Effects of Arugula Vermicompost on the Root-Knot Nematode (Meloidogyne javanica) and the Promotion of Resistance Genes in Tomato Plants
Article information
Abstract
Root-knot nematodes are the most important plant-parasitic nematodes worldwide. Many efforts have been made to find non-chemical, risk-free, and environmentally friendly methods for nematode control. In this study, the effects of compost and vermicompost of arugula (Eruca sativa) on Meloidogyne javanica were investigated in three glasshouse experiments. In addition, the expression of the defense-related genes nonexpressor of pathogenesis-related 1 (NPR1) and lipoxygenase 1 (LOX1) was detected in tomato plants treated with vermicompost of arugula at 0, 2, 7, and 14 days after nematode inoculation. The result showed that the vermicompost of arugula significantly reduced the reproduction factor of the nematode by 54.4% to 70.5% in the three experiments and increased the dry weight of shoots of infected tomato plants. Gene expression analysis showed that LOX1 expression increased on the second and seventh day after nematode inoculation, while NPR1 expression decreased. The vermicompost of arugula showed stronger nematode inhibitory potential than the vermicompost of animal manure. The vermicompost of arugula is superior to arugula compost in suppressing the activity of M. javaniva and reducing its impact. It manipulates the expression of resistance genes and could induce systemic resistance against root-knot nematodes.
Root-knot nematodes (RKNs; Meloidogyne spp.) are the most important plant-parasitic nematodes (PPNs) worldwide. They are high on the list of plant pathogens affecting crop production (Sasser, 1977). Nematodes are traditionally controlled with chemical nematicides (Sikora et al., 2008), which are expensive and pose a high risk to the environment and human health (Dong and Zhang, 2006). Many attempts have been made to find suitable alternatives to chemical nematicides and several methods have been introduced (Kerry, 2000).
A variety of Brassicaceae species have been reported as hosts or associated with a wide range of economically important PPNs, including RKNs. On the other hand, they can reduce the destructive effects of the most harmful nematodes (Fourie et al., 2016). Eruca sativa Mill. (arugula, rocket) is a winter annual plant in the Brassicaceae family and is a poor host for M. hapla, M. incognita, and M. javanica. As a cover or trap crop, it has the potential to control RKNs (Curto et al., 2005; Daneel et al., 2018; Edwards and Ploeg, 2014; Melakeberhan et al., 2006). It is a source of glucosinolates that have suppressive effects against PPNs (Sarıkamıs et al., 2017). Compounds such as erucin, pentyl isothiocyanate, hexyl isothiocyanate, (E)-2-hexenal, 2-ethylfuran, and methyl thiocyanate present in E. sativa were toxic to M. incognita (Aissani et al., 2015). Growing arugula as a winter crop before sowing the susceptible main crop reduced damage by M. hapla and M. arenaria (Aydinli and Mennan, 2018; Melakeberhan et al., 2006). In addition, incorporation of above-ground parts of E. sativa cv. Nemat significantly reduced the population densities of M. incognita and M. javanica in tomato roots (Daneel et al., 2018). The use of arugula for soil amendment significantly reduced the number of M. incognita females and galls in tomato roots and second-stage juveniles (J2s) in soil (Ntalli et al., 2019).
RKNs are obligate sedentary parasites that manipulate plant defense mechanisms to establish permanent feeding sites, the multinucleate giant cells. Strong suppression of hormone pathways associated with plant defense, particularly salicylic acid (SA) and ethylene (ET) pathways, has been observed in giant cells and galls at the early stage of nematode infection (Kyndt et al., 2013). SA is an essential molecule of systemic acquired resistance (SAR) (An and Mou, 2011). Suppression of SA signaling in the plant helps in nematode infection (Molinari et al., 2014). The protein nonexpressor of pathogenesis-related 1 (NPR1) is a receptor of SA. The binding of SA to NPR1 promotes the expression of downstream defense genes (Wu et al., 2012; Zhang and Li, 2019). NPR1 is a major component of the complex network of regulatory interactions between the SA and jasmonic acid (JA)/ET responses, a positive regulator of the SAR pathway and the major regulator of plant defense signaling (Backer et al., 2019).
JA and its derivatives are involved in the induced systemic resistance (ISR) pathway (Ghasemi Pirbalouti et al., 2014). Lipoxygenases (LOXs) present in eukaryotes play an important role in JA biosynthesis and in plant response to various biotic and abiotic stresses, including nematodes. The JA pathway has been reported to play a key role in the immunity of rice roots to M. graminicola. Blocking its biosynthesis by the application of LOX inhibitors increases the infection and susceptibility of rice to the nematode (Nahar et al., 2011). On the other hand, artificially increasing the concentrations of JA impairs the ability of nematodes to suppress JA biosynthesis and signal transduction, significantly reducing the number of developing females (Kammerhofer et al., 2015). It was shown that the mutant tomato plants overexpressing JA had lower infection with M. incognita than the control plants (Wubie and Temesgen, 2019). Similarly, application of methyl-JA to Arabidopsis leaves reduced infection with Heterodera schachtii, while infection of two mutants deficient in JA biosynthesis lox6 and dde2 (delayed dehiscence 2) enhanced female development compared to wild type (Kammerhofer et al., 2015). Lipoxygenase 1 (LOX1) synthesizes the JA in Arabidopsis during leaf senescence (Viswanath et al., 2020). The level of LOX1 expression was down-regulated in tomato roots inoculated with the fungus Trichoderma atroviride, the RKN M. javanica, or both, 4 and 6 days after treatment (de Medeiros et al., 2017). On the other hand, a number of JA-, ET- and SA-biosynthetic or dependent genes were overexpressed 7 days after inoculation with M. incognita, and the nematode juveniles and eggs increased in the roots of maize lox3-4 mutants (Gao et al., 2008).
Combining the nematicidal properties of arugula with those of vermicompost is likely to increase their efficacy. As far as we know, there is no report on the nematicidal activity of arugula vermicompost and its effects on plant response to nematode infection. Therefore, this study investigated the effects of compost and vermicompost of arugula on the RKN M. javanica in tomato roots under glasshouse conditions. In addition, the expression of defense-related genes NPR1 and LOX1 was analyzed in nematode-infected tomato plants treated with arugula vermicompost.
Materials and Methods
Collection and inoculum of Meloidogyne javanica
Cucumber roots heavily infected with the RKN M. javanica were collected from an infested greenhouse. The single egg mass technique was used to grow the RKN on tomato roots (Solanum lycopersicum L. cv. Early Urbana). Specific primers for M. javanica, M. incognita, and M. arenaria were used to confirm morphological identification of the nematode species (Dong et al., 2001). The eggs of M. javanica were extracted from infected roots using sodium hypochlorite (Hussey and Barker, 1973).
Preparation of compost and vermicompost
Arugula seeds (local cultivar) were sown at the end of summer. The plants were harvested in spring at flowering time and used to make compost and vermicompost. To prepare arugula compost, the above-ground plant parts were cut into small pieces and 10 kg of the shredded plants were poured into a 20-liter plastic container with some holes at the bottom. The plant material was mixed every week with a garden fork to aerate it, stored for three months and watered as needed.
Arugula and common vermicomposts were prepared by adding red worm (Eisenia fetida) to the shredded plants and cow manure in separate containers. The mesh basket containing the vermicompost with 30 ± 5 red worm was placed on top of the containers. The worms gradually penetrated and fed on the plant tissue and manure. After three months, the vermicompost was sieved and used for the experiments.
Chemical analysis of compost and vermicompost of arugula
The chemical properties of the compost and vermicompost of arugula were determined by analyzing a sample of 200 g each. The pH was measured using a pH meter in an aqueous suspension ratio of 1:10 (w/v). Total nitrogen was determined by the Kjeldahl method (Bremner and Mulvaney, 1982), phosphorus (P) by the Vanadate/molybdate method (yellow method) (Chapman and Pratt, 1961). Cationic elements (Zn, Fe, Cu, Mn, Ca, and Mg) were measured using an atomic absorption spectrophotometer (AA-670, Shimadzu Scientific Instruments, Kyoto, Japan) (Hoenig, 2005).
Glasshouse experiments
Three experiments were conducted to investigate the effects of compost and vermicompost of arugula, and common vermicompost on nematode indices of M. javanica and growth parameters of infected tomato plants (cv. Early Urbana). In the first experiment, the effects of compost and vermicompost of arugula were compared with the common vermicompost in two trials. Then, the best treatment was selected and used in the second experiment, and its effect on the expression of defense-related genes LOX1 and NPR1 was evaluated in the third experiment.
Effects of arugula compost and vermicompost on Meloidogyne javanica and infected tomato plants
First trial: tomato seeds (cv. Early Urbana) were sown in plastic pots (19 cm diameter) filled with 3 kg of pasteurized mixed soil (field soil and river sand in a ratio of 1:2; N = 0.10%, P = 7 mg/kg, K = 420 mg/kg; pH = 6.98). Based on soil analysis, 40 mg P/(kg soil)/(250 mg triple superphosphate (kg soil)) and 60 mg N/(kg soil)/(360 mg potassium nitrate (kg soil)) were thoroughly mixed with the soil before sowing. The treatments were arugula compost, arugula vermicompost, common vermicompost, and soil without organic matter as control. Sixty grams (2% of potting soil) of the compost or vermicompost was thoroughly mixed with the soil before sowing. Tomato plants in half of the treated pots were inoculated at the four-leaf stage by pouring a suspension containing about 6,000 M. javanica eggs (two eggs/gram of soil) into the soil at a depth of 5 cm around the plant roots. The experiment was laid out on a glasshouse bench in a completely randomized design with four replicates. The pots were observed daily and watered as needed. During the experiment, the average values of minimum and maximum air temperatures in the glasshouse were 25°C and 30°C, respectively.
Sixty days after nematode inoculation, the plant in each pot was harvested and its shoots were dried in an oven at 65 ± 3°C for 48 h and weighed. The J2s of RKN in 100 g of mixed soil of each pot were extracted by tray method (Whitehead and Hemming, 1965) and counted, and the number of J2s in the potting soil was calculated. In addition, the number of galls and egg masses in one gram of tomato roots in each pot was counted after staining with fuchsin acid (Coyne et al., 2007). Then the final population (Pf) and the reproduction factor (Rf) of M. javanica were calculated (Rf = Pf/Pi).
Second trial: the second trial of the experiment was conducted similarly to the first, but there were eight replicates and the average values of the minimum and maximum air temperatures of the glasshouse were 26°C and 32°C, respectively.
The effect of arugula vermicompost on nematode activity
The second experiment was conducted to confirm the effect of the best treatment from the first experiment. The vermicompost of arugula, which proved to be the most effective treatment against M. javanica in the first experiment, was used in the second experiment, while the soil without amendment served as control. Sixty grams of vermicompost of arugula was added to 3 kg of soil in each plastic pot and mixed thoroughly. Tomato seeds (cv. Early Urbana) were sown and the plants were inoculated at the four-leaf stage by pouring a suspension of about 6,000 M. javanica eggs (two eggs/gram of soil) into the soil at a depth of 5 cm around the roots of each plant. Glasshouse conditions were the same as in the first experiment. The experiment was laid out in a completely randomized design with four replicates. The plants were kept up to 60 days after inoculation, and then the dry weight of the shoots of the tomato plants, as well as the reproduction factor (Rf) and the final population of the nematode were determined.
Expression of defense-related genes LOX1 and NPR1
This experiment was conducted to evaluate the expression of LOX1 and NPR1 in tomato plants 0, 2, 7, and 14 days after nematode inoculation. The treatments were soil with arugula vermicompost and soil without organic material as control. The experiment was conducted in 1 kg plastic pots (12 cm in diameter) filled with pasteurized mixed soil (field soil and river sand in a ratio of 1:2). Vermicompost of arugula (20 g; 2% of soil pot) was mixed with the pasteurized soil before sowing. Then tomato seeds (cv. Early Urbana) were sown in each pot. The experiment was laid out on the glasshouse bench in a completely randomized design with three replicates for each time point. At the four-leaf stage, the roots of tomato plants were inoculated with a suspension of approximately 2,000 M. javanica eggs. Pots were observed daily, watered as needed, and maintained in the glasshouse at 25–30°C. To evaluate the expression of defense genes, leaf samples were collected from each treatment 0, 2, 7, and 14 days after nematode inoculation and stored at −80°C, and their dry weight was measured at the same time points.
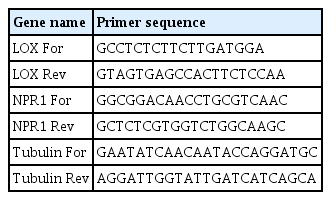
Stored leaf samples were ground in liquid nitrogen using a mortar and pestle. RNA was extracted from the leaves of each plant. Total RNA was extracted using Topazol plus Top RNA purification kit (Topazgene, Karaj, Iran) according to the manufacturer’s protocol and used for first-strand cDNA synthesis with oligo dT primers and the M-MuLV reverse Transcriptase cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA) from DNase-treated (Thermo Scientific) total RNA extracts. PCR reactions were performed using Bioneer Real-Time Quantitative PCR (Daejeon, Korea) and Top 2× Real-Time PCR Master Mix (Topazgene) in a total volume of 12.5 μl with reactions containing 20 ng cDNA and 10 μM of each primer. The cDNA from three biological replicates and two technical replicates was used and analyzed for each sample. All reaction conditions were 94°C for 2 min, 40 cycles of 94°C for 20 s, 57°C for the 20 s, and 72°C for 20 s. Threshold cycle (Ct) values were calculated and transcript abundance was calculated in Microsoft Excel from Ct values and normalized to β-Tubulin gene signal. Relative expression values were calculated using 2−ΔΔCT (Livak and Schmittgen, 2001). Expression of the desired genes in tomato plants treated with arugula vermicompost and M. javanica, and in control plants (sown in soil with M. Javanica) was measured at 0, 2, 7, and 14 days after nematode inoculation. The LOX1 and NPR1 genes were selected as representative defense pathway SA (NPR1) and JA (LOX1). The primers and their corresponding sequences are listed in Table 1 (de Medeiros et al., 2017; Rubio et al., 2014).
Statistical analysis
SAS statistical software was used for data analysis (SAS ver. 9.1, SAS Institute Inc., Cary, NC, USA). The analysis of parametric indices (plant indices) was performed using the Proc ANOVA method and the non-parametric indices (nematode indices) were performed using the Friedman rank test method. Mean values were compared using a posthoc Tukey honestly significant difference test (P < 0.05). In addition, the means of the treatments in the second experiment were compared using a t-test.
Results
Chemical properties of compost and vermicompost of arugula
The chemical properties of compost and vermicompost of arugula are shown in Table 2. The results show that the pH of vermicompost of arugula was lower but the total nitrogen, P, Fe, and Mg contents were higher than that of compost.
Effects of compost and vermicompost of arugula on the growth of Meloidogyne javanica and infected tomato plants (first and second experiment)
In the first and second trials of the first experiment, both the common and arugula vermicomposts significantly (P < 0.05) increased the dry weight of the healthy and infected tomato plants, but the arugula vermicompost showed the greatest effect on the dry infected plants (Tables 3 and 4). The arugula vermicompost showed the same effect in the second experiment, increased the dry weight of the infected tomato plants by more than 2.8 times compared to the control (Table 5).
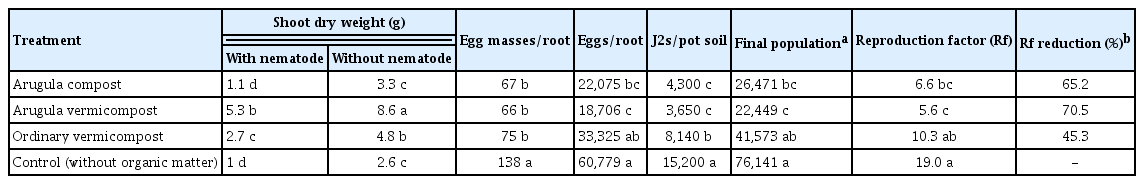
Effects of common vermicompost, compost and vermicompost of arugula on shoot dry weight and nematode indices of Meloidogyne javanica in infected tomato roots, under greenhouse conditions (first trial of the first experiment)

Effects of common vermicompost, compost and vermicompost of arugula on shoot dry weight and nematode indices of Meloidogyne javanica in infected tomato roots, under greenhouse conditions (second trial of the first experiment)
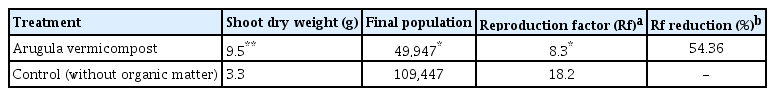
Effects of vermicompost of arugula on nematode indices and growth parameters of tomato plants infected with Meloidogyne javanica (second experiment)
The results of the first experiment showed that the treatments had different effects on nematode indices. In the first trial, all treatments significantly reduced the number of egg masses and J2s in the soil. Vermicompost and compost of arugula reduced eggs in the root system and the final population and reproduction factor of M. javanica, but the number of galls/root and eggs/egg mass were similar to the control in all treatments (Table 3). In the second trial, the arugula compost significantly reduced the number of egg masses on the root system, and the arugula vermicompost reduced the number of eggs in the egg masses and roots. All treatments significantly reduced the number of J2s in the soil. Among them, the arugula vermicompost was the most effective and reduced the reproduction factor by 70.5 and 58.17% in the first and second trials, respectively, compared to the control (Tables 3 and 4). In the second experiment also, the arugula vermicompost reduced the reproduction factor by 54.36% (Table 5).
Expression of defense genes (third experiment)
The expression of NPR1 and LOX1 genes in tomato plants was studied to better understand the response of plants to RKN infection and treatment with arugula vermicompost. In this experiment, arugula vermicompost increased the shoot dry weight of infected plants on all days studied (Fig. 1), but it was higher on days 7 and 14 (Fig. 2).

Effect of arugula vermicompost on shoot dry weights of tomato plants 0, 2, 7, and 14 days after inoculation with Meloidogyne javanica. Data are the means of three replicates. Bars with the same letters are not significantly different (P < 0.05).
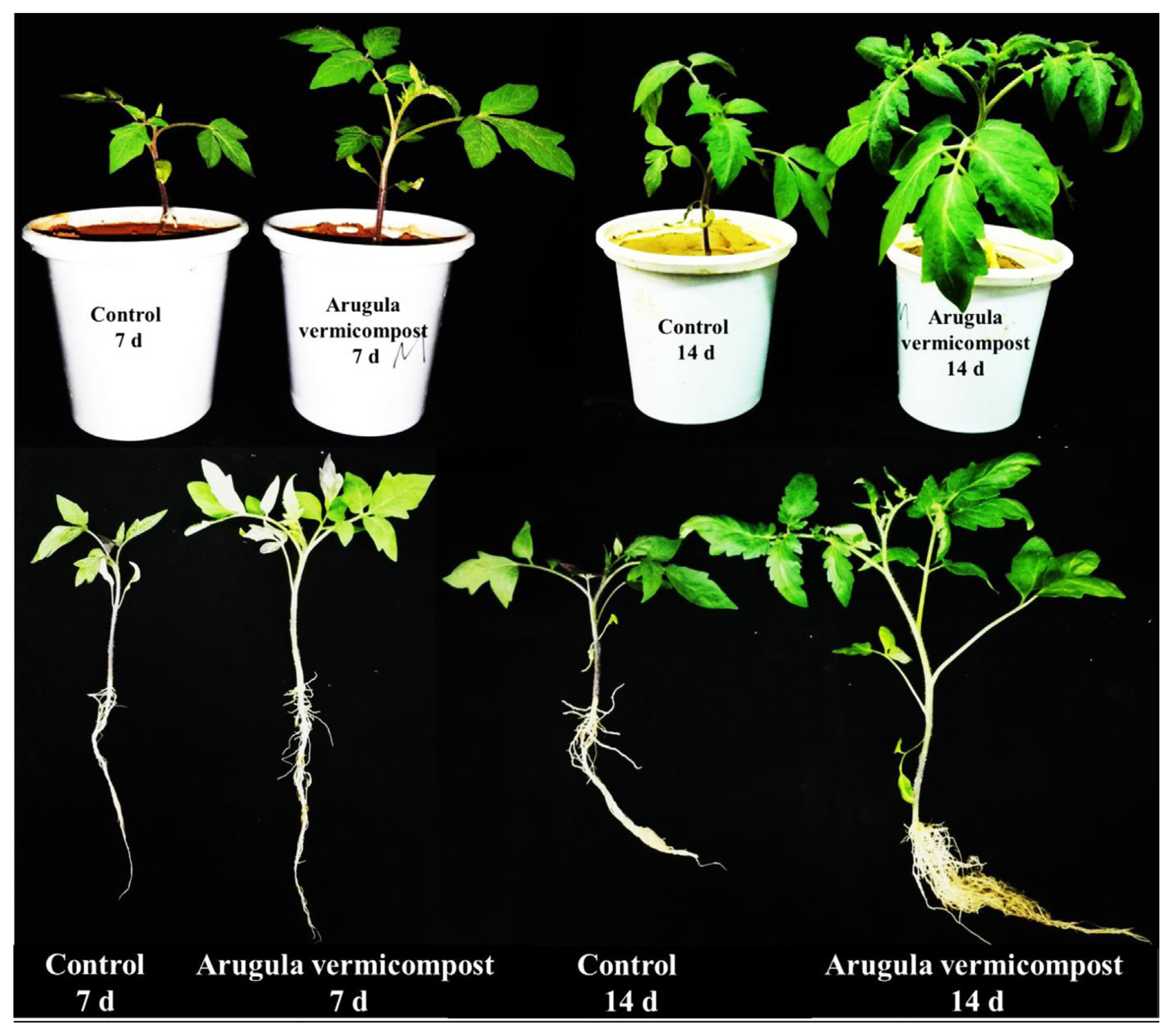
Effect of arugula vermicompost on growth of tomato plants 7 and 14 days after inoculation with Meloidogyne javanica.
Gene expression in the tomato plants treated with arugula vermicompost and control plants is shown in Fig. 3. The results showed that the expression of NPR1 and LOX1 genes were almost the same at the beginning of nematode inoculation, but later changed differently. The expression of NPR1 in the treated plants was down-regulated at 2 and 7 days, and then increased slightly 14 days after inoculation (Fig. 3A). On the other hand, the expression of LOX1 was increased at 2 and 7 days, and then decreased significantly at 14 days (Fig. 3B).
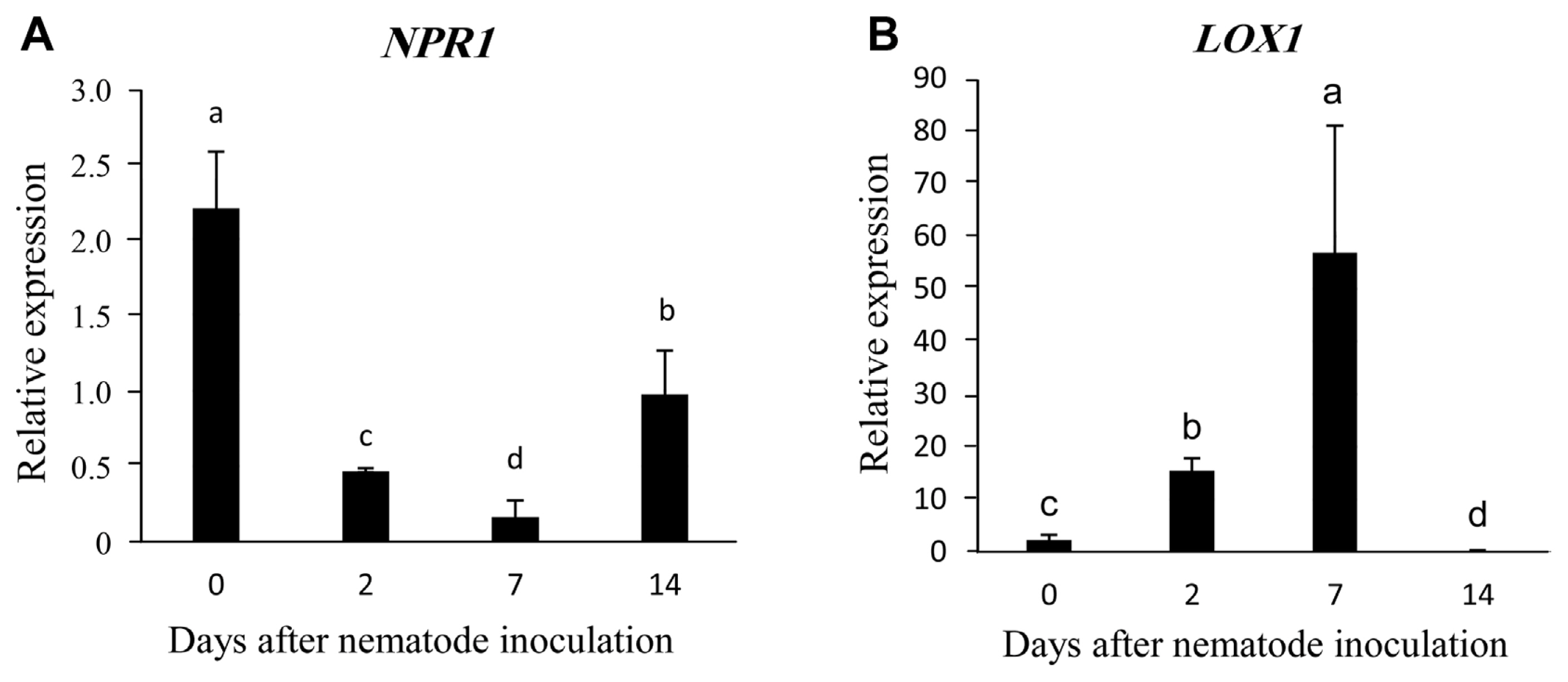
Relative expression of nonexpressor of pathogenesis-related 1 (NPR1) (A) and lipoxygenase 1 (LOX1) (B) genes in leaves of tomato plants treated with arugula vermicompost at 0, 2, 7, and 14 days after inoculation with Meloidogyne javanica. Expression of the desired genes in the plants was measured relative to the control (plants treated with M. javanica only; without vermicompost) (2−ΔΔCT method). Data are means of three replicates. Bars with the same letters are not significantly different (P < 0.05).
Discussion
The present study showed the inhibitory effect of arugula vermicompost against RKN. Arugula vermicompost was superior to arugula compost and common vermicompost in reducing the population of M. javanica. Two experiments confirmed that arugula vermicompost was more effective in increasing the shoot dry weight of nematode-infected plants and reducing the final population and reproduction factor of M. javanica. The growth of tomato plants in the second trial of the first experiment was better than the first. The first experiment was conducted in winter and the second in spring with longer days. Previous studies reported that vermicompost suppressed the population of PPNs in a field experiment (Arancon et al., 2002). Application of vermicompost in a pot experiment against Meloidogyne spp. reduced the number of galls and improved relative control efficacy (Liu et al., 2019; Rostami et al., 2014). Our results suggest that the chemical properties of vermicompost of arugula differ from those of its compost and that earthworms improve the nematicidal properties of arugula. The vermicomposting process increases the levels of N, P, K, Ca, and Mg in the worm manure, while decreasing the C:N ratio, pH, and total organic carbon (Raphael and Velmourougane, 2011). Earthworm activity on organic material alters the chemical, physical and biological properties of these materials. Garg et al. (2006) recorded an increase EC, which could be related to the production of ions and minerals during vermicomposting. Mineralization of N and P, decomposition of organic matter and CO2 production lead to a decrease in pH in vermicompost (Pathma and Sakthivel, 2012). The N content in worm manure increases due to the mucus, hormones, and enzymes of earthworms (Tripathi and Bhardwaj, 2004). Carbon loss is higher than N loss due to CO2 biooxidation during decomposition of the materials (Lazcano et al., 2008). The increase in total P is probably related to the phosphatase activity of the microorganisms in the vermicomposting process. In addition, vermicomposting causes a significant increase in Ca2+, Mg2+, and K2+ in the decomposed material (Pathma and Sakthivel, 2012).
The vermicompost is effective in improving plant growth and resistance to agricultural pests. It increases the size of the microbial population in the soil, adds various nutrients including macronutrients, micronutrients and humus, and improves the physical properties of the soil (Adhikary, 2012; Tognetti et al., 2005). In addition, vermicompost is rich in microbial activity that can suppress nematode infection. It includes fungi such as Trichoderma spp., chitinolytic bacteria, Pseudomonas spp., and predatory mites (Edwards et al., 2011; Pathma and Sakthivel, 2012). Microbial activity induces systemic resistance (ISR) to RKNs (Adam et al., 2014; Siddiqui and Shaukat, 2002).
Arugula plants belong to the family Brassicaceae. The effectiveness of some plant species of this family in reducing damage by RKNs is due to the non-glucosinolate and glucosinolate compounds, which are toxic to nematodes (Matthiessen and Kirkegaard, 2006). The oil of arugula inhibited the root-knot development and reduced the nematode population (Akhtar and Mahmood, 1993). Isothiocyanates are prominent compounds that have nematicidal activity and are derived from glucosinolates (Ntalli and Caboni, 2017). Leaf tissue of Eruca species contains glucosativin (4-mercaptobutyl), glucoerucin (4-methylthiobutyl), and glucoraphanin (4-methylsulfinylbutyl), which are kinds of glucosinolates (Pasini et al., 2011; Villatoro-Pulido et al., 2013), could suppress RKNs.
Vermicompost treatment caused the highest expression of polyphenol oxidase D (PPOD) and flavonol synthase (FLS) genes, and increased the content of phenols and flavonoids in the roots of tomato plants inoculated with M. incognita. The PPOD gene, which plays a role in polyphenol metabolism, protects plants from pathogens and environmental stress (Xiao et al., 2016). A number of plant growth-promoting rhizobacteria (PGPR) such as Bacillus, Pseudomonas, Rhizobium, and Azotobacter in vermicompost induce plant resistance (Gopinath and Prakash, 2014; Hoitink and Grebus, 1997). The physicochemical properties of vermicompost have led to its introduction as a suitable substrate for plant growth (Singh et al., 2020). Moreover, earthworms have the potential to stimulate the root growth of an Arabidopsis thaliana mutant with impaired auxin transport. Regulation of gene expression in the presence of earthworms is known to be responsive to biotic and abiotic stresses or the application of exogenous hormones, and has strong analogies with systemic resistance induced by signaling molecules released by PGPR and elicitors by non-virulent pathogens (Puga-Freitas et al., 2012).
In our study, plants treated with arugula vermicompost and M. javanica showed increased expression of LOX1. It has been suggested that LOXs may play a role in the interaction between pea root-Heterodera goettingiana and in resistance mechanisms (Leone et al., 2001). LOX1 encodes LOX, which is probably the first enzyme to activate JA synthesis (Melan et al., 1993). LOXs are involved in wound response and pest resistance in plants and increase volatile compounds. LOX products such as hexanal have a negative effect on plant pathogens (Hildebrand, 1989). Pseudomonas putida induced resistance of tomato plants to Botrytis cinerea by stimulating the LOX pathway (Akram et al., 2008). In agreement with the present study, it was shown that the expression of LOX3 in maize gradually increased and peaked 7 days after inoculation with the RKN M. incognita, indicating that LOX3 is required for normal plant development (Gao et al., 2008). Considering the increase in LOX1 expression in the present study and the role of LOX1 in the defense system JA, it can be concluded that vermicompost of arugula may play a role in the induction of ISR. Vermicompost is rich in PGPRs and several reports suggest that they induce systemic resistance after infestation by PPNs (Burkett-Cadena et al., 2008; Ramamoorthy et al., 2001).
Expression of NPR1, which has been evaluated as a representative of the SA pathway, was decreased in infected tomato plants treated with arugula vermicompost. NPR1 is the major protein of the SA signaling pathway. NPR1 expressed in some cotton lines exhibits resistance to various fungal, bacterial, and nematode pathogens (Parkhi et al., 2010). This gene regulates the SA defense pathway against H. schachtii (Wubben et al., 2008). Expression of AtNPR1 increased the resistance of transgenic tobacco plants to the RKN M. incognita, which was associated with a reduction in the number of galls and egg-masses numbers in the transgenic plant compared to the wild type (Priya et al., 2011). Downregulation of NPR1 showed that vermicompost of arugula did have no effect on the SA pathway. Our result is consistent with the use of vermicompost tea against M. incognita on cucumber. It showed that the nematode penetration into the plant roots treated by vermicompost tea was lower than that in the control. Moreover, the expression of defense-related genes such as CHIT-1, PAL-1, and LOX1 was up-regulated, while the expression of PR-1 decreased (Mishra et al., 2018).
The relative expression of both genes in this study was almost the same before nematode incubation, but the comparison of LOX1 and NPR1 expression at other time points after nematode incubation showed that the increased expression of one gene coincided with the decreased expression of the other gene. Antagonism between the ET/JA- and the SA-defense pathways causes mutual inhibition, demonstrating crosstalk between SA and the ET/JA signaling pathway. JA-biosynthesis genes are repressed by SA, and activation of the ET/JA-pathway also represses the SA-response (Li et al., 2019).
In conclusion, vermicompost of arugula (Eruca sativa) is superior to arugula compost in suppressing Meloidogyne javaniva activity and reducing its impact. It manipulates the expression of resistance genes and could induce ISR against RKNs. Arugula is a suitable earthworm food to produce suppressive vermicompost for nematode suppression.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors gratefully acknowledge the financial support from Shiraz University and Iran National Science Foundation (INSF).
The authors declare that the present article is part of the result of the first author’s doctoral dissertation project approved by the Vice-Chancellor of Education and Postgraduate Studies, Shiraz University. Furthermore, they declare that all relevant ethical issues have been taken into consideration in conducting the research and writing the manuscript.