 |
 |
| Plant Pathol J > Volume 32(2); 2016 > Article |
Abstract
Acknowledgments
Fig.┬Ā1
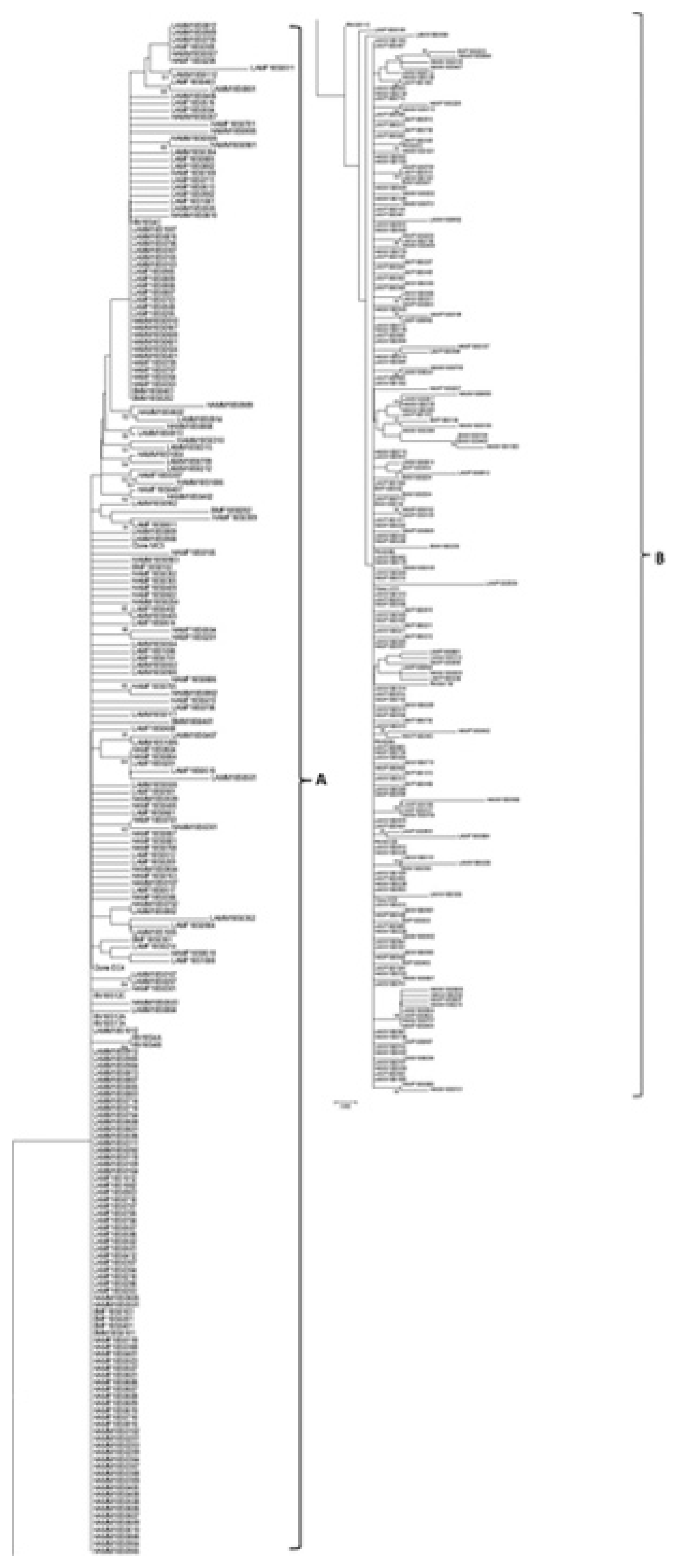
Fig.┬Ā4
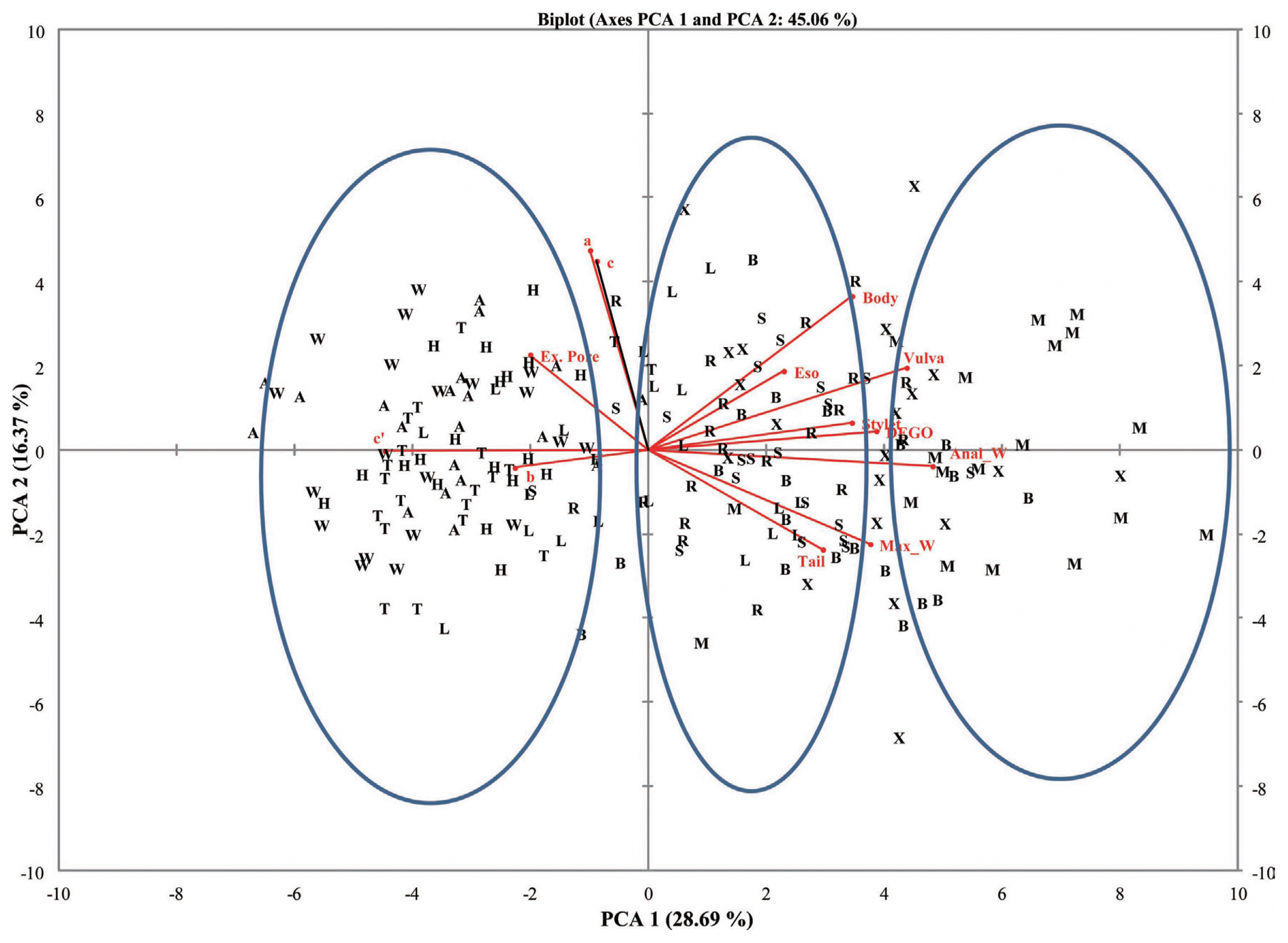
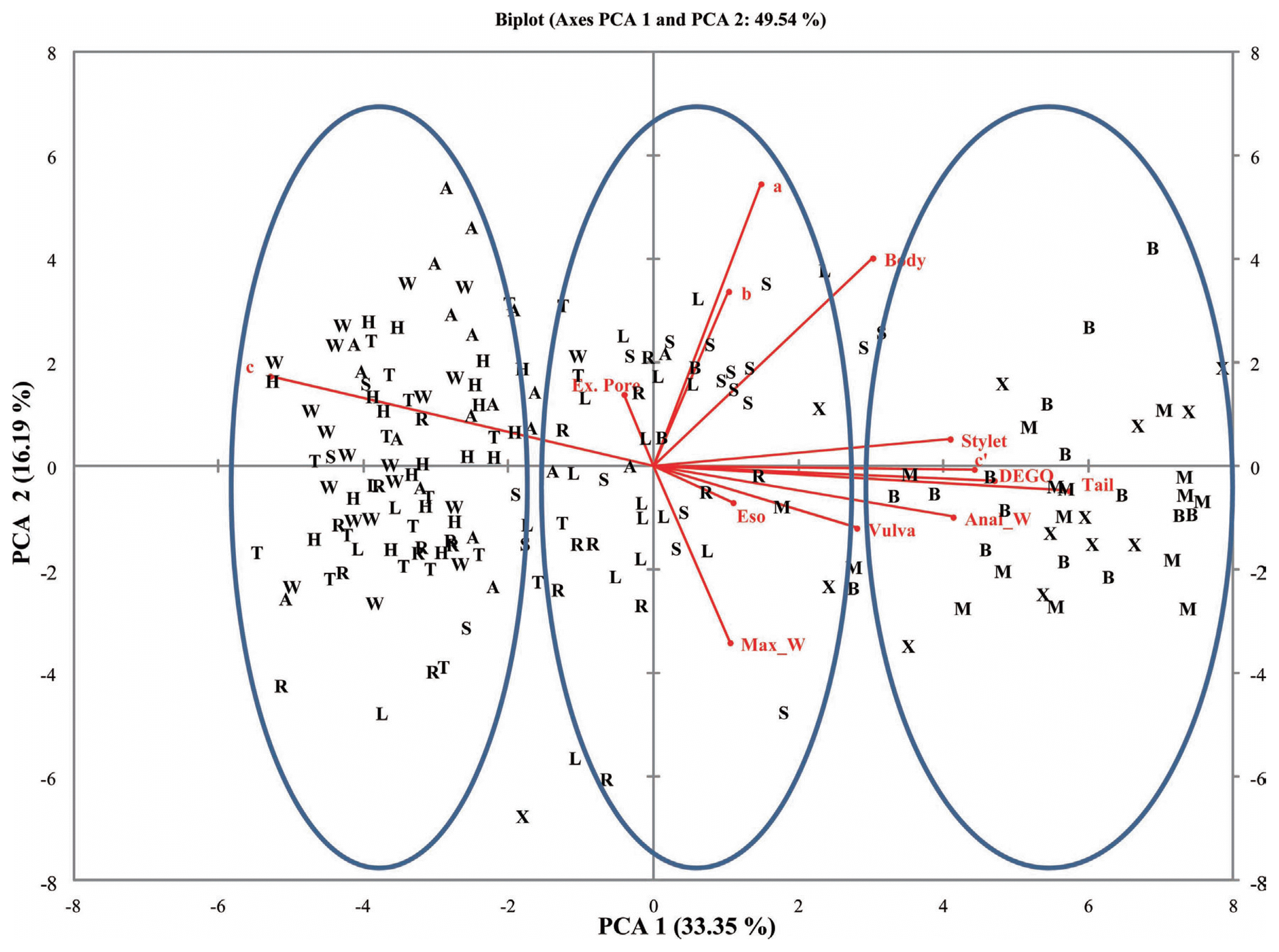
Table┬Ā1
Table┬Ā2
Table┬Ā3
L = body length; SL = stylet length; V = Position of vulva; TL = tail length; DEGO = dorsal esophageal gland orifice; EP excretory pore; MW = maximum width; ESOP = Esophageal length; AW = Anal Width; a = body length/maximum body width; b = body length/esophageal length; c = body length/tail length cŌĆ▓ = tail length/anal body width
Table┬Ā4
L = body length; SL = stylet length; V = Position of vulva; TL = tail length; DEGO = dorsal esophageal gland orifice; EP excretory pore; MW = maximum width; ESOP = Esophageal length; AW = Anal Width; a = body length/maximum body width; b = body length/esophageal length; c = body length/tail length cŌĆ▓ = tail length/anal body width
Table┬Ā5
L = body length; SL = stylet length; TL = tail length; DEGO = dorsal esophageal gland orifice; EP excretory pore; MW = maximum width; ESOP = Esophageal length; AW = Anal Width; a = body length/maximum body width; b = body length/esophageal length; c = body length/tail length cŌĆ▓ = tail length/anal body width
Table┬Ā6
| POPULATION | L | SL | TL | DEGO | EP | MW | ESOP | AW | a | b | c | cŌĆ▓ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BELLMINA | 1.4 | 14.8* | 47.8** | 1.5 | 1.1 | 36.3** | 3.9 | 9.5* | 34.8** | 4.3 | 41.9** | 148.4** |
| SHAW | 2.3 | 84.5** | 0.7 | 7.9* | 4.9* | 13.4* | 49.6** | 2.3 | 12.0* | 73.1** | 3.5 | 91.6** |
| MURPHY | 2.8 | 123.1** | 17.0* | 4.7* | 0.6 | 16.5* | 7.5* | 0.4 | 2.3 | 1.7 | 6.9* | 149.7** |
| HARGRAVE | 1.6 | 184.4** | 10.6* | 20.4** | 14.0* | 12.7* | 33.1** | 1.0 | 13.9* | 35.4** | 10.9* | 11.4* |
| THORTON | 2.5 | 78.8* | 12.4* | 9.0* | 13.1* | 8.9* | 45.9** | 1.0 | 11.4* | 36.7** | 14.7* | 6.7* |
| HAMILTON | 2.6 | 74.5** | 3.2 | 3.9 | 6.7* | 4.4* | 12.8* | 0.0 | 11.2* | 18.6* | 6.7* | 3.1 |
| LAMONS | 1.6 | 65.6** | 0.5 | 0.1 | 0.5 | 7.7* | 7.0* | 1.8 | 8.1* | 13.3* | 1.5 | 114.6** |
| WHITEHEAD | 4.2* | 84.6** | 1.3 | 8.6* | 8.6* | 7.3* | 8.4* | 1.0 | 11.8* | 15.3* | 4.1* | 0.5 |
| HUXFORD | 0.0 | 0.2 | 80.3** | 15.5* | 0.8 | 8.1* | 0.1 | 26.1** | 4.6* | 1262.4** | 76.2** | 224.3** |
| MSU | 0.2 | 63.6** | 49.9** | 3.0 | 4.7* | 11.1* | 19.8** | 18.0* | 10.3* | 40.3** | 35.8** | 189.6** |
L = body length; SL = stylet length; TL = tail length; DEGO = dorsal esophageal gland orifice; EP excretory pore; MW = maximum width; ESOP = Esophageal length; AW = Anal Width; a = body length/maximum body width; b = body length/esophageal length; c = body length/tail length cŌĆ▓ = tail length/anal body width.
Table┬Ā7
L = body length; SL = stylet length; TL = tail length; DEGO = dorsal esophageal gland orifice; EP = excretory pore; MW = maximum width; ESOP = Esophageal length; AW = Anal Width; a = body length/maximum body width; b = body length/esophageal length; c = body length/tail length cŌĆ▓ = tail length/anal body width,
Table┬Ā8
| Population (n=20) | L (╬╝m) | SL (╬╝m) | TL (╬╝m) | DEGO (╬╝m) | EP (╬╝m) | MW (╬╝m) | ESOP (╬╝m) | AW (╬╝m) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|||||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| BelleMina (F) | 381.0abŌĆĀ | 26.1 | 16.3bcd | 1.8 | 33.4a | 4.7 | 34.4bcd | 2.4 | 66.4bc | 8.1 | 16.9a | 2.0 | 124.5b | 26.3 | 11.4a | 1.5 |
| BelleMina (M) | 390.5a | 24.8 | 14.3ab | 1.5 | 45.9a | 6.6 | 35.6ab | 3.6 | 63.4b | 9.6 | 13.9bc | 0.9 | 112.0ab | 11.1 | 9.9a | 1.5 |
| Shaw (F) | 375.5abc | 22.6 | 17.6ab | 1.6 | 30.8ab | 3.1 | 36.7ab | 4.3 | 69.1ab | 5.1 | 15.8abc | 1.2 | 125.0b | 10.0 | 9.6b | 1.2 |
| Shaw (M) | 387.5a | 27.3 | 13.6abc | 1.1 | 29.9b | 4.2 | 33.3bc | 3.1 | 65.0ab | 6.7 | 14.7ab | 0.9 | 104.0b | 8.8 | 9.0ab | 1.3 |
| Murphy (F) | 381.5ab | 30.3 | 16.9bc | 1.1 | 29.6abc | 2.8 | 36.1bc | 5.2 | 68.8ab | 9.7 | 16.2abc | 1.0 | 126.0b | 10.5 | 8.7bc | 1.2 |
| Murphy (M) | 366.0a | 28.2 | 12.6cd | 1.4 | 26.2bc | 2.4 | 33.1bc | 3.3 | 70.8a | 5.8 | 14.9ab | 1.1 | 117.0a | 10.3 | 8.4bc | 1.2 |
| Hargrave (F) | 364.0abc | 26.8 | 15.6cde | 1.2 | 28.4bc | 2.5 | 30.8de | 2.5 | 73.4a | 4.2 | 15.2bcd | 1.2 | 123.0b | 12.6 | 7.1d | 0.0 |
| Hargrave(M) | 373.5a | 20.3 | 11.9d | 0.0 | 26.1bc | 2.1 | 27.7d | 1.8 | 68.2ab | 4.7 | 14.3abc | 0.0 | 105.0b | 6.1 | 7.3d | 0.5 |
| Thorton (F) | 356.0bc | 23.7 | 14.3e | 0.0 | 29.0bc | 1.7 | 30.1e | 2.5 | 73.7a | 5.3 | 15.5abcd | 1.2 | 123.5b | 9.3 | 7.3d | 0.5 |
| Thorton (M) | 368.0a | 24.6 | 11.9cd | 0.0 | 26.5bc | 2.7 | 28.2d | 1.4 | 68.1ab | 4.5 | 14.5ab | 0.7 | 106.0b | 6.8 | 7.1d | 0.0 |
| Hamilton (F) | 361.0abc | 26.5 | 15.0de | 1.1 | 28.3bc | 2.4 | 32.1de | 2.4 | 72.0ab | 4.1 | 14.3d | 1.3 | 121.0b | 10.2 | 7.1d | 0.0 |
| Hamilton (M) | 375.0a | 28.2 | 12.3cd | 0.9 | 27.0bc | 2.2 | 30.3cd | 3.3 | 68.3ab | 4.9 | 13.4c | 1.2 | 110.5ab | 8.3 | 7.1d | 0.0 |
| Lamons (F) | 359.5abc | 25.6 | 16.7bcd | 1.1 | 29.3bc | 4.0 | 33.3bcde | 2.9 | 71.0ab | 6.3 | 15.4bcd | 1.2 | 125.0b | 17.0 | 8.2cd | 1.2 |
| Lamons (M) | 370.0a | 27.5 | 13.4bc | 1.4 | 28.4bc | 3.6 | 32.9bc | 3.8 | 69.7ab | 4.9 | 14.4abc | 0.9 | 112.5ab | 12.5 | 7.7cd | 1.1 |
| WhiteHead (F) | 349.5c | 26.3 | 14.4e | 1.2 | 25.9c | 3.3 | 31.4de | 3.3 | 72.1ab | 4.8 | 14.9cd | 1.3 | 116.0b | 10.0 | 7.3d | 0.5 |
| WhiteHead (M) | 365.0a | 21.6 | 11.9d | 0.0 | 25.0c | 2.0 | 28.7d | 2.1 | 67.9ab | 4.1 | 13.9bc | 0.9 | 105.5b | 12.8 | 7.1d | 0.0 |
| Huxford (F) | 384.0a | 29.8 | 15.4cde | 3.4 | 31.1ab | 5.3 | 32.4cde | 4.0 | 70.8ab | 9.9 | 16.3abc | 2.2 | 107.9b | 18.8 | 12.7a | 2.5 |
| Huxford (M) | 384.5a | 32.7 | 14.9a | 2.7 | 48.2a | 6.6 | 38.6a | 5.8 | 67.9ab | 10.0 | 14.6ab | 1.4 | 109.0ab | 7.9 | 9.5a | 1.3 |
| MSU (F) | 386.5a | 28.2 | 18.9a | 2.0 | 33.4a | 5.9 | 39.9a | 5.5 | 59.9c | 6.6 | 16.4ab | 1.3 | 166.0a | 55.3 | 11.5a | 2.2 |
| MSU (M) | 390.0a | 24.7 | 14.2ab | 1.8 | 48.7a | 7.7 | 37.0a | 4.9 | 64.1b | 5.6 | 15.1a | 1.2 | 110.0ab | 10.3 | 9.2ab | 1.2 |
References
- TOOLS
-
METRICS

- Related articles
-
Molecular Characterization of Hypernodulation in Soybean2003 February;19(1)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print



