Large-Scale Screening of the Plant Extracts for Antifungal Activity against the Plant Pathogenic Fungi
Article information
Abstract
Plants produce chemicals of immense diversity that provide great opportunities for development of new antifungal compounds. In search for environment-friendly alternatives to the fungicide of current use, we screened plant extracts obtained from more than eight hundred plant materials collected in Korea for their antifungal activity against the model plant pathogenic fungus, Magnaporthe oryzae. This initial screening identified antifungal activities from the eleven plant extract samples, among which nine showed reproducibility in the follow-up screening. These nine samples were able to suppress not only M. oryzae but also other fungal pathogens. Interestingly, the plant extracts obtained from Actinostemma lobatum comprised five out of eight samples, and were the most effective in their antifungal activity. We found that butanol fraction of the A. lobatum extract is the most potent. Identification and characterization of antifungal substances in the A. lobatum extracts would provide the promising lead compounds for new fungicide.
Over the past two decades, the incidence of plant diseases caused by fungal pathogens has dramatically increased (Fisher et al., 2012). To date, the use of agrochemicals remains one of the most effective and important agricultural practices in controlling fungal diseases of crops, although synthetic chemicals are associated with negative environmental impacts and human health concerns via direct exposure to pesticides and deposition of chemicals on agricultural products (Surapuram et al., 2014). Thus, there has been a growing need for better fungicides with less environmental burden and human health hazard. Naturally-occurring compounds are potential source of lead compounds for new agrochemicals, although being natural compounds does not necessarily guarantee being better fungicides. As plants are a cornucopia of bioactive secondary metabolites, exploiting such metabolic capacity would possibly open doors for identification and development of new disease control agents (Arif et al., 2009).
Plant extracts from many different species of plants were tested for antifungal activity against the fungal pathogens of important crops (Adnew et al., 2022; Hernández-Ceja et al., 2021; Schoss et al., 2022). In particular, some medicinal plants were heavily used for exploring possibilities of finding new lead compounds (Mohammadi et al., 2014). However, few studies attempted to survey the plant extracts sampled longitudinally from geographically diverse regions. The Nakdonggang National Institute of Biological Resources (NNIBR) has been systematically collecting and archiving diverse plant species in Korea over the years. These resources should offer opportunities to tap into the chemical repertoire of those plants.
Magnaporthe oryzae is a model plant pathogenic fungus that causes the rice blast disease. The disease is one of the most serious threats to food security in the rice-cultivating regions where the consumption of rice takes up the major calorie intakes (Talbot, 2003). In addition to social-economic impact of the disease it causes, M. oryzae undergoes pathogenic development that is common to many plant pathogenic fungi, making it a model for investigating fungal development and pathogenesis. Infection usually starts with dissemination of asexual spore called conidium. The conidium that lands on leaf surface of the rice plants germinates and, upon recognition of surface hydrophobicity and hardness, differentiates an infection-specific structure, appressorium (Dean, 1997). The appressorium can build up an enormous chemical pressure that can be channeled into mechanical pressure to breach the cuticular layer of the host plants (Howard and Valent, 1996). Once inside the host cell, the fungus colonizes the plant tissues by growing inside the plant cells, leading to the formation of visible disease symptoms within a week (Kankanala et al., 2007). As the germination and subsequent development of appressorium are the critical steps that determine the onset of the disease, chemical interference with these processes is a highly effective disease control measure.
In this study, we took advantage of over eight hundred plant materials that are sampled from diverse habitats across Korea over different time points. Using collected plant materials, we initially evaluated plant extracts for the in-vitro antifungal activity against M. oryzae KJ201. With the selected extracts from the first screening, and the second screening was performed with the following important crop pathogens: Aspergillus flavus NRRL3357, Botrytis cinerea, Rhizoctonia solani, Sclerotinia minor, Cochliobolus miyabeanus, Fusarium graminearum, Colletotrichum gloeosporioides MGCF10, C. gloeosporioides YCJF8, C. gloeosporioides YCJF10, Fusarium oxysporum f. sp. conglutinans, and F. oxysporum f. sp. niveum. These were grown on potato dextrose agar (PDA) medium at 25°C. M. oryzae KJ201 and Phytophthora infestans T30-4 were grown on oatmeal agar (OMA; 5% oatmeal (w/v) and 2% agar (w/v)) medium at 25°C and V8 agar medium at 16°C, respectively. For antifungal activity assays, the fungal strains were inoculated on a PDA, OMA, and V8 agar medium plate (90 mm × 15 mm Petri dish) at 25°C, 25°C and 16°C, respectively, suitable for each strain. The sterile antibiotic assay discs (6 mm diameter; Whatman, Little Chalfont, UK) were placed on the periphery of the mycelial colony, when each fungal mycelium had grown to a diameter of 20–30 mm. In addition, the 15 μl of dissolved fractionated extracts in dimethyl sulfoxide (DMSO) of 20 mg/ml were added to each antibiotic assay discs, and 15 μl of DMSO was added to the antibiotic assay disc as a negative control. After that, plates were continuously cultured at 25°C and 16°C, suitable for each strain. The growth status of each fungal mycelium was observed within 3 days.
A total of 804 plant extracts, which was obtained from the NNIBR, was first screened for their antifungal activity against the rice blast fungus (Supplementary Table 1). These 804 plant extracts were from 192 different plant species that had been collected for three years. Depending on the plant species and timing of collection, plant extracts were obtained from different tissues or organs using one of the followings: DMSO, 70% ethanol, and distilled water. For efficient screening of a large number of plant extracts, a modified disk diffusion method was used (Fig. 1A and B).

Screening of plant extracts for their antifungal activity against Magnaporthe oryzae. (A) Flow chart for screening of plants extracts, candidate selection, and in-depth analysis. (B) Representative image of screening results based on a modified disk diffusion method. The inset figure provides magnified view of the hyphal growth inhibition by a plant extract.
During our screens, antifungal activity was assessed not in quantitative but in binary manner (yes or no) in order to expedite the screening. The initial screening of the plant extracts led to the identification of eleven candidates. The second round of screening was performed for the eleven, and ascertained the antifungal activity for the nine candidates (Table 1). These candidates are from only five genera, and interestingly, five out of the nine candidate plant extracts were from Actinostemma lobatum (common name: lobed Actinostemma), although sampling time and solvent used for extraction differ from one sample to another.
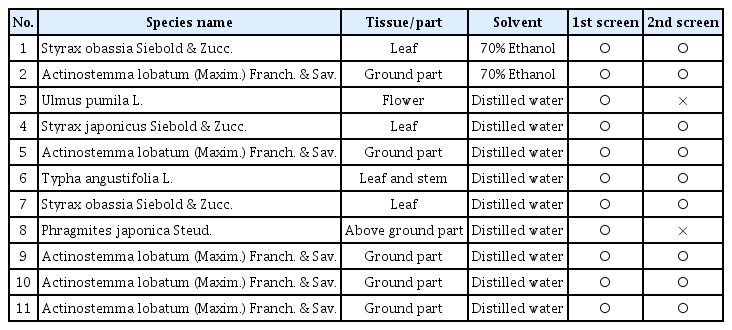
List of 11 candidate plant extracts and their antifungal activity test results in the first and second round of screening
As our initial screening of the plant extracts were tested only against the single fungal pathogen, M. oryzae, we tested how broad the inhibitory effects of nine candidates are against the common fungal plant pathogens and an oomycete pathogen, using the modified disk diffusion method (Fig. 2A). All the extracts were effective against S. minor and C. miyabeanus. However, hyphal growth of many pathogens was inhibited only by a few plant extracts. Importantly, the plant extract #1 and #2, which correspond to 70% ethanol extracts of S. obassia and A. lobatum, respectively, showed a broad range of inhibitory effect against the pathogens tested here (Table 1, Fig. 2A).
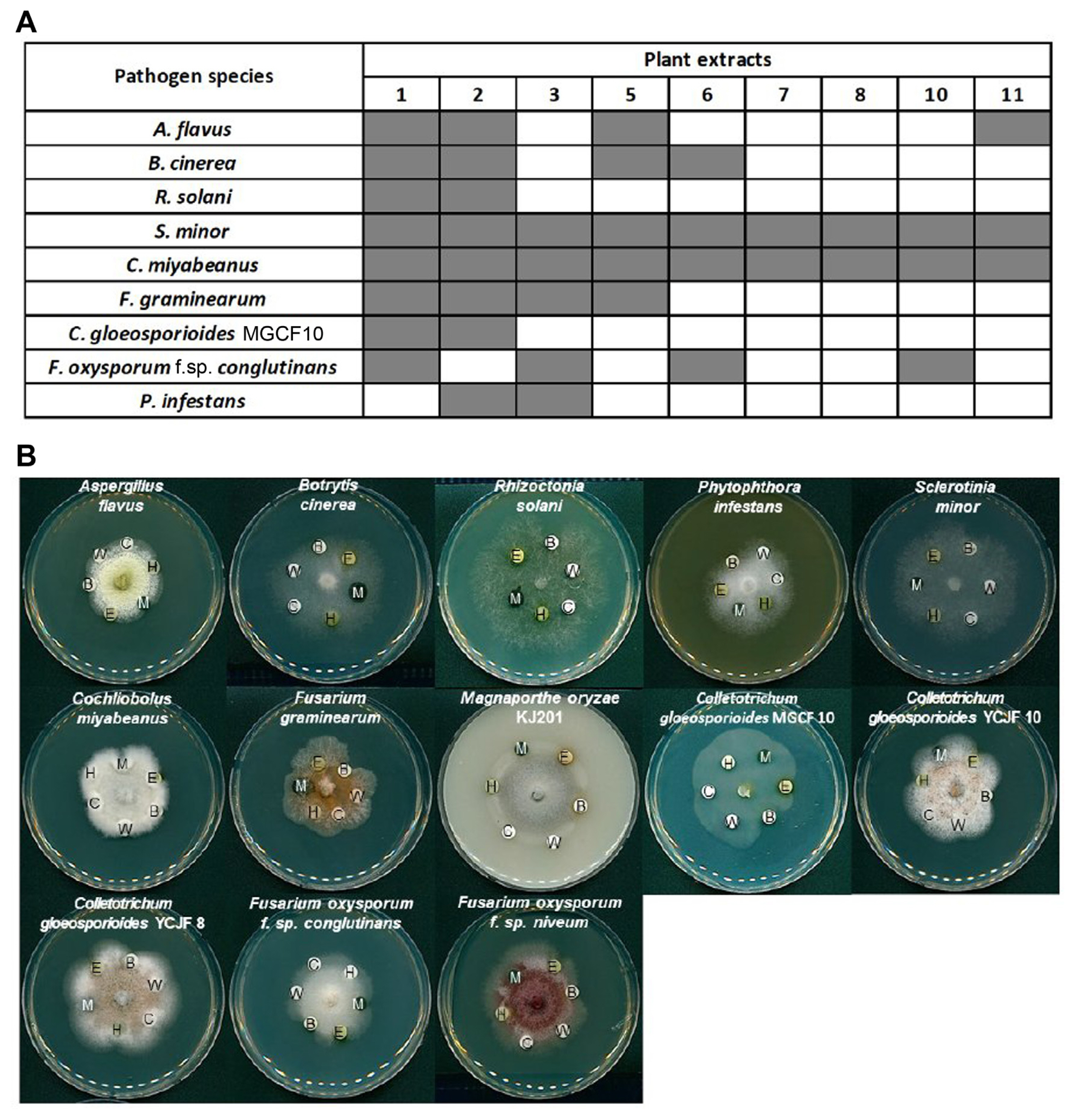
Antifungal activity of selected plant extracts against a set of common plant pathogens. (A) A heatmap summarizing antifungal activity test results against a set of common plant pathogens. Results were shown as binary outcome: inhibition, gray-filled block; no inhibition, white-block. A. flavus, Aspergillus flavus; B. cinerea, Botrytis cinerea; R. solani, Rhizoctonia solani; S. minor, Sclerotinia minor; C. miyabeanus, Cochliobolus miyabeanus; F. graminearum, Fusarium graminearum; C. gloeosporioides, Colletotrichum gloeosporioides; F. oxysporum, Fusarium oxysporum; P. infestans, Phytophthora infestans. (B) Effects of solvents used in extraction on antifungal activity of Actinostemma lobatum extract. C, dimethyl sulfoxide; B, A. lobatum butanol extract; E, A. lobatum ethyl acetate extract; H, A. lobatum hexane extract; M, A. lobatum chloroform extract; W, A. lobatum water extract.
Based on the abundance of samples and broad range of inhibitory effect, we prioritized the A. lobatum for further analysis using different solvents. A. lobatum, an annual herb of Cucurbitaceae family, is widespread in Asia (Kim, 2010; Li et al., 2012) and it has traditionally been used as a medicinal herb for the treatment of arthritis, cardiovascular disease and detoxification of snake bite (Cao et al., 2015; Kim et al., 2008; Li et al., 2012). Although biological activities of A. lobatum such as anti-tumor activity (Fujioka et al., 1996; Li et al., 2012), anti-thrombotic activity (Kim et al., 2008), anti-oxidative activity (Kim, 2010), and anti-bacterial activity (Chen et al., 2005) have been reported, its anti-fungicidal activity has not been investigated to date. Dried and powdered A. lobatum (3.3 kg) was extracted successively with 70% ethanol (2 × 10 l) at room temperature and filtered. The filtrate was evaporated under a vacuum to obtain a 70% EtOH extract. The crude extract (390 g, yield to 11.8%) was re-suspended in water and partitioned successively with hexane (107.7 g), chloroform (11.6 g), ethyl acetate (21.1 g), and normal butanol (60.1 g).
When we tested the inhibitory effect of individual extracts obtained by different solvents on a number of common plant pathogens (Fig. 2B), the butanol extract showed the strongest inhibitory effect on almost all the pathogens tested, suggesting the presence of active compound(s) in the butanol fraction. To gauge the potency of butanol fraction, we compared the butanol fraction with hygromycin B, which is a commonly used aminoglycoside antibiotic produced by Streptomyces hygroscopicus (Eustice and Wilhelm, 1984; González et al., 1978; Mann and Bromer, 1958). Such comparison showed that butanol fraction is able to inhibit hyphal growth of the fungus more effectively than the equivalent amount of hygromycin (Supplementary Fig. 1A). We also compared the antifungal activity of butanol extract with that of a fungicide, benomyl. This indicated that butanol extract has weaker antifungal activity than benomyl in our plate assay. These results suggest that butanol fraction of A. lobatum contains compound(s) having higher potency than hygromycin B.
Since our analyses focused only on inhibition of hyphal growth by the plant extracts, we next tested the effects of the butanol fraction on pathogenic development of the rice blast fungus. The M. oryzae KJ201 strain cultured on OMA medium at 25°C under constant light to promote conidium production. Conidia were harvested from 9-day-old colonies using 5 ml of sterilized distilled water and counted using a hemocytometer under a light microscope. To monitor germination and appressorium formation, the concentration of conidial suspension was adjusted to ~5 × 104 conidia/ml. A 40 μl suspension was placed on a hydrophobic surface of plastic coverslips and then incubated in a humidity chamber at 25°C. The germination and appressorium formation rate were calculated by counting the number of germinating spores and appressorium-forming conidia, respectively, out of at least 100 spores at 2, 4, and 6 h post-incubation. All the assays were conducted with three technical replicates in three independent experiments. Slides observed under a DM2500 microscope (Leica, Wetzlar, Germany) and pictured with a DFC7000 T digital camera (Leica) using LAS X software (Leica).
The harvested spores were incubated with varying concentrations of the butanol fraction on inductive surface (plastic coverslip) (Fig. 3A). In contrast to the DMSO control in which spores germinate and develop appressoria over time, a high concentration (300 μg/ml and 150 μg/ml) of the butanol fraction completely inhibited the spore germination itself (Fig. 3A and B). At a much lower concentration (30 μg/ml), although inhibition of spore germination was significantly alleviated, appressorium formation was rarely observed, indicating that the butanol fraction has inhibitory effect on fungal development as well (Fig. 3A–C).
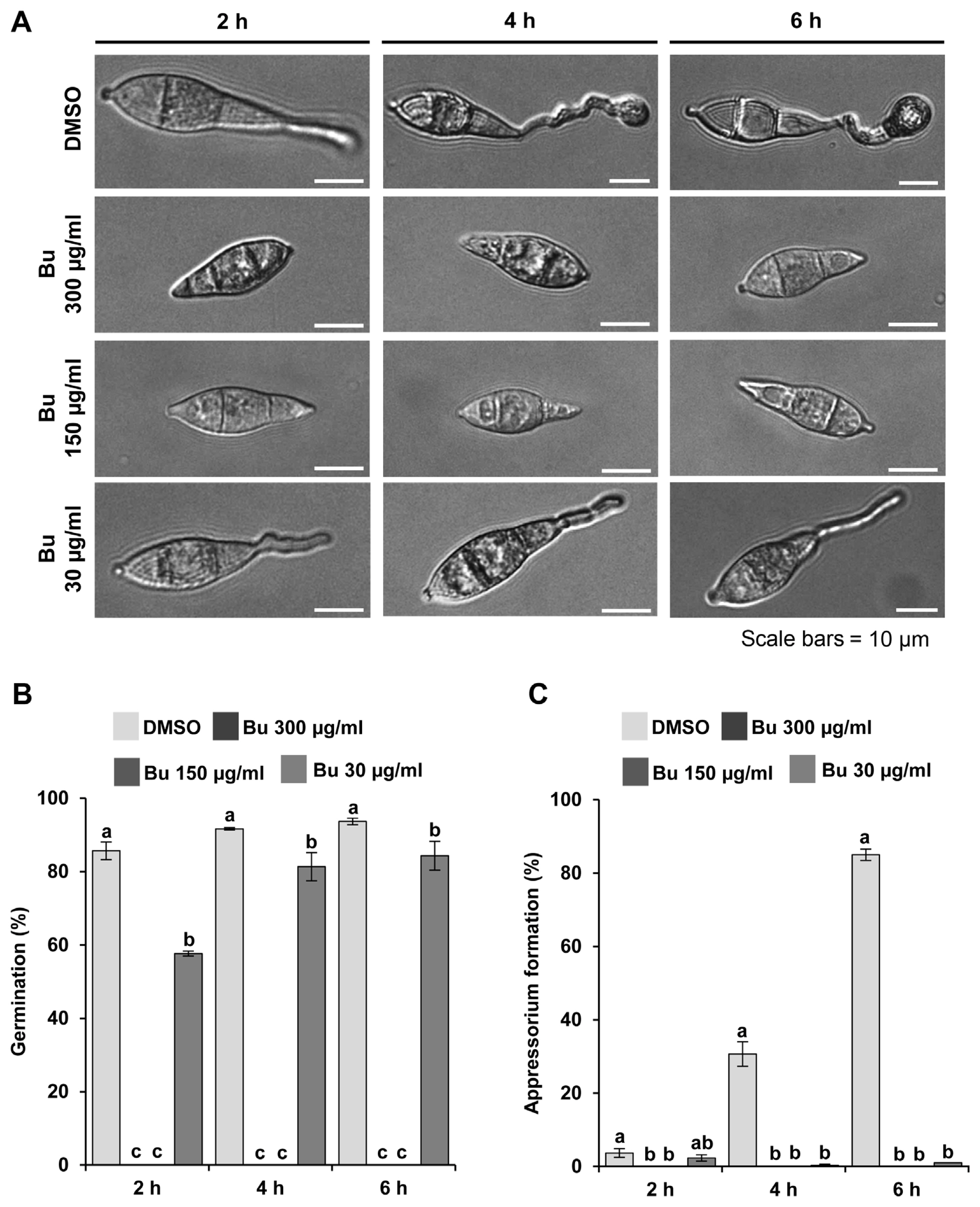
Effects of Actinostemma lobatum butanol extracts (Bu) on fungal development. (A) Images showing the effects of varying concentration of Bu on spore germination and appressorium of Magnaporthe oryzae KJ201 at 2, 4, and 6 h post-incubation of spores on hydrophobic surface. (B) Quantitative measurement of germination under Bu treatment. (C) Quantitative measurement of appressorium formation rates under Bu treatment. Different letters on the bars indicate significant difference in mean values (P < 0.001, Tukey honestly significant difference test, n = 3).
Furthermore, pathogenicity was carried out to test whether the extract is effective in suppressing disease development. For pathogenicity assays, rice plants (Oryza sativa cv. Dongjin) were used in three- to four-leaf stages grown at 25°C for 4 weeks. To carry out wound inoculation, 40 μl of spore suspension (~5 × 105 spores/ml) was directly placed onto wounded site of rice leaves. Inoculated plants were incubated in a humidity chamber without light for 24 h and then transferred to an incubator for growth at 25°C with a 16 h photoperiod of fluorescent light. After 7 d post-inoculation, lesion development was assessed and photographed. Plants grown in three pots were used for a pathogenicity assay in three independent experiments. Our pathogenicity assay using detached rice leaves showed that butanol fraction is able to significantly suppress the disease symptom development with little side-effect on the plants even at higher concentration (Fig. 4A and B). Importantly, the butanol extract showed disease control activity that is almost comparable to benomyl (Fig. 4A and B).

Effects of Actinostemma lobatum butanol extracts (Bu) on fungal pathogenicity. (A) Assessment of pathogenicity of Magnaporthe oryzae KJ201 using wound inoculation with or without the Bu. (B) Quantitative analysis of pathogenicity assay results obtained by measurement of lesion length. Be, benomyl. Different letters on the bars indicate statistically significant difference in mean values (P < 0.001, Tukey honestly significant difference test, n = 3).
In this work, we have screened a large number of plant materials covering diverse geographical locations and species in Korea in order to identify the extracts having antifungal activities. This effort has shortlisted a few plant species including A. lobatum as potential candidates for devel oping plant-derived agrochemicals. Molecular identification and characterization of active compound(s) in the butanol fraction might yield a novel agrochemical(s) in the years to come.
Acknowledgments
This work was supported by a grant from the Nakdonggang National Institute of Biological Resources (NNIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NNIBR202202110), and by a grant from the National Research Foundation of Korea (NRF-2018R1A5A1023599).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).