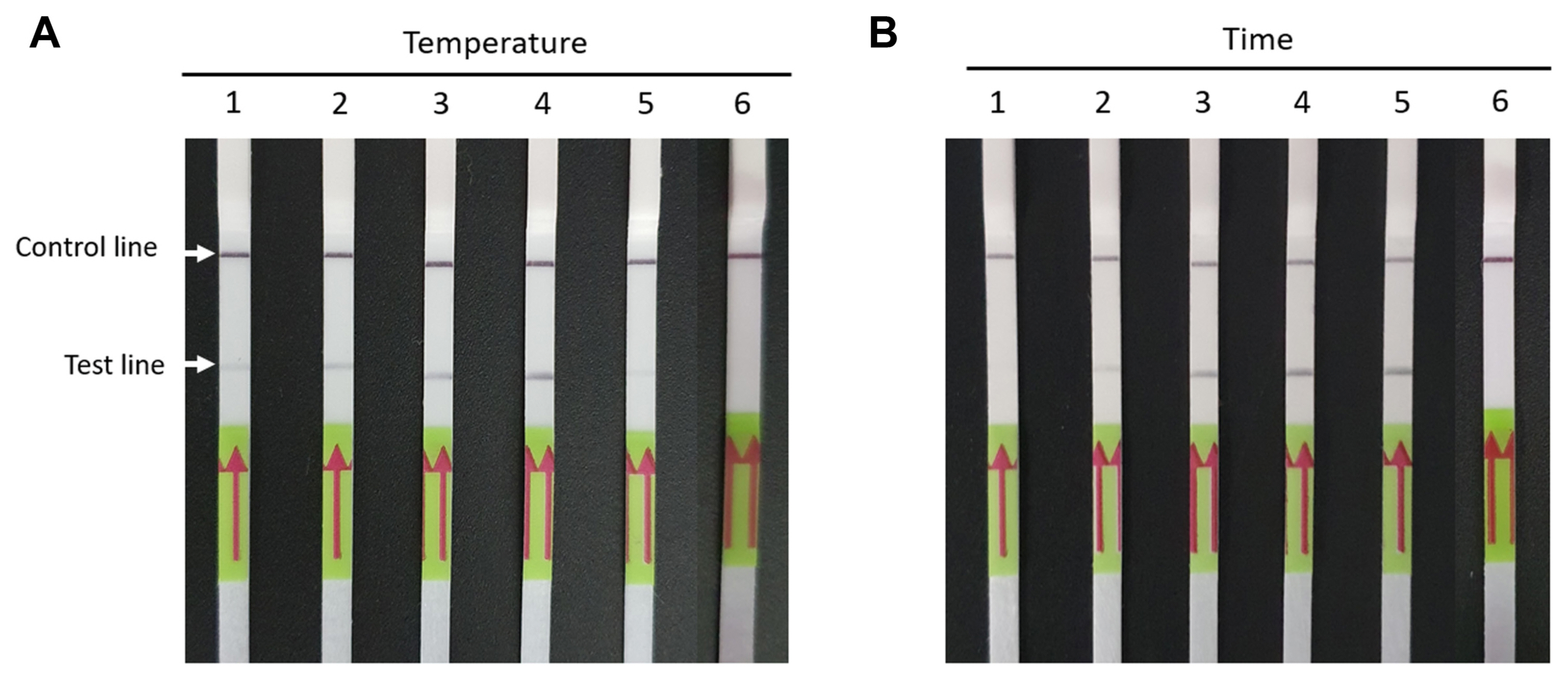
To determine the specificity of the RT-RPA-LFS assay, major viruses, such as barley mild mosaic virus (BaMMV), barley yellow mosaic virus (BaYMV), and rice black streaked dwarf virus (RBSDV) that infect cereal crops, including oats, in Korea were used. RT-PCR assays were performed using primers previously established for the detection of BaMMV, BaYMV, and RBSDV (Lee et al., 2005, 2017) (Fig. 3A). The results indicated no cross-reactivity among the viruses at the test band of the LFS, and only BYDV-infected samples showed a positive result at the test band (Fig. 3A).
Specificity and sensitivity of RT-RPA-LFS assay for detection of BYDV. (A) Lane 1, BYDV-infected sample (positive sample); lane 2, BaMMV and BaYMV co-infected sample; lane 3, RBSDV-infected sample; lane 4, healthy oat sample (negative sample); lane 5, no template control contained water. RT-PCR was performed to confirm the presence of tested viruses. RT-PCR products were visualized on 1.5% agarose gels. M, DNA marker; lane 1, BYDV-infected sample (positive sample); lane 2, BaMMV and BaYMV co-infected sample; lane 3, RBSDV-infected sample; lane 4, healthy oat sample (negative sample); lane 5, no template control contained water. Oat actin was used as an internal control. (B) Ten-fold serial dilutions of transcripts from BYDV CP were performed using RT-RPA- LFS and RT-PCR. The RT-PCR products (~595-bp) were visualized on 1.5% agarose gels. Lanes 1–8, BYDV transcript 100 ng/μl to 100 fg/μl. RT-RPA-LFS, reverse transcription recombinase polymerase amplification combined with a lateral flow strips; BYDV, barley yellow dwarf virus; BaMMV, barley mild mosaic virus; BaYMV, barley yellow mosaic virus; RBSDV, rice black streaked dwarf virus; RT-PCR, reverse transcription polymerase chain reaction; CP, coat protein.
To confirm detection sensitivity of the RT-RPA-LFS assay, the limit of detection was tested using 10-fold serial dilutions (100 ng/μl to 100 fg/μl) of BYDV CP transcripts and by comparing its results with those of RT-PCR. The BYDV CP transcripts were transcribed in vitro using T7 RNA polymerase (Promega). Both RT-RPA-LFS and RT-PCR detected BYDV to a template concentration of 1 pg/μl, indicating similar sensitivities of the RT-PRA-LFS assay and RT-PCR (Fig. 3B). Three independent reactions were conducted and the results were similar.
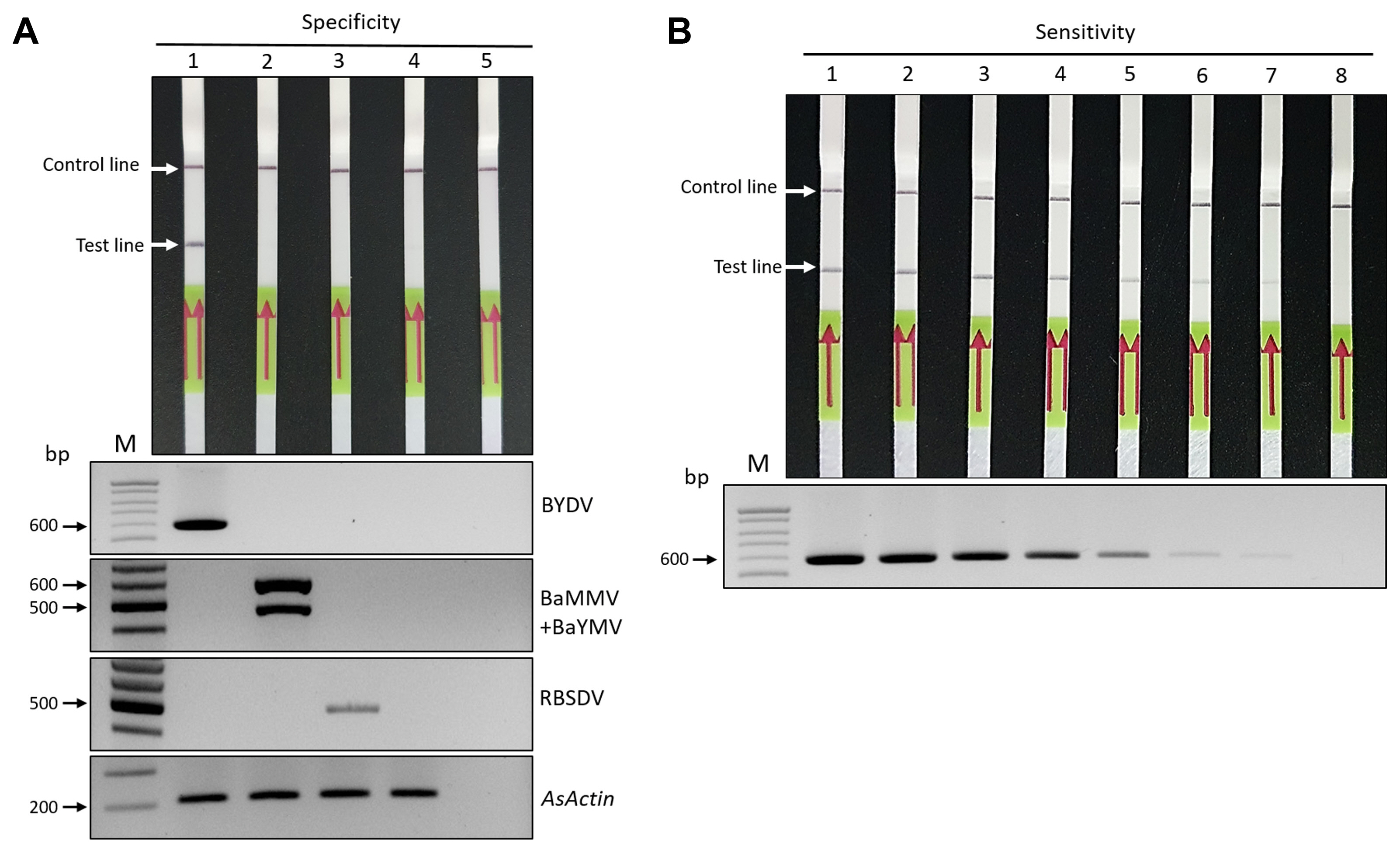
The optimized RT-RPA-LFS assay was validated for the detection of BYDV using 20 oat leaf samples suspected to be BYDV-infected that were collected from fields in Korea; these samples were subjected to the RT-RPA-LFS assay and RT-PCR. Fourteen of the 20 samples were positive, according to both the assays (Fig. 4A). To further confirm reliability of the BYDV-RPA-LFS assay, 52 BYDV-infection suspected oat samples from six different geographical regions of Korea were tested, which indicated that BYDV detection accuracy of the RT-RPA-LFS assay was similar to that of RT-PCR (Table 1).
Application of the RT-RPA-LFS assay for BYDV-infected oat leaves and detection of BYDV in individual aphids using the RT-RPA- LFS assay. (A) RT-RPA-LFS and RT-PCR assays were performed to detect BYDV in oat samples collected from six different regions. Lanes 1–8, samples from Jeongeup; lanes 9–12, samples from Gangjin; lanes 13–15, samples from Haenam; lanes 16–18, samples from Iksan; lanes 19–20, samples from Yeoncheon. RT-PCR products (approximately 595 bp) for BYDV detection were visualized on 1.5% agarose gels. Oat actin level was used as an internal control. (B) BYDVs were detected in eight aphid samples using the RT-RPA-LFS assay. The same samples were positive in both the assays. RT-PCR products (approximately 595 bp) for BYDV detection were visualized on 1.5% agarose gels. An actin gene from Rhopalosiphum padi was used as an internal control (Kim et al., 2020). RT-RPA-LFS, reverse transcription recombinase polymerase amplification combined with a lateral flow strips; BYDV, barley yellow dwarf virus; RT-PCR, reverse transcription polymerase chain reaction.
The application of the RT-RPA-LFS was further extended to the detection of BYDV in a major aphid vector of BYDV, Rhopalosiphum padi. These aphids were collected in Gangjin, Korea, from oat leaves exhibiting BYDV disease symptoms. Total RNA was extracted from each individual aphid using the Clear-S Total RNA Extraction Kit (InVirusTech Co., Gwangju, Korea), and the isolated RNA was subjected to RT-RPA-LFS and RT-PCR assays. The results of the RT-RPA-LFS assay showed clear lines at the test band, which suggested that out of seven individual aphid samples, six were positive, and the remaining one was negative. As expected, there was no amplification in BYDV-free aphids (Fig. 4B). These results were confirmed by RT-PCR. Amplicons in RT-PCR were detected for the same samples which were positive according to the RT-RPA-LFS assay (Fig. 4B). These results indicate that the optimized RT-RPA-LFS assay can be used for detecting BYDV in individual aphids.
BYDV is the causative of one of the most severe diseases of cereals worldwide. Due to the complexity of BYDV-aphid vector-plant host-environmental interactions, it is difficult to predict this viral disease in the field (Choudhury et al., 2017). In particular, unexpected occurrence of BYDV-vector aphids due to increasing global warming and lack of cereal crop varieties that are resistant to BYDV can result in severe cereal diseases. Common management strategies of BYDV and other viral pathogens, such as regular plant disease monitoring and identification of resistant varieties, mainly rely on early rapid and sensitive detection of the respective virus in crops. In the current study, we developed an RT-RPA-LFS assay to detect BYDV in oat, barley, wheat, and its vector aphids and compared the results with those of RT-PCR. The main advantage of the RT-RPA-LFS assay for BYDV detection is the simple indicator of confirming viral presence, a line on the LFS that is visible by the naked eye and can be observed without any training. Our results demonstrate that the RT-RPA-LFS assay takes 10 min for detection, whereas the RT-PCR assay requires at least 1.5 h. In addition to the advantage of shorter reaction time, the RT-RPA-LFS assay can be completed at a constant temperature of 42°C using a water bath or a heat block, whereas RT-PCR requires a thermocycler and an electrophoresis system for amplification and visualization of PCR products, respectively. The RT-RPA-LFS assay developed in this study showed no cross-reactivity with other major cereal viruses, and its sensitivity for BYDV detection in oat leaves was similar to that of RT-PCR. In addition, the RT-RPA-LFS assay was validated with BYDV-infected field samples and aphids. In a previous study, a conventional RT-RPA assay was developed for the detection of BYDV in oat plants (Kim et al., 2020); however, this assay requires more time for BYDV detection because it was developed for agarose gel-based electrophoresis detection systems. Moreover, RT-LAMP assay, an isothermal nucleic acid amplification method, was developed for the detection of BYDV in oat plants (Zhao et al., 2010); however, it requires six primers, causing difficulty regarding primer design from the BYDV genome and is performed at 65 °C for 80 min. The RT-RPA-LFS for BYDV detection developed in the present study requires a shorter reaction time and lower reaction temperature, compared to those in the RT-LAMP assay; its reaction products can be easily observed on LFS, whereas RT-LAMP products need to be confirmed using agarose gel-based electrophoresis or a fluorescent dye. Nonetheless, there are some limitations to the use of the RT-RPA-LFS assay, such as it is comparatively expensive due to the need for specific probes and strips, performs unspecific amplification, and has low specificity due to contamination (Wang et al., 2019).
In conclusion, the RT-RPA-LFS assay for BYDV detection established in the present study is a rapid, simple, specific, and reliable method. Thus, it can be used for rapid detection in epidemiology surveys, identification of resistant varieties, and testing the presence of BYDV in aphids on crop cereals.
Acknowledgments
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01499502) Rural Development Administration, Republic of Korea.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).