NADPH Oxidases Are Required for Appressorium-Mediated Penetration in Colletotrichum scovillei-Pepper Fruit Pathosystem
Article information
Abstract
NADPH oxidase (Nox) complexes are known to play essential roles in differentiation and proliferation of many filamentous fungi. However, the functions of Noxs have not been elucidated in Colletotrichum species. Therefore, we set out to characterize the roles of Nox enzymes and their regulators in Colletotrichum scovillei, which causes serious anthracnose disease on pepper fruits in temperate and subtropical and temperate region. In this study, we generated targeted deletion mutants for CsNox1, CsNox2, CsNoxR, and CsNoxD via homologous recombination. All deletion mutants were normal in mycelial growth, conidiation, conidial germination, and appressorium formation, suggesting that CsNox1, CsNox2, CsNoxR, and CsNoxD are not involved in those developmental processes. Notably, conidia of ΔCsnox2 and ΔCsnoxr, other than ΔCsnox1 and ΔCsnoxd, failed to cause anthracnose on intact pepper fruits. However, they still caused normal disease on wounded pepper fruits, suggesting that Csnox2 and CsnoxR are essential for penetration-related morphogenesis in C. scovillei. Further observation proved that ΔCsnox2 and ΔCsnoxr were unable to form penetration peg, while they fully developed appressoria, revealing that defect of anthracnose development by ΔCsnox2 and ΔCsnoxr resulted from failure in penetration peg formation. Our results suggest that CsNox2 and CsNoxR are critical for appressorium-mediated penetration in C. scovillei-pepper fruit pathosystem, which provides insight into understanding roles of Nox genes in anthracnose disease development.
The NADPH oxidases (Noxs) are membrane-bound enzymatic complexes, which catalyze reduction of dioxygen (O2) to the superoxide anion (O2−) using electrons provided by NADPH (Lambeth, 2004; Rastogi et al., 2017; Scott, 2015; Sumimoto, 2008). The Noxs are widely distributed among eukaryotic organism and play essential roles in multiple cellular processes, including signaling transduction, host defense, and hormone response (Bedard and Krause, 2007; Sahoo et al., 2022; Sumimoto, 2008). The first catalytic subunit (gp91phox) of Noxs was found in mammalian phagocytes with a molecular mass of about 91 kDa (Sumimoto, 2008). Further research revealed that the human genome contains six additional gp91phox homologs (Sumimoto, 2008). The plant also contains several gp91phox homologs, named as Rboh (Torres and Dangl, 2005). Molecular genetic study suggests that each catalytic subunit of Noxs has its specific regulatory components (Rada and Leto, 2008). For example, the representative gp91phox is stabilized by p22phox and activated by Rac1 GTPase, p47phox, p67phox, and p40phox (Rada and Leto, 2008).
Several plant pathogenic fungi were found to contain two gp91phox homologs (Nox1 and Nox2), a p22phox homolog (NoxD), and a p67phox homolog (NoxR) (Takemoto et al., 2007). Recently, a tetraspanin-like protein was suggested as an adaptor protein of Nox2 (Zhao et al., 2016). Activation of Noxs is regulated by a small GTPase Rac1 (Takemoto et al., 2007). Genetic analysis demonstrated that Nox1 and Nox2, together with the regulatory components, play important and divergent roles in hyphal growth, sexual and asexual reproduction, and penetration and colonization in host (Cano-Domínguez et al., 2008; Kayano et al., 2013; Ryder et al., 2013; Yang and Chung, 2012; Zhao et al., 2016). For example, deletion of the NoxA (Nox1) caused severe defects in asexual developments in Aspergillus nidulans and Neurospora crassa (Cano-Domínguez et al., 2008; Lara-Ortíz et al., 2003). The bcnoxB (Nox2) plays an essential role in appressorium penetration, whereas bcnoxA (Nox1) is dispensable for penetration but involves in host colonization of Botrytis cinerea (Segmüller et al., 2008). The adapter protein NoxD/Pro41, homologous to human p22phox, directly interacts with bcnoxA/Nox1 and shows similar functions to bcnoxA/Nox1 (Siegmund et al., 2015). In Magnaporthe oryzae, inactivation of Nox2 or NoxR abolished formation of penetration peg (Ryder et al., 2013). The deletion mutant of Nox1 was able to form penetration peg but failed in elongating the penetration peg (Ryder et al., 2013). The NoxD gene is involved in appressorium-mediated penetration (Galhano et al., 2017).
Chili pepper (Capsicum annum L.) is a valuable and important vegetable crop, widely cultivated in tropical and subtropical countries (Giacomin et al., 2020; Kim et al., 2014). However, the pepper production is seriously constrained by chilli anthracnose disease, caused by Colletotrichum species (Oo and Oh, 2016). The chilli anthracnose is a seed borne, soil borne, water borne and airborne disease, which infects stem, branch, leave, and fruit of chilli plant, finally causing dark-brown necrotic lesions on the infected area (Saxena et al., 2016; Srinivasan et al., 2014). The Colletotrichum scovillei, belonging to Colletotrichum acutatum species complex, was reported as a major agent of pepper fruit anthracnose (Fu et al., 2021). Anthracnose caused by this fungus initiates with emergence of germ tubes from conidia adhering to host surface (Lee et al., 2021). Upon recognition of surface signals, the tips of germ tubes become swollen and then differentiate into appressoria, which subsequently generate specialized penetration pegs to rupture pepper fruits cuticles (Shin et al., 2021). During penetration process, unique dendroid structures appear in the cuticle layer of pepper fruits (Fu et al., 2021; Liao et al., 2012).
Although Nox complexes have been studied in several filamentous fungi, they have not yet been functionally characterized in Colletotrichum species. Therefore, we set out to investigate the roles of Nox complexes in anthracnose development of C. scovillei-pepper pathosystem by phenotypical comparison of targeted deletion mutants (ΔCsnox1, ΔCsnox2, ΔCsnoxR, and ΔCsnoxD), which were generated via homologous replacement. Our results showed that all deletion mutants are normal in mycelial growth, conidiation and appressorium formation. However, ΔCsnox2 and ΔCsnoxr failed to infect intact pepper fruits, caused by their defect in forming penetration peg from appressorium, which suggest that both CsNox2 and CsNoxR are essential for appressorium-mediated penetration in pepper fruit anthracnose by C. scovillei.
Materials and Methods
Fungal strains and culture conditions
The C. scovillei KC05 strain was used as wild-type strain. The wild-type and all transformants were routinely grown on minimal media agar (MMA) and oatmeal agar (OMA) at 25°C, as previously described (Fu et al., 2019, 2021). liquid complete media was used to culture mycelia for genomic DNA isolation, total RNA extraction, and protoplast generation (Han et al., 2018; Wang et al., 2020). Fungal transformants were grown on transformation agar (TB3) agar (Shin et al., 2019).
Sequence and phylogenetic analysis
The amino acid sequences of Nox proteins (CsNox1, CsNox2, CsNoxR, and CsNoxD) and their homologs were obtained from National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov) and Comparative Fungal Genomics Platform (CFGP, http://cfgp.riceblast.snu.ac.kr) (Han et al., 2016). Phylogenetic relationship, identity, and domain structures among Nox proteins were analyzed by using MEGA X software, NCBI BLASTP (https://blast.ncbi.nlm.nih.gov/), and InterPro Scan (http://www.ebi.ac.uk/interpro/), respectively.
Targeted gene deletion and complementation
Targeted deletion constructs were generated by a modified double joint polymerase chain reaction (PCR) (Yu et al., 2004). Each flank (about 1.5 kb) in upstream and downstream of targeted gene was amplified using corresponded paired primers (5F/5R and 3F/3R) (Supplementary Table 1). The HPH cassette, which contains the hygromycin phosphotransferase gene, was amplified using the primers HPHF/HPHR from pBCATPH (Choi et al., 2009). The upstream and downstream flanks of each gene and HPH cassette were fused using corresponded primers 5F/3R, and then amplified using the corresponded primers NF/NR (Supplementary Table 1) to generate the deletion constructs. The protoplasts of wild-type were transformed with deletion constructs, and next grown on TB3 agar medium containing hygromycin B (Sigma, St. Louis, MO, USA). The colonies observed on TB3 agar medium were selected using screening PCR with primers SF/SR (Supplementary Table 1), and finally confirmed with Southern blotting and reverse transcription polymerase chain reaction (RT-PCR). To generate complemented strains, the genomic copies of each targeted gene were amplified using primers NF/NR, and then co-introduced with pII99 vector into protoplasts of corresponded deletion transformants (Fu et al., 2021). Complemented strains were selected through screening PCR and confirmed by RT-PCR (Lee et al., 2021).
Nucleic acid manipulation
The genomic DNA was extracted through a quick and safe method for screening of transformants (Chi et al., 2009). Genomic DNA used in Southern blotting was extracted through a standard method (Park et al., 2013). Total RNA was isolated from the frozen fungal tissues using Easy-Spin Total RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea). The cDNA was synthesis using SuperScriptTM III First-Strand Synthesis System Kit (Invitrogen Life Technologies, Carlsbad, CA, USA) (Shin et al., 2022). Quantitative reverse transcription PCR was performed with Real-Time PCR 2× Master Mix (Elpis Bio, Daejeon, Korea) using a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) (Fu et al., 2021). The target gene expression was expressed as 2−ΔΔCt, where ΔΔCt = (Ct, target gene − Ct, β-tubulin) control − (Ct, target gene − Ct, β-tubulin) test condition (Livak and Schmittgen, 2001). This experiment was performed in three-independent experiments with two replicates per experiment.
Phenotypic characterization of transformants
To measure mycelial growth, three-day old mycelial agar plugs (5 mm in diameter) from MMA were inoculated onto potato dextrose agar (PDA) media and incubated at 25°C for 5 days without light (Fu et al., 2021). To evaluate conidiation, three-day old mycelial agar plugs (5 mm in diameter) were inoculated onto V8 agar medium and incubated at 25°C for 5 days without light and then 2 days with light (Lee et al., 2021). The conidia were harvested with 5 ml distilled water (DW) and counted using a hemocytometer. To perform appressorium formation assay, conidia were collected from 7-day-old OMA with 2 ml DW. The conidial suspensions were filtered through three layers of Miracloth (Calbiochem, San Diego, CA, USA) and next washed for three times by centrifuge at 5,000 rpm for 10 min. Conidial suspension (5 × 104 conidia/ml) were dropped on the hydrophobic surface of coverslips, and incubated in a humid box at 25°C. To perform pathogenicity assays, the washed conidial suspensions (5 × 105 conidia/ml) were dropped onto intact and wounded pepper fruits. To perform microscopic observation of infection, the inoculation areas were sliced using a razor and observed using a light microscope. All phenotypes were evaluated at least in three-independent experiments with three repeats per experiment.
Scanning electron microscope observation
To observe the appressorium penetration site of C. scovillei on pepper fruit, the conidia suspensions (5 × 104 conidia/ml) were inoculated onto intact pepper fruits. After incubated in humid box at 25°C for 3 days, the inoculation areas were sliced using a razor and fixed in 4% paraformaldehyde at 4°C for 24 h (Fu et al., 2022). The samples were subsequently washed using 1× phosphate buffered saline. The washed samples were dehydrated in series of increasing concentrations of ethanol (30%, 40%, 50%, 60%, 70%, 80%, 90%, and 95%) for 30 min at each concentration, and then in 100% ethanol for 1 h and for three times (Pariona et al., 2019). The dehydrated samples were sonicated to remove the dust and detach appressoria from the pepper surface. After sonication, the samples were dried according to critical-point dried method, and mounted on the carbon tapes, and coated with gold (Schroeckh et al., 2009). The samples were observed through a scanning electron microscope (SEM).
Results
Phylogenetic analysis and domain predictions of Nox proteins
The phylogenetic analysis was performed with amino acid sequences of Nox proteins (Nox1, Nox2, NoxR, and NoxD) from C. scovillei and other fungal species, including Alternaria alternata, B. cinerea, Colletotrichum higginsianum, Epichloë festucae, M. oryzae, N. crassa, and V. dahliae. The result showed that all proteins were divided into four clades, representing Nox1, Nox2, NoxR, and NoxD clades (Fig. 1). Each Nox protein within the corresponded clade was closely related to its homolog in C. higginsianum, but distantly related to its homolog in A. alternata (Fig. 1). The domain prediction showed that Nox1, and Nox2 proteins contain a ferric reductase transmembrane component-like domain (IPR013130), FAD-binding 8 domain (IPR013112), and a ferric reductase-NAD binding domain (IPR013121); NoxR proteins contain a PB1 domain (IPR000270); NoxD proteins do not contain domain (Fig. 1). These results suggest that Nox proteins are highly conserved among filamentous fungi.
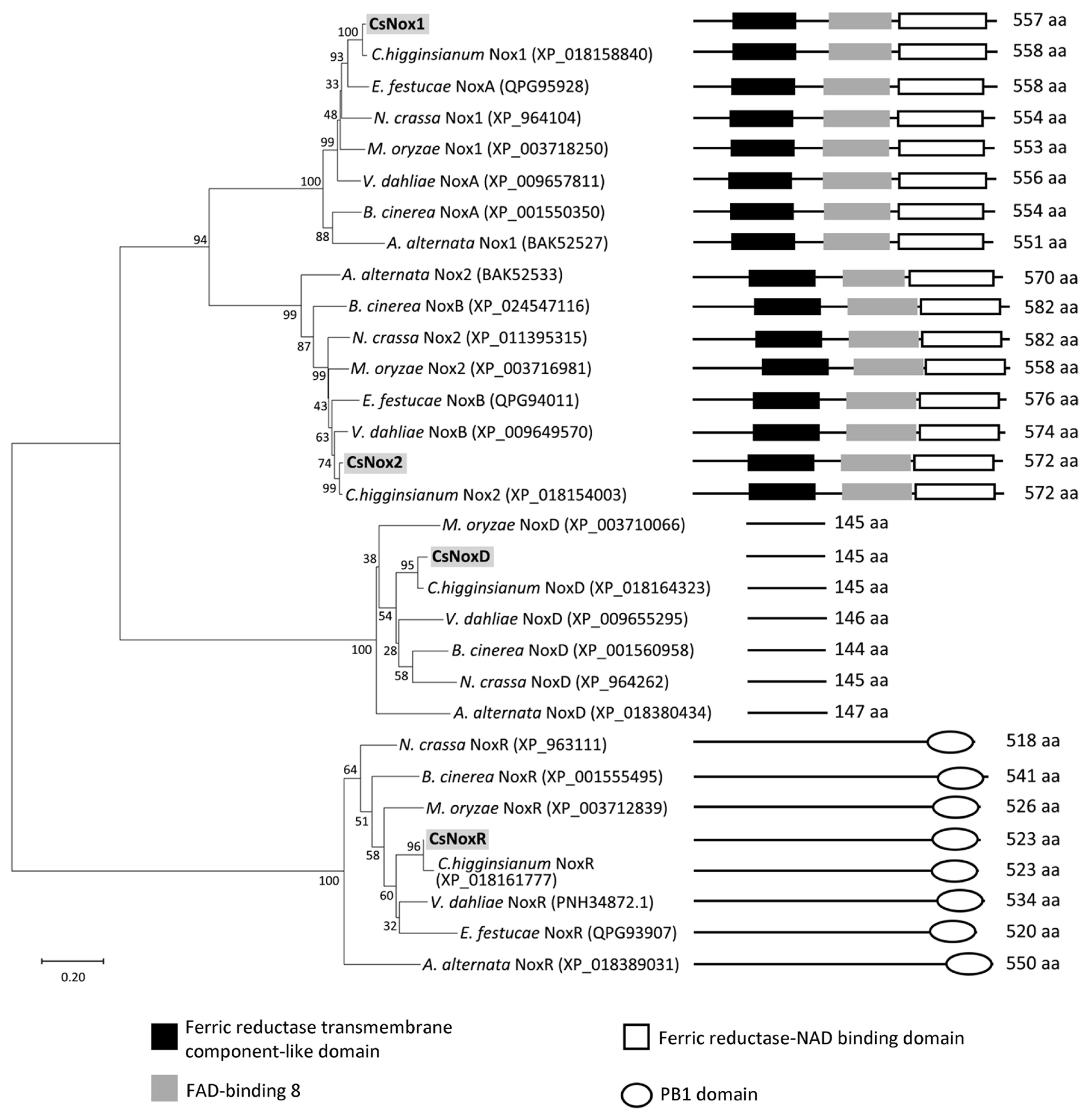
Phylogenetic analysis and domain prediction of NADPH oxidases (Noxs) among several fungi. The phylogenetic tree was generated using maximum likelihood estimation with 1,000 bootstraps. Domains of Noxs, including ferric reductase transmembrane component-like domain (IPR013130), FAD-binding 8 (IPR013112), ferric reductase, NAD binding domain (IPR013121), and PB1 domain (IPR000270), were predicted through InterPro.
Targeted gene deletion of Nox genes
To investigate the functional roles of Nox proteins in C. scovillei, we generated the deletion mutants (ΔCsnox1, ΔCsnox2, ΔCsnoxr, and ΔCsnoxd) via targeted gene replacement (Fig. 2A). The targeted gene deletions were confirmed with Southern blotting (Fig. 2B). Expressions of target gene were found to be not detected in the corresponded deletion mutant (Fig. 2C). The recovery of expressions of target gene was verified in the corresponded complemented strain in RT-PCR (Fig. 2C).
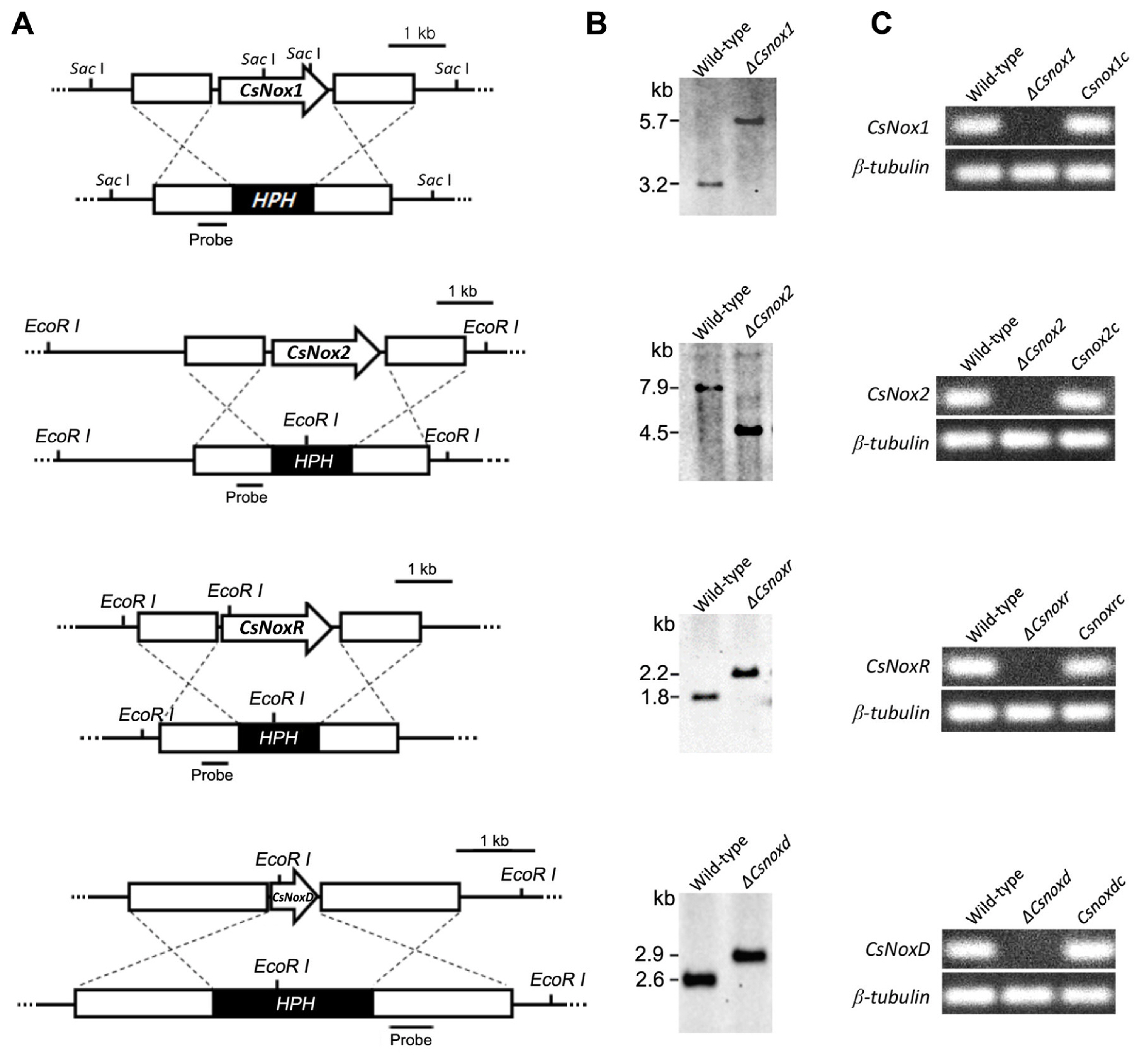
Targeted deletion and complementation of Nox genes (CsNox1, CsNox2, CsNoxR, and CsNoxD). (A) Targeted gene deletion. Each Nox gene was replaced with the HPH cassette via homologous recombination. (B) Verification of deletion mutants. Genomic DNA of wild-type and candidates of deletion mutant was digested with restriction enzymes and hybridized to a probe (about 500 bp). (C) Expression of targeted gene in deletion mutant and complemented strain. Expression of each targeted gene was detected in wild-type and corresponded complemented strain, but not deletion mutant.
Dispensable role of Nox genes in mycelial growth and conidiation
To investigate the roles of CsNoxs in mycelial growth, the diameters of colony growth were measured. The diameter of colony growth in wild-type was 42.4 ± 1.1 mm on PDA (Table 1). All deletion mutants exhibited similar colony growth, compared to the wild-type (Fig. 3), suggesting that CsNoxs are not involved in mycelial growth. To test whether CsNoxs are associated with conidiation, we counted the number of reproduced conidia in wild-type and transformants. The wild-type produced 117.8 ± 9.6 conidia/ml (Table 1). All deletion mutants produced similar amount of conidia compared to wild type, suggesting that CsNoxs are not associated with conidiation. Collectively, these results suggest that the CsNoxs are dispensable for mycelial growth and conidiation of C. scovillei.
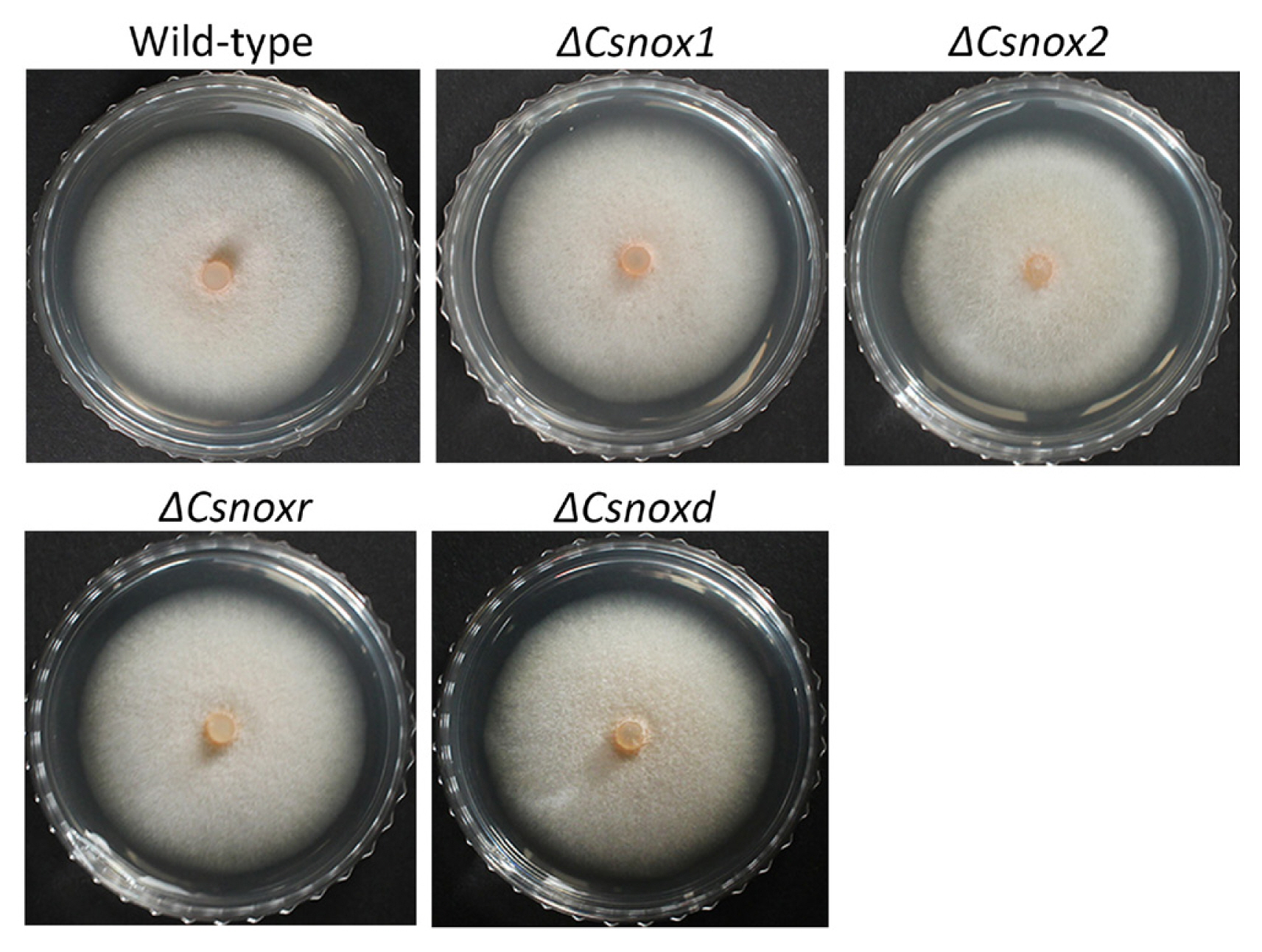
Mycelial growth and conidiation assays. (A) Photographs of mycelial growth. Three-day old mycelial agar plugs (5 mm in diameter) from minimal media agar were inoculated onto potato dextrose agar (PDA) and incubated at 25°C for 5 days. (B) Measurement of mycelial growth. Diameters of colony growth on PDA were measured after 5-day incubation at 25°C.
Requirement of Nox2 and NoxR for internal light spot formation during appressorium development
We further investigated whether CsNoxs are related to appressorium development in response to the hydrophobic surface. The result showed that 94.1 ± 2.2% and 84.9 ± 3.4% of conidia in wild-type germinated and developed appressoria after 12 and 16 h, respectively (Table 1). All deletion mutants were identical in conidial germination and appressorium formation (Table 1), suggesting that CsNoxs are not related to appressorium formation of C. scovillei, in response to hydrophobic surface. During appressorium development, the internal light spot (ILS) was reported to correspond to appressorium pore and penetration peg development in Colletotrichum acutatum (Diéguez-Uribeondo et al., 2003; Wharton and Schilder, 2008). Therefore, we observed the ILS during appressorium development in response to hydrophobic surface. The wild-type, ΔCsnox1, ΔCsnoxd, and all complemented strains were found to from the ILS in appressoria, while ΔCsnox2 and ΔCsnoxr did not show ILS (Fig. 4), suggesting that CsNox2 and CsNoxR are required for ILS formation. These results suggested that Nox genes are dispensable for appressorium formation, whereas CsNox2 and CsNoxR are required for ILS formation during appressorium development.
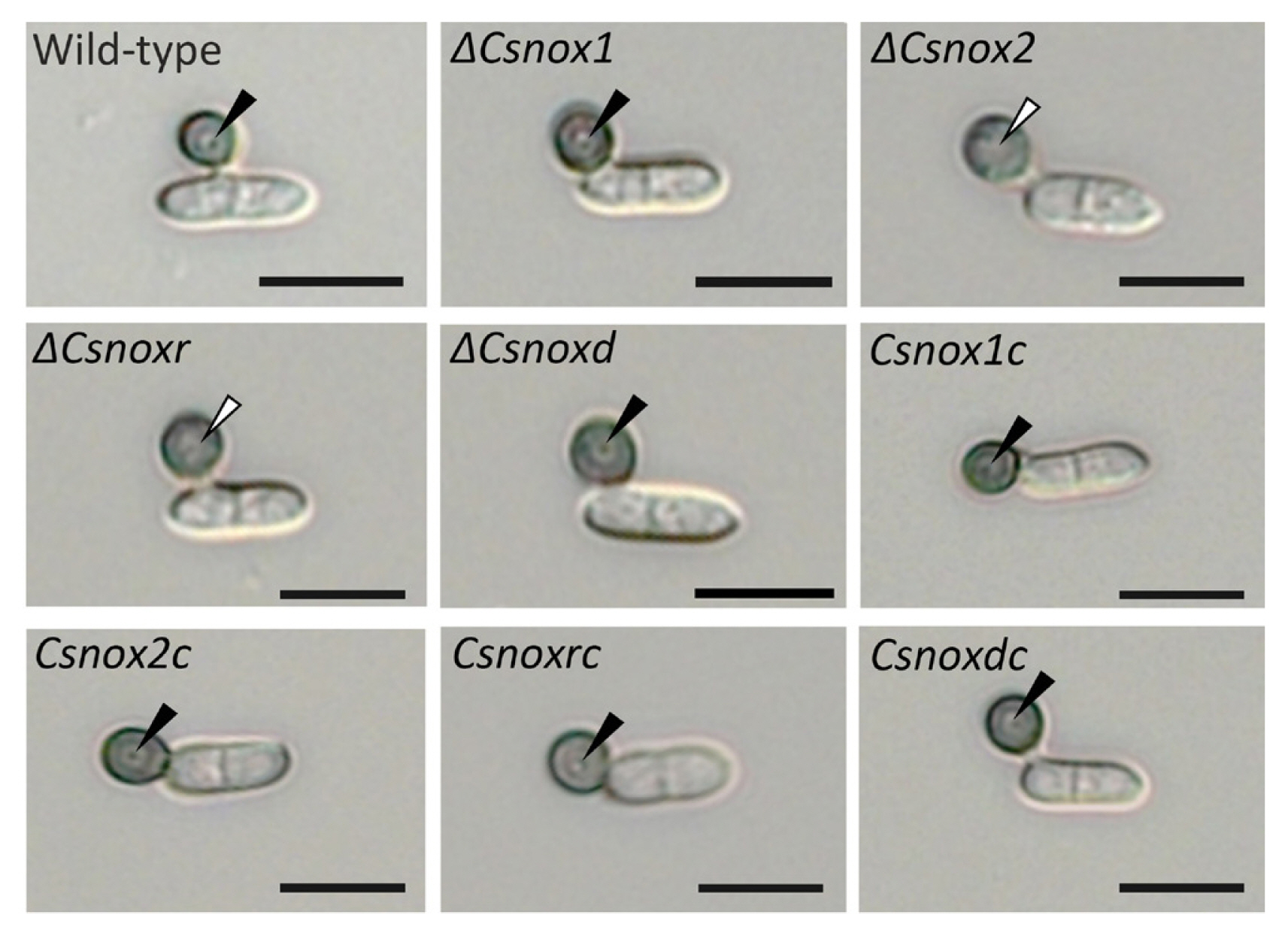
Appressorium formation assay. (A) Observation of appressorium development. Conidia obtained from 7-day-old oatmeal agar were placed on the hydrophobic surface of coverslips to form appressoria. Photographs were taken after 16-h incubation in humid box at 25°C. Black and white triangle indicates the presence and absence of internal light spot (ILS), respectively. Scale bars = 10 μm.
Requirement of Nox2 and NoxR for anthracnose disease development
To determine whether Nox genes are related to anthracnose development, the conidial suspensions were inoculated onto both intact and wounded pepper fruits. The ΔCsnox1 and ΔCsnoxd caused typical anthracnose disease on both intact and wounded pepper fruits, similar to that caused by wild-type and each corresponded complemented strain (Fig. 5), suggesting that CsNox1 and CsNoxR are not related to pathogenicity of C. scovillei. ΔCsnox2 and ΔCsnoxr caused anthracnose disease on wounded pepper fruits, similar to that caused by the wild-type and the corresponded strains (Fig. 5). However, ΔCsnox2 and ΔCsnoxr failed to cause lesions on intact pepper fruits (Fig. 5), suggesting that CsNox1 and CsNoxR are required for anthracnose development.
Requirement of Nox2 and NoxR for penetration on host and artificial surface
We further perform microscopic observation to investigate the penetration process of ΔCsnox2 and ΔCsnoxr on intact pepper fruits. The wild-type, ΔCsnox1, ΔCsnoxd, and all complemented trains induced dendroid structures in cuticle layers of pepper fruits (Fig. 6). However, ΔCsnox2, and ΔCsnoxr failed to induce dendroid structures, while they adequately formed appressoria (Fig. 6). We further performed SEM to study the appressorium-mediated penetration. The penetration hole in the wild-type, ΔCsnox1, and ΔCsnoxd infected pepper fruits could be clearly observed, while ΔCsnox2 and ΔCsnoxr failed to generate penetration holes (Fig. 7A). This result suggests that CsNox2 and CsNoxR are critical for appressorium-mediated penetration on host surface.

Observation of appressorium-mediated penetration. The conidial suspensions were inoculated onto intact pepper fruits and incubated in a humid box at 25°C for 3 days. Thin segments of inoculated area were sliced and observed. Co, Ap, and DS indicates conidium, appressorium, and dendroid structure, respectively. Scale bar = 10 μm.
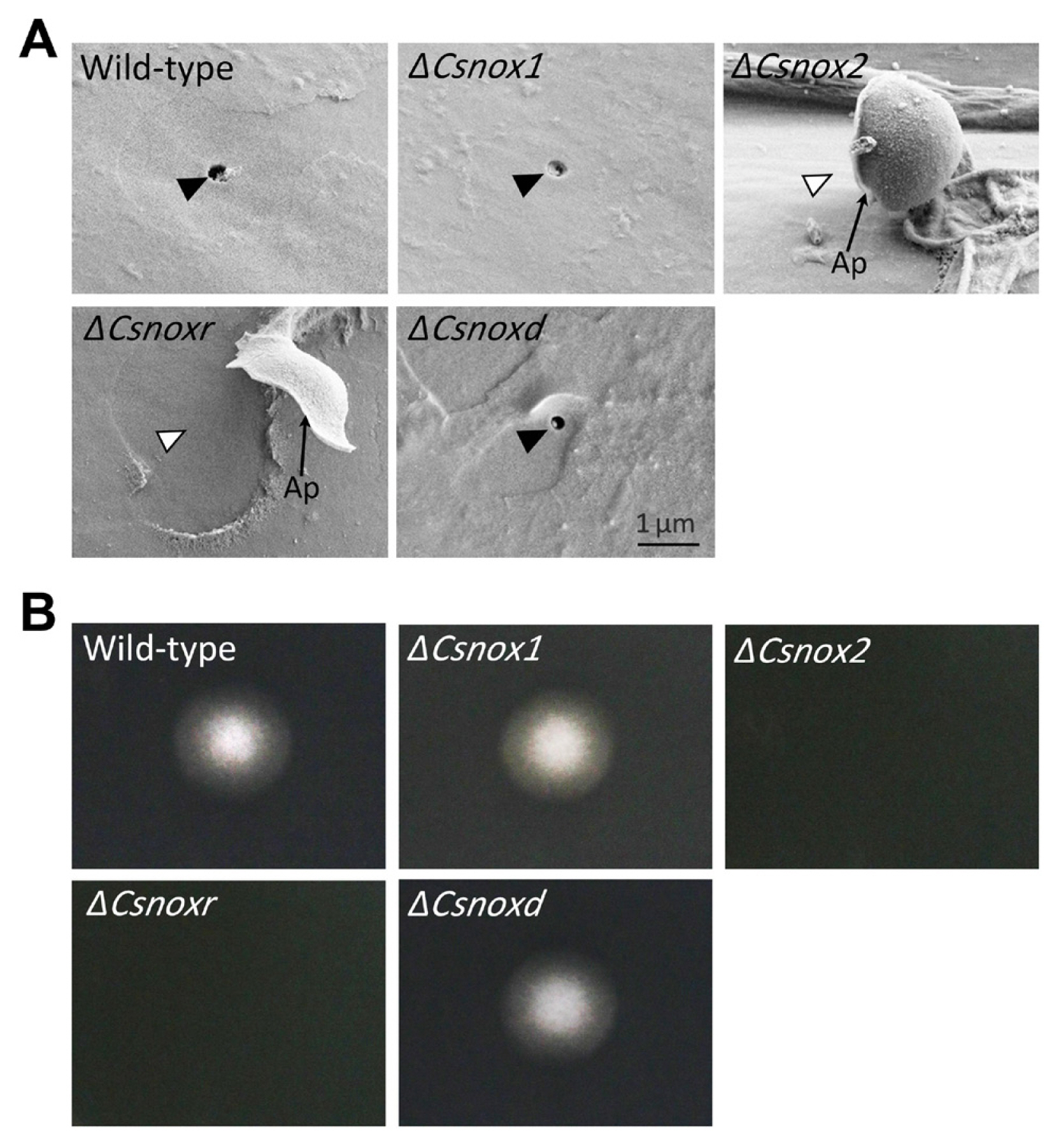
Penetration on host and artificial surfaces. (A) Penetration onto pepper fruits. Conidial suspensions were inoculated onto intact pepper fruits and incubated in a humid box at 25°C for 3 days. The samples were observed using a scanning electron microscope (Analytical HR-SEM). Ap indicates appressorium. Black and white triangle indicates presence and absence of holes generated by penetration peg, respectively. Scale bar = 1 μm. (B) Penetration onto cellophane membrane. Conidial suspensions were inoculated onto cellophane membranes, which are placed on potato dextrose agar (PDA). The cellophane membranes were removed after a 3-day incubation at 25°C. The PDA medium was additionally cultured for 2 days.
To further investigate roles of CsNox2 and CsnNoxR in appressorium-mediated penetration of C. scovillei. We dropped conidia suspensions onto cellophane membranes, which were placed on PDA. After removing cellophane membrane and culturing 2 days more, the wild-type, ΔCsnox1 and ΔCsnoxd showed mycelial growth on PDA, revealing that they successfully penetrated onto cellophane membrane (Fig. 7B). However, ΔCsnox2 and ΔCsnoxr failed to penetrate onto cellophane membrane, because no mycelial growth could be observed on PDA (Fig. 7B), suggesting that CsNox2 and CsNoxR are required for penetration onto cellophane membrane. Together, these results suggest that CsNox2 and CsNoxR are essential for appressorium-mediated penetration of C. scovillei on both host and artificial surface.
Discussion
The Nox genes (CsNox1, CsNox2, CsNoxR, and CsNoxD) are dispensable for fungal developments, including mycelial growth, conidiation, conidial germination, and appressorium formation of C. scovillei. However, those genes were previous demonstrated to be essential for growth, proliferation, and differentiation in many endophytes (Cano-Domínguez et al., 2008; Kayano et al., 2013; Lara-Ortíz et al., 2003). For example, noxA and noxR are associated with hyphal fusion and conidiation in E. festucae (Kayano et al., 2013). Deletion of noxA blocked development of fruiting body in A. nidulans, whereas inactivation of noxA did not affect sexual development but reduced conidiation and hyphal growth in N. crassa (Cano-Domínguez et al., 2008). These results suggest that Nox gens may play specific roles in fungal developments among different fungal species.
The ΔCsnox2 and ΔCsnoxr were normal in appressorium formation and anthracnose formation on wounded pepper fruits (Table 1, Fig. 5), suggesting that ΔCsnox2 and ΔCsnoxr may be defective in penetration peg development. The failures in developing ILS (Fig. 4) and penetrating cellophane membrane and host cuticle layer (Figs. 6 and 7) strongly indicate that ΔCsnox2 and ΔCsnoxr are defective in penetration peg development. The ILS is known to associate with penetration pore and is required for penetration peg differentiation of Colletotrichum acutatum (Diéguez-Uribeondo et al., 2003; Wharton and Schilder, 2008). Molecular mechanisms underlying Nox2 and NoxR-dependent penetration were extensively studied in M. oryzae (Ryder et al., 2013; Segmülle et al., 2008). The penetration peg initiates after the reorganization of F-actin during turgor pressure buildup in appressorium (Dagdas et al., 2012). Treatment with a Nox inhibitor (diphenyleneiodonium chloride), or deletion of Nox2 or NoxR resulted in abnormal localizations of gelsolin and septin, which are regulators of F-actin reorganization (Ryder et al., 2013). Later, the Nox2 and NoxR-dependent penetration was found to be controlled by a Zn(II)2Cys6 transcription factor Tpc1 via directly regulating the expression of NoxD (Galhano et al., 2017). Tpc1 physically interacts with Mst12, which is regulated by Pmk1-type MAP kinase during appressorium repolarization (Galhano et al., 2017). Thus, the Noxs should function in the downstream of Pmk1-dependent signaling pathway during appressorium repolarization and penetration peg initiation (Kou et al., 2019). Furthermore, Noxs-dependent appressorium repolarization is associated with autophagy in M. oryzae (Galhano et al., 2017). Although Nox2 and NoxR are required for penetration peg initiation in both C. scovillei and M. oryzae, generation of high turgor pressure inside appressorium and autophagic cell death of conidium do not occur in C. scovillei (Fu et al., 2021). Therefore, the underlying mechanisms of CsNox2 and CsNoxR in C. scovillei are still attractive for further investigation.
Unlike Nox2, which is essential for penetration peg differentiation, deletion of CsNox1 did not cause obvious defects in developments of ILS, penetration peg, and anthracnose disease of C. scovillei (Figs. 4–7). Intriguingly, the ΔbcnoxA in B. cinerea could successfully penetrate but failed to colonize in host tissues (Segmüller et al., 2008). The Δnox1 in M. oryzae was non-pathogenic because it was unable to elongate the penetration peg (Ryder et al., 2013). The Δnoxa in V. dahlia still caused disease but severely reduced in its virulence (Zhu et al., 2021). These findings suggest that Nox1 may play divergent roles among different plant pathogenic fungi, and the Nox1 may be a functional redundancy of the Nox2 in C. scovillei. The adapter protein NoxD was reported to directly interact with Nox1, and both of Δnox1 and ΔnoxD in B. cinerea showed same phenotypes, including defects in fusion of conidial anastomosis tubes, formation of sclerotia and conidia, and pathogenicity (Siegmund et al., 2015). In M. oryzae, the ΔnoxD showed similar phenotypes on mycelial growth in response to chemical stresses compared to Δnox1 and was significantly impaired in appressorium-mediated penetration and (Ryder et al., 2013). In our study, ΔCsnoxd was normal in fungal developments and pathogenicity, which is in accordance with phenotypes of ΔCsnox1. These findings reveal that Nox1 and the adapter NoxD may play same functional roles within a fungal species, but their orthologs are functionally diverse among different fungi.
Our study represents the first attempt to investigate functional roles of Nox complexes in C. scovillei. Notably, the CsNox2 and CsNoxR regulate penetration peg differentiation, whereas CsNox1 and CsNoxD are not associated with fungal developments and pathogenicity of C. scovillei. Our research would contribute to better understand of Nox proteins in anthracnose development by Colletotrichum fungi.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea grant (NRF-2020R1A2C100550700) funded by the Ministry of Education, Science and Technology, and by a grant (918019043HD020) from the Strategic Initiative for Microbiomes in Agriculture and Food, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).


