Cytochrome b Gene-Based Assay for Monitoring the Resistance of Colletotrichum spp. to Pyraclostrobin
Article information
Abstract
Resistance to pyraclostrobin due to a single nucleotide polymorphism at 143rd amino acid position on the cytochrome b gene has been a major source of concern in red pepper field infected by anthracnose in Korea. Therefore, this study investigated the response of 24 isolates of C. acutatum and C. gloeosporioides isolated from anthracnose infected red pepper fruits using agar dilution method and other molecular techniques such as cytochrome b gene sequencing, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), and allele-specific polymerase chain reaction (PCR). The result showed that four isolates were resistant to pyraclostrobin on agar dilution method and possessed GCT (alanine) codon at 143rd amino acid position, whereas the sensitive isolates possessed GGT (glycine). Furthermore, this study illustrated the difference in the cytochrome b gene structure of C. acutatum and C. gloeosporioides. The use of cDNA in this study suggested that the primer Cacytb-P2 can amplify the cytochrome b gene of both C. acutatum and C. gloeosporioides despite the presence of various introns in the cytochrome b gene structure of C. gloeosporioides. The use of allele-specific PCR and PCR-RFLP provided clear difference between the resistant and sensitive isolates. The application of molecular technique in the evaluation of the resistance status of anthracnose pathogen in red pepper provided rapid, reliable, and accurate results that can be helpful in the early adoption of fungicide-resistant management strategies for the strobilurins in the field.
Red pepper (Capsicum annuum) is believed to have originated from American Tropics (Pickersgill, 1997) from where it was spread to other parts of the World by early explorers in the sixteenth century (Greenleaf, 1986). The importance of red peppers to human health cannot be overemphasized. This is because it contains various vitamins and minerals which are beneficial to human health (Ganguly et al., 2017; Howard et al., 2000; Pawar et al., 2011). Over the years, red pepper production has been faced with a big problem of anthracnose disease caused by Colletotrichum species which causes significant yield loss and a decrease in fruit marketability (De Silva et al., 2017). Reports showed that Colletotrichum can cause a yield loss of 50% to 100% under severe conditions (Prusky, 1996; Ramchandran et al., 2007). In Korea, red pepper anthracnose caused by Colletotrichum species resulted in a monetary loss of approximately $100 million which accounted for almost 10% of the total annual production (Kang et al., 2009).
Colletotrichum species such as C. fructicola, C. siamens, C. truncatum, C. capsici, C. gloeosporioides, C. acutatum and many more are associated with red pepper anthracnose in different parts of the world (Harp et al., 2008; Kim et al., 1999; Ranathuge et al., 2012; Sharma and Shenoy, 2014; Than et al., 2008). Colletotrichum species are considered as one of the major destructive fungal pathogens causing anthracnose of fruits in the tropical regions (Agrios, 2005; Cannon et al., 2012; Noireung et al., 2012). A survey conducted by Dean et al. (2012) ranked Colletotrichum species as the eighth most economically and scientifically important plant pathogenic fungi. Initially, Colletotrichum species were differentiated into C. acutatum and C. gloeosporioides based on morphological characteristics such as conidia shape (Simmonds, 1965). However, it was observed by various researchers that it was difficult to differentiate some species due to the presence of intermediate strains and overlapping characteristics (Kim et al., 2008; Van der Aa et al., 1990). Recently, Damm et al. (2012) and Weir et al. (2012) revised the classification of Colletotrichum species using molecular techniques by the comparison of DNA sequences of some regions and genes such as the internal transcribed spacer, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-tubulin (TUB2), actin (ACT), chitin synthase 1 (CHS-1), and/or histone3 (HIS3) genes. They reported C. acutatum and C. gloeosporioides as species complexes with 31 and 22 species respectively. Members of the C. acutatum species complex includes C. acutatum, C. fioriniae, C. nymphaeae, C. scovillei while Colletotrichum gloeosporioides species complex include C. fructicola, C. gloeosporioides, C. siamense, C. horii, etc.
To control red pepper anthracnose, there are some control methods such as a chemical fungicide application, a cultural control method, and the use of resistant varieties. Among them, since the excellent controlling activity appears quickly in the field where pepper is grown, the use of fungicides is regarded as the easiest control method. As fungicides for controlling red pepper anthracnose, protective fungicides, benzimidazoles, triazoles, and strobilurins have been registered for field treatment. Among the strobilurin fungicides that showed excellent efficacies in controlling red pepper anthracnose, azoxystrobin and kresoxim-methyl were first marketed worldwide in 1996, and pyraclostrobin has been used since 2002. Although pyraclostrobin had an excellent efficacy against red pepper anthracnose, there have been reports that resistant pepper anthracnose pathogen has been developed in Korea from 2015 (Kim et al., 2019). Therefore, there is the need to monitor the resistant isolates of Colletotrichum spp. causing red pepper anthracnose in the field for effective management strategy.
Strobilurin fungicides known as quinone outside inhibitors (QoIs) were designed to target the respiratory system of the fungal organisms by inhibiting electron transfer at the quinone outside site of the cytochrome b of the mitochondria complex III (Brent, 2012). In these group of fungicides, mutation at the 143 amino acid position from glycine to alanine (G143A) or at 129 amino acid position from phenylalanine to leucine (F129L) and/or substitution of glycine by arginine at 137 amino acid position (G137R) confers a fungicidal resistance of the pathogen (Edin and Torriani, 2012). Among these three mutation sites, G143A is the strongest and most frequent which confers complete resistance while F129L and G137R confer partial resistance. Strobilurin fungicides have been listed by Fungicide Resistance Action Committee as fungicides with ‘High Risk’ of resistance occurrence.
The use of an appropriate evaluation method to determine the sensitivity or resistance of plant pathogens to a certain fungicide is crucial in plant disease management. Thus, various methods have been developed for the monitoring and evaluation of fungicide resistance for field and laboratory application. These methods include in-vivo and in-vitro tests such as micro-titer plate test, agar plate test, leaf segment test (detached leaf test), germ tube elongation test, spore germination test, whole plant test, as well as molecular detection of mutation using allele-specific polymerase chain reaction, allele-specific real-time polymerase chain reaction (PCR), PCR-restriction fragment length polymorphism (PCR-RFLP), primer-introduced restriction analysis PCR, and quantitative sequencing (Fungicide Resistance Action Committee, 2012; Ma and Michailides, 2005). If the fungicide resistance monitoring is carried out using the traditional methods for example the agar dilution method, which directly uses the isolates of Colletotrichum spp. collected through a single spore isolation method from the diseased plant tissue, a lot of time and effort are required. For this reason, if traditional methods are used to monitor fungicides resistance, it may be too late for the results to be fed back to the field where the fungicides are used. Compared to the traditional resistance monitoring method, the molecular technology uses an isolated pathogen, but has the advantage of being able to obtain accurate and rapid results by testing the fungicide resistance gene. Cytochrome b gene amplification and sequencing, PCR-RFLP and allele-specific PCR have already been established for monitoring the resistance of pyraclostrobin on C. acutatum, the causal pathogen of red pepper anthracnose in Korea (Kim et al., 2019). However, since the allele-specific PCR method by Kim et al. (2019) was established for only C. acutatum, there is the need to evaluate the same method on C. gloeosporioides, which has been identified as the causal pathogen of anthracnose in various crops including red pepper. Therefore, in this study, the cytochrome b gene structure of C. acutatum and C. gloeosporioides isolated from diseased red pepper fruits was identified, and allele-specific PCR and PCR-RFLP methods that could simultaneously detect resistance to pyraclostrobin in both pathogens were established.
Materials and Methods
Fungal isolates used
Colletotrichum species isolated from pepper in 2015/2016 using single spore isolation method and stored in the Fungal laboratory, Department of Plant Medicine, Chungbuk National University were used for the experiment as shown in Table 1.
Strobilurin fungicide (QoI) sensitivity test
The response of the Colletotrichum isolates was investigated using the ADM with pyraclostrobin (a.i. 20%, SC). The pyraclostrobin fungicide was serially diluted in sterile distilled water (SDW) to a pre-determined concentration (10, 2, 0.4, 0.08 and 0.016 μg/ml) and used to amend the potato dextrose agar (PDA; Becton, Dickinson and Company, Difco, USA). A 3-mm mycelial disc taken from the edge of a 7-day-old colony of the isolates was transferred onto the PDA plates amended with each of the above concentrations. The mycelial disc was inverted on the freshly amended PDA. The plates were incubated for 7 days at 25°C under the dark condition. Untreated PDA served as a standard check and three replicates were maintained for each isolate. The colony diameter for each isolate at various concentrations was measured and recorded. The mycelial growth inhibitory effect of each fungicide concentration was calculated by comparing the colony diameter of isolates grown on pyraclostrobin amended PDA with the unamended control using the formula below.
Fungal genomic DNA extraction
Total genomic gDNA was extracted from mycelia obtained from PDA culture of Colletorichum spp. grown at 25°C for 7 days. Aerial mycelia were harvested from culture plates using a sterile transfer needle and placed in a sterile 1.5 ml microcentrifuge tube containing 300 μl of extraction buffer (0.2 M Tris-HCl, 0.25 M NaCl, 25 mM EDTA, and 2 ml sodium dodecyl sulfate, pH 8.5). Uncapped tubes were placed on a boiling water bath for 5 min and then cooled to 25°C. Two hundred μl of phenol that was calibrated with extraction buffer (v/v) and 200 μl of chloroform was added. The tubes were vortexed for 4 min and then centrifuged at 13,000 rpm for 15 min. The supernatant was extracted with 200 μl of isopropanol and centrifuged at 13,000 rpm for 15 min. The nucleic acid pellet, after washing with 70% ethanol, followed by air-drying for 15 min, was re-suspended in 50 μl of TE buffer (10 mM Tris-HCl and 0.1 mM EDTA, pH 8.5). DNA was finally treated with ribonuclease A.
Amplification of the cytochrome b gene using the gDNA
The cytochrome b gene of C. acutatum was amplified using the primer Cacytb-P2 (CAT AGT AAY ACA GCT TCT G) and Cacytb-R (GGA ATA GAT CTT AAT ATA GC) (Kim et al., 2019). A 20 μl PCR mixture containing 4 μl master mix (EzPCR 5× PCR master mix, Elpis Biotech, Daejeon, Korea), 1 μL reverse and forward primer each, 2 μl gDNA and 12 μl SDW was prepared. The amplification was conducted as follows; initial denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 37°C for 30 s, and extension at 72°C for 1 min, and a final extension at 72°C for 7 min. On the other hand, the gene of C. gloeosporioides was amplified using the primer CF1 (ATC TAC CAA GAC AAG ACC GTC) and CR2 (TCT TGT CCA ATT CAT GGG ATA GC) developed by Hu et al. (2015). The amplification was conducted as follows: initial denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 52°C for 1 min, and extension at 72°C for 1 min, and a final extension at 72°C for 7 min. The PCR products were separated by electrophoresis in a 1% agarose gel in tris-borate (TBE) buffer for 40 min at 100 V. The PCR products were purified using the Expin SV PCR Mini (GeneAll Biotechnology, Seoul, Korea) and sent for sequencing at Macrogen in Korea. The sequence data was analyzed using Mega X software version 10.1.8.
RNA extraction and cDNA synthesis
The RNA was extracted from mycelia harvested from the colony on PDA incubated at 25°C for 7 days. Aerial mycelia were placed in a sterile 2 ml microcentrifuge tube. After putting the glass beads into the tubes with mycelia and sealing it with a parafilm, it was freeze-dried in a freeze dyer (Ilshin BioBase, Yangju, Korea) for 24 h. The dried mycelia were grinded in a bead beater (Next Advance Inc., Raymertown, NY, USA) and 1 ml RiboEx was added and homogenized. Hybrid-R RNA Extraction kit (GeneAll Biotechnology) was used to extract the RNA and the protocols were followed as described in the kit manual. The concentration and quality of the RNA was evaluated using NanoDrop (K Lab Co. Ltd., Gunpo, Korea), the concentration adjusted to 50 μg/ml and stored at −20°C until needed. The cDNA was synthesized from the extracted total RNA using a 20 μl mixture of; 4 μl 5× reverse transcription master mix (Elpis Biotech), 11 μl SDW and 5 μl total RNA. The incubation reaction was performed in a thermal cycler (i-cycler version 3.021, Bio-Rad, Hercules, CA, USA) for 1 h at 37°C and followed by 94°C for 5 min.
Cytochrome b gene PCR using the cDNA
For the amplification of the cytochrome b gene, the primer Cacytb-P2 (CAT AGT AAY ACA GCT TCT G) and Cacytb-R (GGA ATA GAT CTT AAT ATA GC) was used, which were the same used in C. acutatum. Amplification, electrophoresis, and separation of cytochrome b gene were performed by the same method as described above. The nucleotide sequence analysis of cytochrome b gene was performed by requesting the amplified PCR product to Microgen. The sequence data was analyzed using MEGA X software version 10.1.8.
Allele-specific PCR
Allele-specific PCR was performed using the forward primer CacytbF (GGG TAT AGG TTT CCT GGG TTA TG) and the reverse primer S-mmc (ATA AGG TTA GTA ATA ACT GTT GCC C) for the amplification from sensitive isolates and R-mmc (ATA AGG TTA GTA ATA ACT GTT GCC G) for the amplification from resistant isolates (Kim et al., 2019). The primers were designed to specifically amplify a single point mutation at 143 amino acid position. A 20 μl PCR mixture containing 4 μl master mix (EzPCR 5× PCR master mix, Elpis Biotech), 1 μl of each primer (forward and reverse), 2 μl gDNA, and 12 μl SDW was prepared. The amplification was performed in a thermo-cycler (MiniAmp Plus, Thermo Fisher Scientific, Waltham, MA, USA) with an initial denaturation at 95°C for 4 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension 72°C for 1 min 20 s, and a final extension at 72°C for 5 min. To confirm the amplification of the genes by PCR, 1.5% agarose gel was made with 0.5× TBE buffer, and safe view classic (Applied Biological Materials Inc., Richmond, BC, Canada) was added at 5 μl per 100 ml of agarose solution. The PCR products were separated by electrophoresis for 40 min at 100 V and the gel was viewed on a UV-transilluminator.
PCR-RFLP
A PCR-RFLP was conducted using representative sample of resistant C. gloeosporioides (16CAYY19, 16CACY14, and 15KJ1), sensitive C. gloeosporioides (16CACY8, 16CASJ3, 15JD8, 16CACC1, and 16CACS10), resistant C. acutatum (15CY12) and sensitive C. acutatum (16CABH1, 15JE5, and 16GS4). The SatI (Fnu4HI) restriction enzyme that restrict 5′GC/NGC3′ and 3′CG/NCG5′ was used for the experiment. The PCR was conducted using the primer Cacytb-P2 and Cacytb-R as described above. Furthermore, a 30 μl volume reaction consisting of 17 μl SDW, 10 μl PCR product, 10× buffer and 1 μl Fun4HI restriction enzyme was prepared. The mixture was incubated in a thermal cycler at 37°C for 1 h, followed by inactivation at 65°C for 20 min. The products were separated by electrophoresis for 40 min at 100 V and the gel was viewed in a UV-transilluminator.
Results
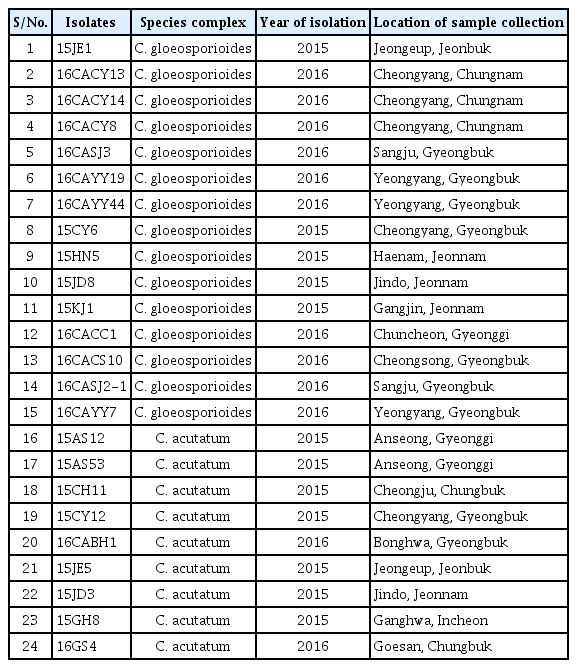
Response of Colletotrichum species to pyraclostrobin
Experiments conducted using ADM to evaluate the response of Colletotrichum species isolated from diseased red pepper fruits in Korea showed that four isolates were resistant to pyraclostrobin as shown in Fig. 1. The sensitive isolates had a mycelial inhibition rate of greater than 70% at 10 μg/ml, while the resistant isolates had an inhibition rate of less than 40% at the same concentration. Further analysis of the result based on earlier identification showed that three of the resistant isolates (16CACY14, 16CAYY19, and 15KJ1) were identified as C. gloeosporioides (Fig. 1A). On the other hand, one of the resistant isolates (15CY12) was identified as C. acutatum (Fig. 1B).
Response of Colletotrichum gloeosporioides (A) and C. acutatum (B) isolated from diseased fruits of red pepper to pyraclostrobin investigated by agar dilution method. Isolates of C. gloeosporioides and C. acutatum were inoculated on potato dextrose agar (PDA) amended with/without the fungicide at indicated concentrations and incubated for 7 days at 25°C. The effect of pyraclostrobin was investigated by calculating the ratio of the colony diameter (mm) on PDA with the fungicide at each concentration to that of the PDA without the fungicide.
Amplification of the cytochrome b gene of Colletotrichum species
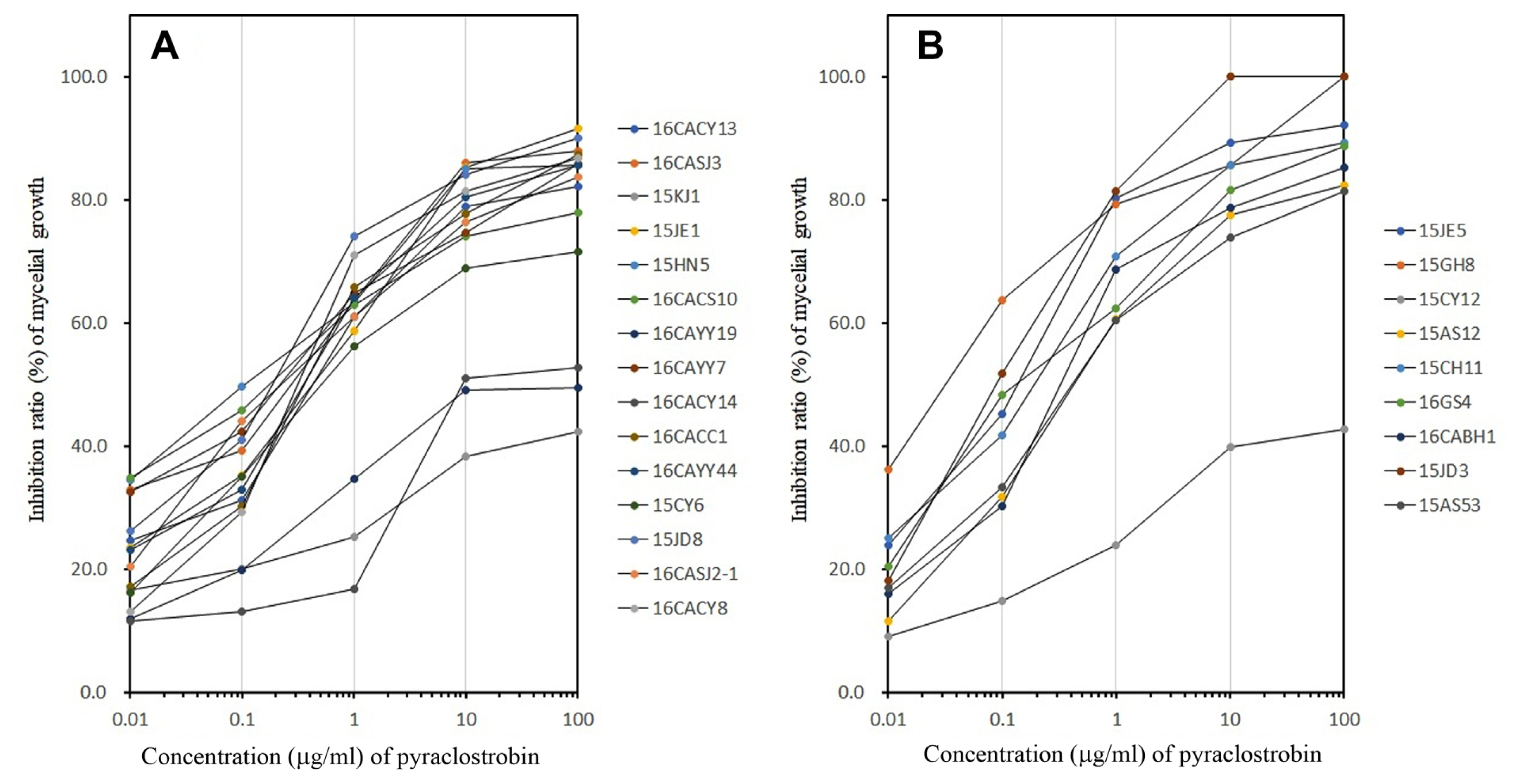
The amplification of the cytochrome b gene of Colletotrichum species was conducted using the primers Cacytb-P2/Cacytb-R and CF1/CR2 for C. acutatum and C. gloeosporioides, respectively. The result of the gel electrophoresis showed that 617 bp fragments were amplified from nine isolates identified as C. acutatum as shown in Fig. 2A. On the other hand, 295 bp fragments were amplified from 14 isolates identified as C. gloeosporioides whereas 936 bp fragment was amplified from one isolate as shown in Fig. 2B. To overcome the challenge of using different primers in the amplification of the cytochrome b gene of both C. acutatum and C. gloeosporioides, the cDNA synthesized from the RNA was used to amplify 617 bp fragments from both species as shown in Fig. 3.

Amplification of cyt b gene of Colletotrichum gloeosporioides and C. acutatum using the primer pair CacytbP2/CacytbR (A) and CF1/CR2 (B). From the mycelia cultured for 7 days on potato dextrose agar at 25°C, gDNA was extracted and used as a template for cyt b gene amplification. Gene amplification was performed using the CacytbP2/CacytbR primer pair designed in C. acutatum and the CF1/CR2 primer pair designed in C. gloeosporioides. N, negative control; M, DNA size marker (100 bp plus DNA ladder).
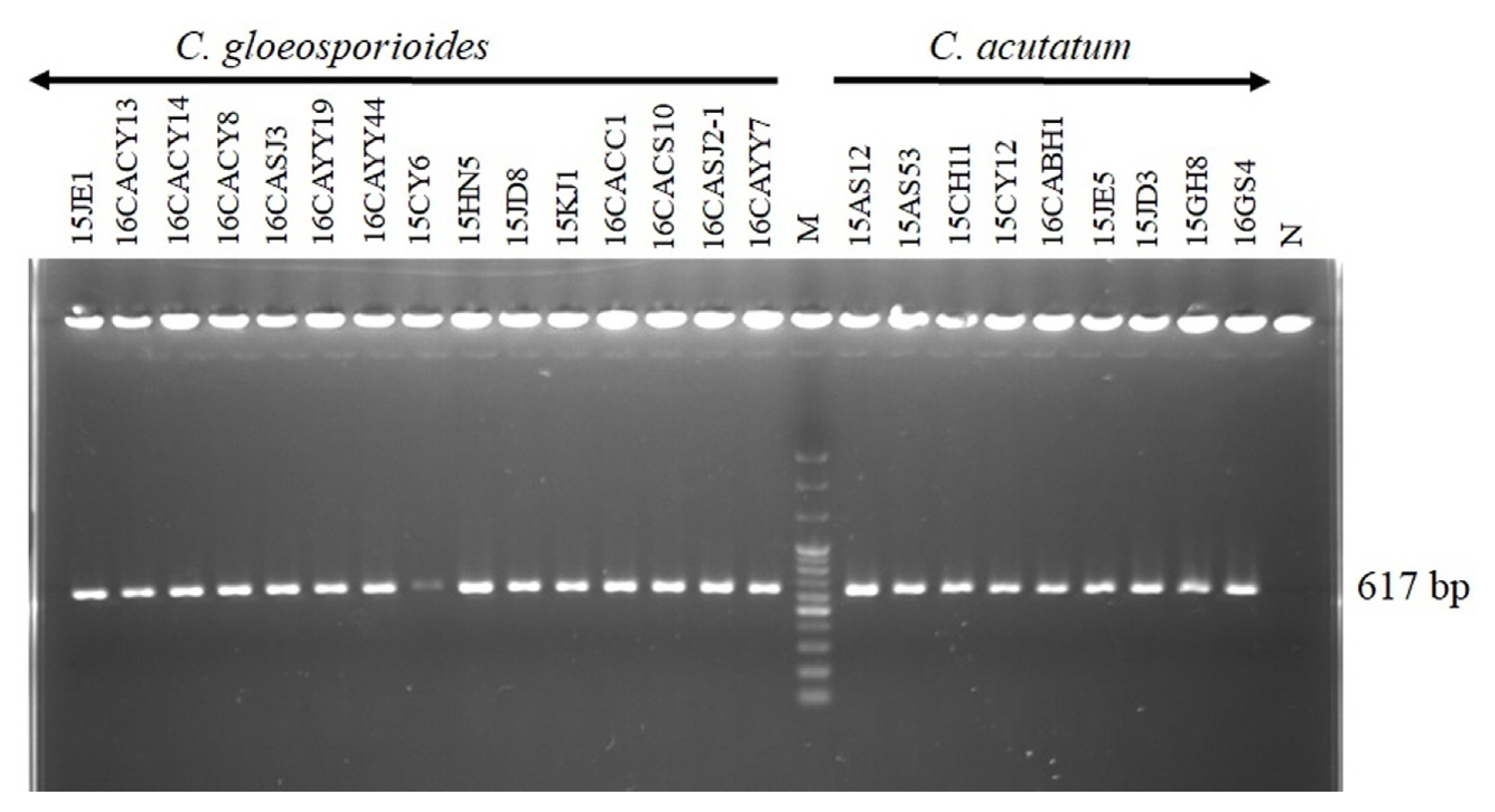
Amplification of cyt b gene of Colletotrichum gloeosporioides and C. acutatum using cDNA as a template. To amplify a gene, cDNA was synthesized using RNA extracted from C. gloeosporioides and C. acutatum and used as a template. Cytochrome b gene was amplified using CacytbP2/CacytbR primer pair designed in C. acutatum. N, negative control; M, DNA size marker (100 bp plus DNA ladder).
Analysis of the cytochrome b gene of Colletotrichum species
Analysis of the aligned sequences of the cytochrome b gene of C. gloeosporioides and C. acutatum showed that all 4 isolates (16CACY14, 16CAYY19, 15KJ1, and 15CY12) that showed resistance to the pyraclostrobin possessed a single point mutation at 143rd amino acid position, regardless of the species of Colletotrichum. The nucleotide sequence analysis revealed that the resistant isolates possessed GCT (alanine) while the other 20 isolates that were sensitive to pyraclostrobin possessed GGT (glycine) at the same position as shown in Fig. 4.

Aligned nucleotide (A) and amino acid (B) sequences of the cytochrome b gene for Colletotrichum species isolated on anthracnose infected pepper fruits in Korea. A mutation at codon 143 from glycine to alanine that conferred resistance to pyraclostrobin was observed on 16CACY14, 16CAYY19, 15KJ1, and 15CY12.
Allele-specific PCR
The result showed that the primer CacytbF/R-mmc successfully amplified 78 bp of PCR product from the resistant isolates 16CACY14, 16CAYY19, 15KJ1, and 15CY12 (Fig. 5A). On the other hand, the primer CacytbF/S-mmc amplified 78 bp of product from the sensitive isolate (16CACY8, 16CASJ3, 16CACY13, 16CAYY44, 15CY6, 15HN5,15JD8, 16CACC1, 16CACS10, 16CASJ2-1, 16CAYY7, 15AS12, 15AS53, 15CH11, 16CABH1, 15JE5, 15JD3, 15GH8, and 16GS4) of the Colletotrichum species used in this study as shown in Fig. 5B.
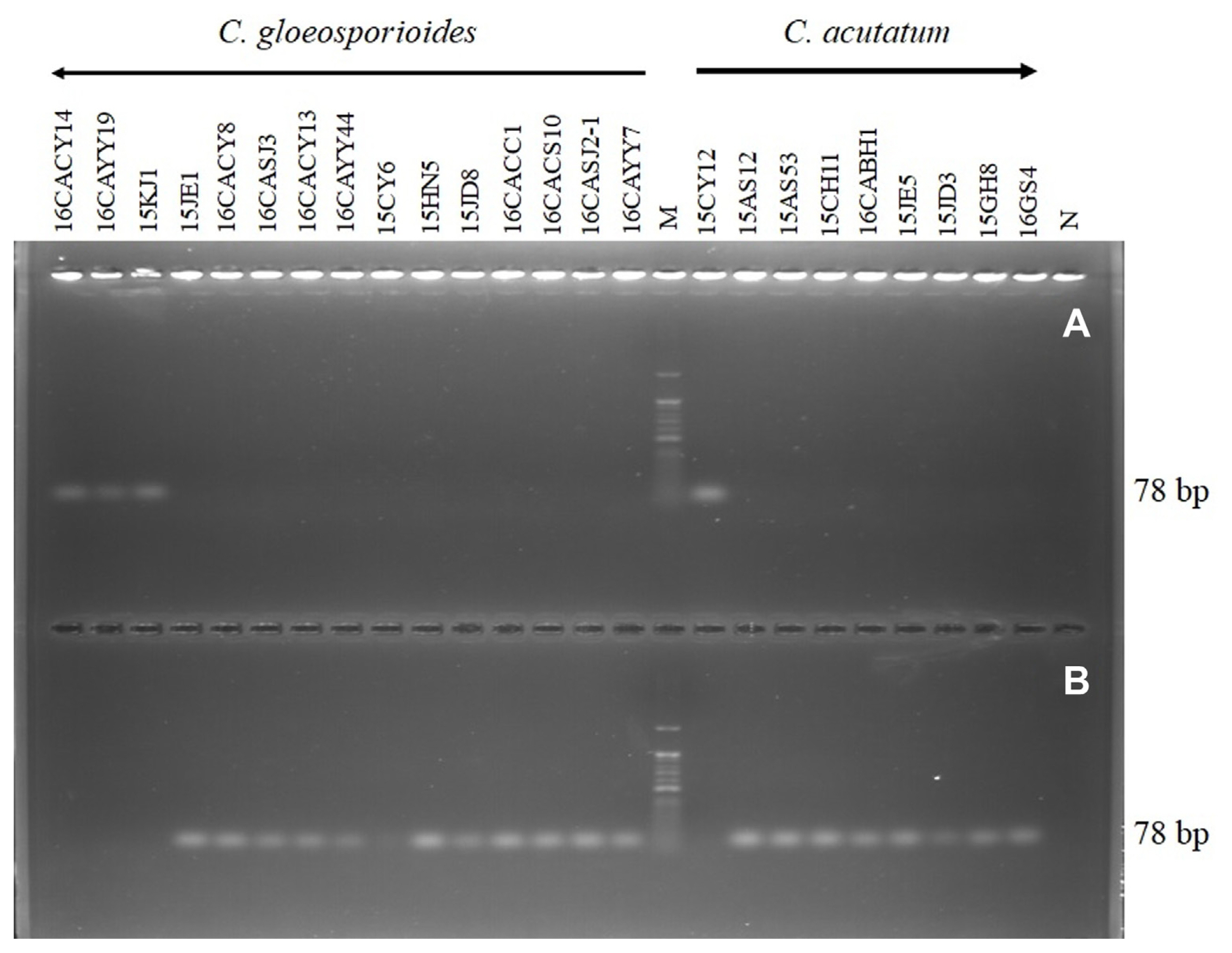
Allele-specific polymerase chain reaction (PCR) amplification of the G143A cyt b allele. DNA extracted from pyraclostrobin resistant (A), and sensitive (B) isolates of Colletotrichum gloeosporioides and C. acutatum respectively was used as templates for the PCR amplifications which used primer CacytbF in combination with allele-specific primers RMMC and SMMC that specifically bind to the mutant cytochrome b sequence. N, negative control; M, DNA size marker (100 bp plus DNA ladder).
PCR-RFLP
The primer Cacytb-P2/Cacytb-R amplified 617 bp fragment from Colletotrichum isolates used in this experiment. Furthermore, the restriction enzyme Fnu4HI restricted the PCR products of both the isolates of C. gloeosporioides and C. acutatum resistant to pyraclostrobin to generate three amplicons of 275, 200, and 142 bp as shown in Fig. 6. On the contrary, the sensitive isolates of both C. gloeosporioides and C. acutatum were restricted at one position to generate amplicons of 342 and 275 bp as shown in Fig. 6.
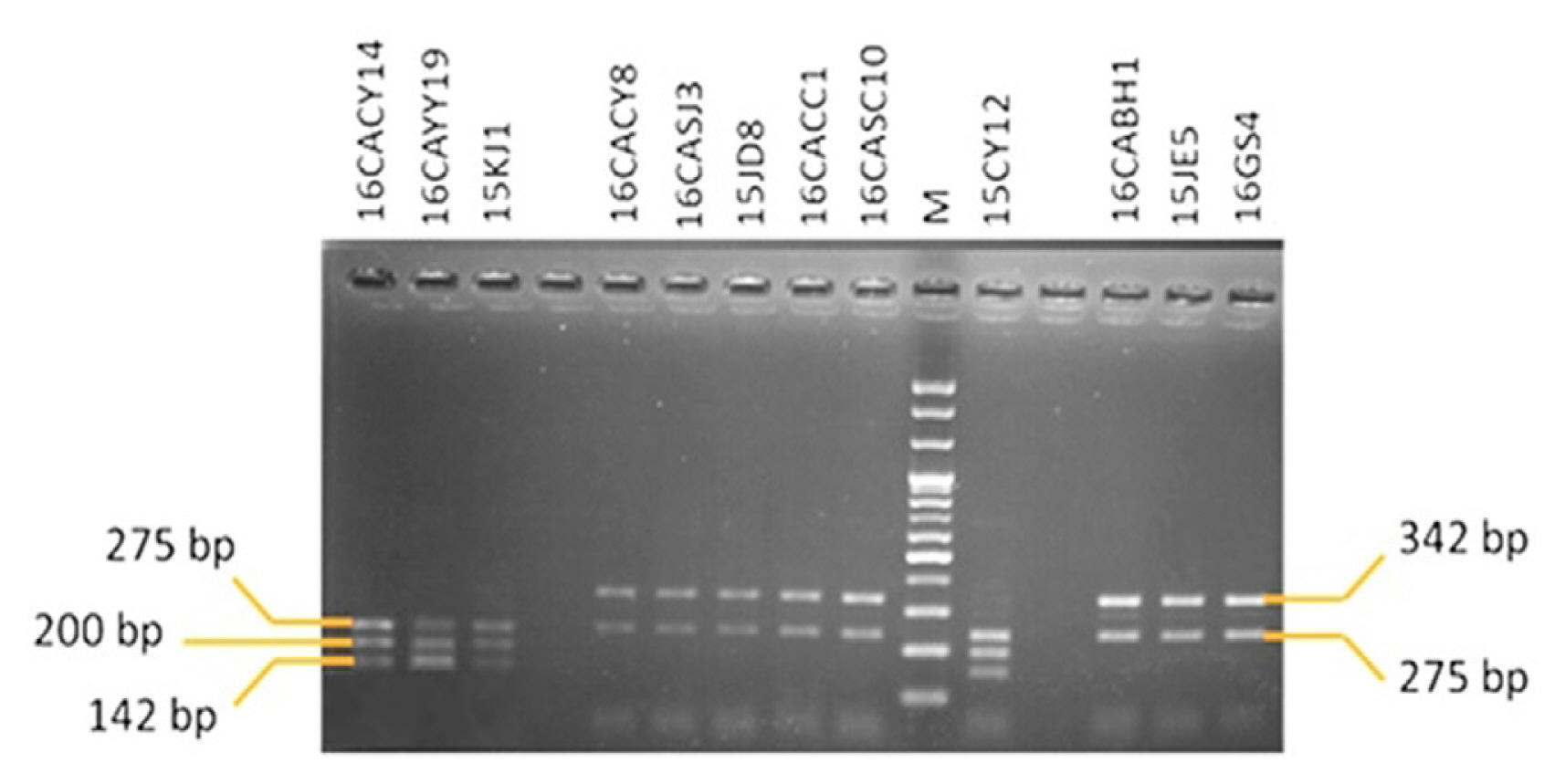
Fnu4HI restriction pattern of a 617 bp long fragment of the cytochrome b gene including the site of the resistance-associated mutation in sensitive (S) and resistant (R) isolates of Colletotrichum spp. Isolates identified as C. gloeosporioides; 16CACY14, 16CAYY19, 15KJ1, 16CACY8, 16CASJ3, 15JD8, 16CACC1, and 16CASC10, Isolates identified as C. acutatum; 15CY12, 16CABH1, 15JE5, and 16GS4. Among 12 isolates, isolates resistant to pyraclostrobin was 16CACY14, 16CAYY19, and 15KJ1 belonging to C. gloeosporioides, and 15CY12 belonging to C. acutatum. M, DNA size marker (100 bp plus DNA ladder).
Discussion
Pyraclostrobin belonging to the strobilurin fungicide group has been registered and used in Korea since 2002 for the control of red pepper anthracnose, and the resistance of the causal pathogen to the fungicide has been a problem from 2015 (Kim et al., 2019). The mechanism of resistance to pyraclostrobin in C. acutatum is due to a point mutation in the cyt b that occurs at the 143rd amino acid codon which changes from GGT (glycine) to GCT (alanine). Point mutations leading to the substitution of amino acid at 143rd of the cyt b have already been reported in many plant pathogens (Gisi et al., 2002; Sierotzki et al., 2000). However, the substitution positions of amino acids exhibiting resistance to strobilurin fungicides by point mutations have been reported not only at position 143, but also at 129 and 137 (Gisi et al., 2002; Kim et al., 2003; Pasche et al., 2004; Sierotzki et al., 2007). In Alternaria solani and Pyricularia grisea, the 129th amino acid was substituted from phenylalanine (TTT or TTC) to leucine (CTT or CTC), resulting in resistance. In addition, in Pyrenophora tritici-repentis, the 137th amino acid is substituted from glycine (GGT) to arginine (CGT). Also, in C. acutatum isolated from strawberries in Florida, USA, substitution at both F129L and G143A has been reported (Forcelini et al., 2016). Through DNA sequencing of cyt b gene of strawberry anthracnose pathogens, 11 of 18 isolates of C. acutatum were resistant to azoxystrobin. Among them, the 129th amino acid (F129L) point mutation was found in 2 isolates whereas the 143rd amino acid (G143A) mutation was found in 9 isolates. In an agar dilution experiment, the EC50 values for azoxystrobin of C. acutatum 13-494 and 14-141 with F129L were 36.9 and 31.4 μg/ml, and the EC50 values for pyraclostrobin were 1.2 and 2.5 μg/ml, which were moderately resistant compared to the sensitive isolates. For the control of these moderately resistant isolates, control was possible when the treatment was performed with higher concentration than the recommended concentration in the field. However, in all isolates of C. acutatum and C. gloeosporioides isolated from red pepper and used in this experiment, the substitution of amino acids 129 or 137 was not found.
In anthracnose of crop plants, one species of Colletotrichum invades several crops, and in other cases, several species of Colletotrichum invade one crop (Agrios, 2005). In Korea, C. gloeosporioides, C. acutatum, C. dematium, and Glomerella cingulate have also been reported to cause anthracnose in red pepper (Park and Kim, 1992). Currently, the major Colletotrichum sp. causing red pepper anthracnose has changed from C. gloeosporioides to C. acutatum (Kim et al., 2008), and most of isolates obtained from the lesions of anthracnose were identified as C. acutatum, but C. gloeosporioides has been isolated still from the weak symptoms of anthracnose (unpublished data). C. gloeosporioides is a pathogen that has not received much attention in terms of control because it has little or no pathogenicity to pepper (unpublished data). However, since it is the major pathogen causing anthracnose in other crops, both C. acutatum and C. gloeosporioides were used in this study.
To accurately test the response to pyraclostrobin, the cyt b gene of C. gloeosporioides was amplified and nucleotide sequence analysis was performed. For cyt b gene amplification, PCR was attempted using the Cacytb-P2/Cacytb-R primer used in C. acutatum, but the cyt b gene of C. gloeosporioides was not amplified at all. Conversely, the CF1/CR2 primer used in C. gloeosporioides did not amplify the C. acutatum cyt b gene (Fig. 2). This result is due to the insertion of an intron into the cyt b gene of C. gloeosporioides. Analysis of the primers using the sequences obtained on the NCBI database showed that the primer Cacytb-P2/Cacytb-R can amplify C. acutatum NC_027280.1 that do not possess intron. The primer was also blasted on the NCBI database against C. gloeosporioides with the accession numbers KX885103.1 and KX885104.1 and a 2,526 bp and 3,136 bp fragments were generated respectively as shown in Fig. 7. However, when the isolates used in this study were amplified with the Cacytb-P2/Cacytb-R primers, the gel electrophoresis result did not show any clear amplification of the C. gloeosporioides (Fig. 2A). The primer CF1/CR2 amplified only the C. gloeosporioides because the primer was developed based on the intron possessed by the C. gloeosporioides (Fig. 8). Further analysis of the structure of C. gloeosporioides Cyt b gene that produced 936 bp fragment showed that the isolate possessed a single intron at 131/132 amino acid position when compared with KX885103.1 whereas the isolates that produced 295 bp fragment possessed two introns at 131/132 and 163/164 when compared with KX885104.1 on the NCBI database (Fig. 8). According to these results, 9 isolates of C. acutatum complex species and 15 isolates of C. gloeosporioides complex species isolated from red pepper could be classified into 3 types as Ca, CgI and CgII according to the number of inserted introns. C. acutatum complex species belongs to Ca type, but intron is not inserted. On the other hand, C. gloeosporioides complex species belonged to CgI and CgII types, and one intron was inserted in CgI type, and two introns were inserted in CgII type. The diversity of the cyt b gene has been reported in several types of phytopathogenic fungi (Grasso et al., 2006; Hu et al., 2015; Yin et al., 2012).
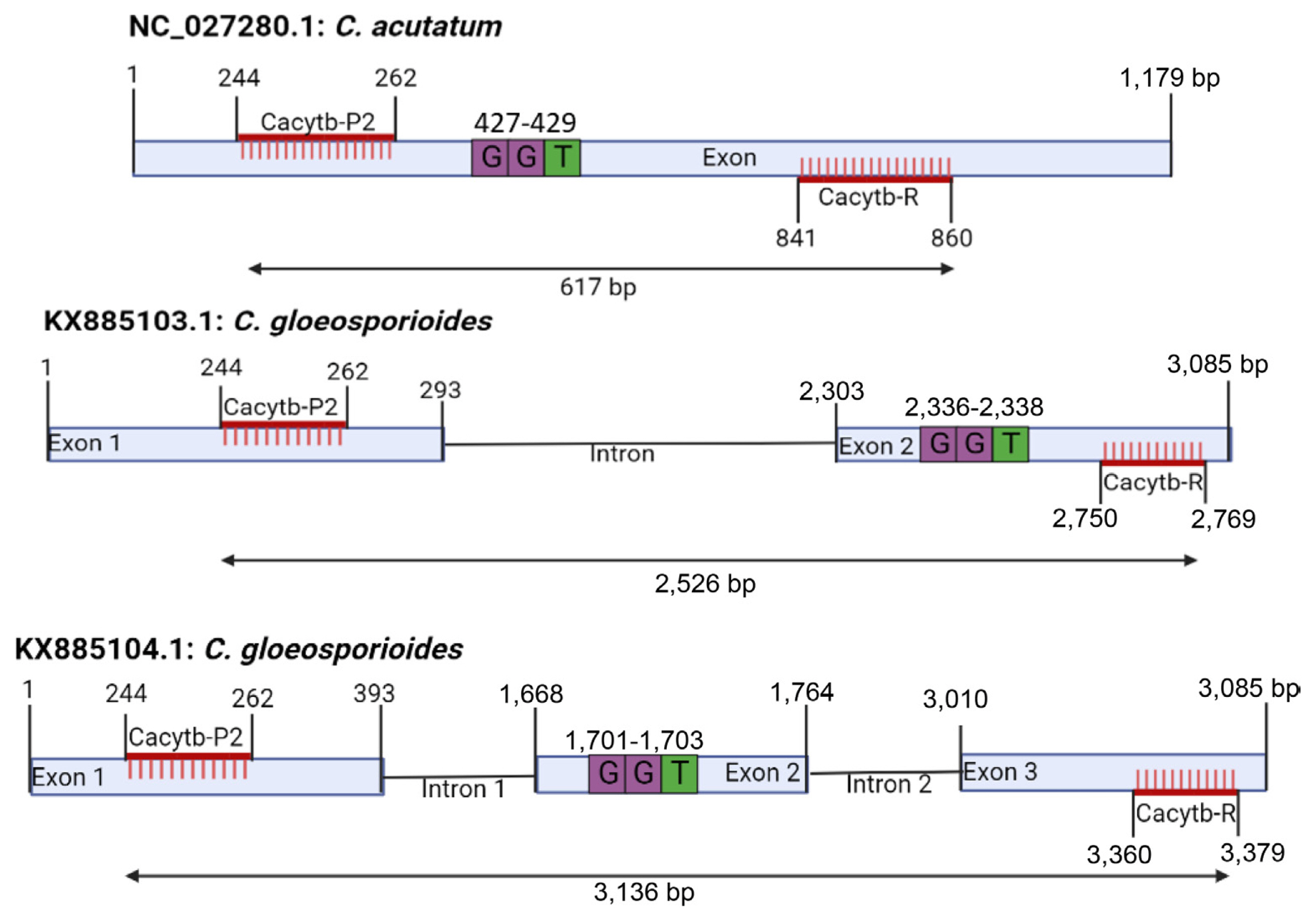
Site of CacytbP2/CacytbR primer pair on Colletotrichum acutatum and C. gloeosporioides. The GGT (glycine) represent the codon of 143rd amino acid position that confer resistance to strobilurin fungicides.
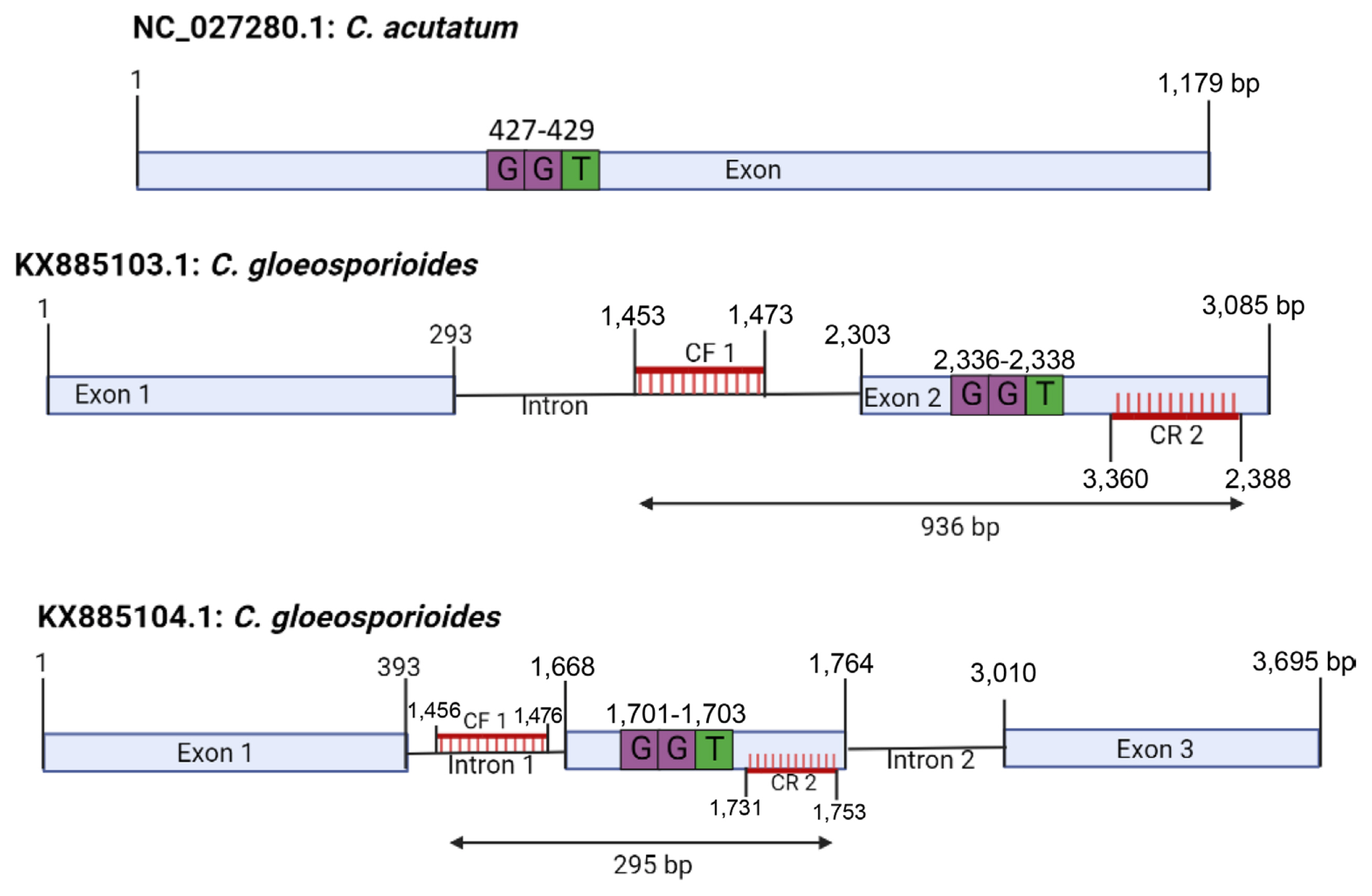
Site of CF1/CR2 primer pair on Colletotrichum acutatum and C. gloeosporioides. The GGT (glycine) represent the codon of 143rd amino acid position that confer resistance to strobilurin fungicides.
Grasso et al. (2006) reported that the intron inserted after codon 143, where a point mutation that induces resistance to strobilurin fungicides, inhibits the expression of resistance to pathogens against the fungicides. In fact, resistance to strobilurin fungicides was not detected in 8 species of Puccinia. As a result of analyzing DNA sequence of the cyt b gene, an intron of over 1,400 bp was inserted after codon 143 of all rust fungi. On the other hand, in Alternaria alternata, Blumeria graminis, Magnaporthe grisea, Mycosphaerella fijiensis, M. graminicola, Venturia inaequalis, and Plasmopara viticola, in which resistance occurred due to substitution of amino acid at position 143, an intron was not inserted after codon at position 143.
The intronic sequence after codon 143 observed in rusts and A. solani is assumed to belong to the group I intron family (Burke, 1988). Group I introns are common in fungal mitochondrial genes encoding components of the electron transport system such as cytochrome oxidase, cytochrome b and ribosomal RNA genes. Group 1 introns are involved in self-splicing and according to the results of Grasso et al. (2006) the codon GGT at position 143 is located exactly at the exon/intron boundary in all rust species as well as in A. solani and is likely part of the signal sequences essential for the recognition of the splice site. If fungicide resistance of plant pathogens is expressed by G143A, the splice site cannot be recognized, and eventually, mRNA splice does not occur, and it dies. Therefore, if an intron is inserted immediately after codon 143, resistance expression by G143A substitution is suppressed (Rosenzweig et al., 2008; Sierotzki et al., 2007).
Such an example was easily found in B. cinerea causing gray mold disease. With the cyt b gene structure, Banno et al. (2009) classified 198 isolates of B. cinerea isolated from several crops cultivated in Osaka and Kanagawa, Japan from 2004 to 2006 into two groups. In group I, two introns were inserted, and in group II, one more intron was inserted after the 143rd codon in addition to the two introns seen in group I. Among them, there were a total of 88 isolates in which an intron was inserted after codon 143, and all these isolates were sensitive to azoxystrobin.
Yin et al. (2012) divided the cyt b gene of B. cinerea isolated from apples in Washington State, USA into 6 types according to the number and location of inserted introns. Among all the strains, the strain in which an intron was not inserted after the 143rd codon accounted for more than 90%, so the risk for the development of resistance to strobilurin fungicides was expected to be high. In resistance monitoring, close to 20% of the isolates were resistant to pyraclostrobin. Although 3 types of cyt b gene were identified in Colletotrichum spp. used in this study, there was no gene in which an intron was inserted after codon 143, so the risk of resistance emergence in Colletotrichum spp. like B. cinerea was thought to be high. Therefore, the rapid and accurate monitoring of resistance to the strobilurin fungicide in Colletotrichum spp., which cause anthracnose in several crops should be continuously performed.
In the traditional method for monitoring resistance to fungicides, individuals of pathogens isolated directly from the disease were used. The traditional methods are labor-intensive and time-consuming if large numbers of isolates are to be tested. To solve the disadvantages, a method that has emerged is a molecular technology method to test a target gene related to fungicides resistance. Advances in molecular biology have provided new opportunities for rapidly detecting fungicide-resistant genotype when the mechanisms of resistance have been elucidated at a molecular level. Several molecular techniques, such as PCR-RFLP and allele-specific PCR, have been used successfully to detect fungicide-resistant genotypes of several plant pathogens.
In this study, we tried to establish molecular technique method such as PCR-RFLP and allele-specific PCR that can simultaneously detect the pyraclostrobin resistance of C. acutatum and C. gloeosporioides. However, when gDNA extracted from mycelia of C. acutatum and C. gloeosporioides was used, the cyt b gene of C. gloeosporioides was not amplified due to the intron inserted into the cyt b gene. These results were also found in B. cinerea when Bccytb-F1/Bccytb-R1 primer pair was used for monitoring azoxystrobin resistance of B. cinerea and it was possible to detect the cyt b gene of resistant isolates (Banno et al., 2009). However, B. cinerea in which genes were not amplified were found to be sensitive isolates. Although the cyt b gene was amplified in B. cinerea isolates sensitive to the fungicide, it was not amplified in the resistant isolates because the binding site of the primer’s changes depending on the inserted intron(s).
In this study, in order to solve the problem associated with the cyt b amplification due to the insertion of introns, cDNA was synthesized using RNA extracted from Colletotrichum spp., and it was performed to monitor the resistance to pyraclostrobin. As shown in Fig. 3, when cyt b gene was amplified using cDNA as a template, the same amplification product of 617 bp was obtained regardless of the cyt b gene type, and allele-specific PCR and PCR-RFLP could be performed using the product. Depending on the crops, there were cases in which C. acutatum and C. gloeosporioides are reported as the causal pathogens of anthracnose. If it is needed to simultaneously perform a resistance monitoring for Colletotrichum spp. against strobilurin fungicides in such plant diseases, the cDNA must be synthesized using the RNA as a template. In addition, the allele-specific PCR and PCR-RFLP established in this study will be molecular techniques that can be used for monitoring the resistance to strobilurin fungicides not only in red pepper but also in other crops in which C. acutatum species complex and C. gloeosporioides species complex cause anthracnose.
As a result of detecting the resistance to azoxystrobin using 114 isolates of C. acutatum species complex collected from apples recently, the ratio of susceptible isolates was 83.3%, resistant isolates were 5.6%, and highly resistant isolates were 11.1% (Moreira et al., 2019). Looking at the nucleotide sequence of the cyt b gene of resistant and highly resistant isolates, substitutions of amino acids at 129th, 137th, and 143rd for which point mutations were reported were not found. These results showed the possibility that there might be other mechanisms despite point mutations as the mechanism by which resistance to azoxystrobin occurs. Therefore, it is necessary not only to establish a molecular technique method using resistance genes to fungicides but also to explore novel fungicides resistance mechanisms.
Acknowledgments
This work was supported by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the Crop Viruses and Pests Response Industry Technology Development Program, funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA; Grant No. 320042-5).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.