 |
 |
| Plant Pathol J > Volume 40(1); 2024 > Article |
|
Abstract
Fusarium graminearum, the causal agent of Fusarium head blight (FHB) in cereal crops, employs the production of sexual fruiting bodies (perithecia) on plant debris as a strategy for overwintering and dissemination. In an artificial condition (e.g., carrot agar medium), the F. graminearum Z3643 strain was capable of producing perithecia predominantly in the central region of the fungal culture where aerial hyphae naturally collapsed. To unravel the intricate relationship between natural aerial hyphae collapse and sexual development in this fungus, we focused on 699 genes differentially expressed during aerial hyphae collapse, with 26 selected for further analysis. Targeted gene deletion and quantitative real-time PCR analyses elucidated the functions of specific genes during natural aerial hyphae collapse and perithecium formation. Furthermore, comparative gene expression analyses between natural collapse and artificial removal conditions reveal distinct temporal profiles, with the latter inducing a more rapid and pronounced response, particularly in MAT gene expression. Notably, FGSG_09210 and FGSG_09896 play crucial roles in sexual development and aerial hyphae growth, respectively. Taken together, it is plausible that if aerial hyphae collapse occurs on plant debris, it may serve as a physical cue for inducing perithecium formation in crop fields, representing a survival strategy for F. graminearum during winter. Insights into the molecular mechanisms underlying aerial hyphae collapse provides offer potential strategies for disease control against FHB caused by F. graminearum.
Fusarium graminearum, a plant pathogenic fungus causing Fusarium head blight (FHB) in major cereal crops, such as wheat, barley, rice, and corn, is prevalent in temperate and subtropical regions, including the United States, Europe, and Asia (McMullen et al., 1997). FHB leads to annual economic losses of hundreds of millions of dollars in the United States, affecting seed and crop quality (Nganje et al., 2002). In Korea, a substantial reduction in wheat yields occurred in the southern region in 1963 (Chung, 1975). The historical 10-year epidemic cycle of FHB in Korea has been influenced by abnormal climatic conditions such as global warming and changes in cropping systems. In addition to its economic impact, F. graminearum poses health risks due to the production of mycotoxins such as trichothecenes and zearalenone in infected cereals, leading to toxicity symptoms in animals and humans (Bennett and Klich, 2003). Current control measures for FHB include drainage management, seed disinfection, chemical treatments, and best management practices during the emergence to flowering period of infection. However, efforts to breed disease-resistant or mycotoxin-resistant crops and develop eco-friendly control agents are still in the early stages (Baek et al., 2020; Goswami and Kistler, 2004). Despite its pathological significance, disease control for F. graminearum heavily relies on chemical agents (Kim et al., 2017).
Upon penetrating the host plant, F. graminearum absorbs nutrients through hyphal growth during the infection process, giving rise to aerial hyphae and conidia through asexual reproduction. These conidia, becoming airborne, initiate infections on new host plants. However, when the fungus perceives an unfavorable environment for its survival (e.g., fall and winter), it shifts from hyphal growth and asexual reproduction to producing black, hard-shelled sexual fruiting bodies called perithecia on the surface of plant debris. The perithecia, within which a total of eight ascospores are formed in a single ascus after meiosis during sexual reproduction, serve both as overwintering propagules (Turkington et al., 2016) and as primary inocula for epidemics of the diseases in crop fields (Trail et al., 2002).
Under laboratory conditions, F. graminearum, like most filamentous ascomycetes, forms three primary hyphae types on solid substrates: aerial, surface, and penetrative hyphae (Fig. 1). Aerial hyphae rise for conidia dispersal, while surface and penetrative hyphae are responsible for nutrient uptake (Balmant et al., 2015). During sexual reproduction conditions, surface and certain penetrative hyphae combine to form a perithecium, a sexual fruiting body. Inoculation of hyphae or conidia onto agar medium, particularly carrot agar, initiates radial growth. Dense aerial hyphae emerge within approximately 3 days, forming clear radial colonies. Subsequently, colonies continue radial growth, but a collapse of some aerial hyphae is occasionally observed in the central colony area of the medium from 1-2 day after inoculation. In most cases, new aerial hyphae rarely emerge in the collapsed region; instead, perithecia form after 20 days. This irreversible collapse of aerial hyphae and subsequent induction of sexual reproduction are reported in other fungi. In 1928, Hein described a similar phenomenon in Sordaria, where the central area collapsed, expanding radially, and young ascocarps developed 5-7 days later (Hein, 1928). Therefore, aerial hyphae collapse appears to be a crucial mycological feature, inducing perithecia formation—a pathologically significant process, considering the role of perithecia in the recurrent cycle of FHB development in crop fields. Additionally, an artificial method for inducing perithecia on carrot medium involves inoculating hyphal fragments or conidia, culturing for a week, and then artificially removing aerial hyphae. After an additional week of re-incubation, new aerial hyphae do not emerge in the removed areas, leading to perithecia formation. Consequently, it is evident that the artificial aerial hyphae removal is an essential condition for inducing sexual reproduction in F. graminearum.
The sexual reproduction of F. graminearum, a homothallic (self-fertile) species, is controlled by master regulators known as mating-type (MAT) loci (Kim et al., 2012). Previous research has suggested that the MAT loci are activated in response to various environmental cues such as light and nutrient conditions, ultimately controlling the expression of over 1,245 target genes throughout the entire sexual development stage (Kim et al., 2015). However, the potential role of the natural aerial phenomenon, as described above is an additional environmental cue for influencing sexual developmental processes in F. graminearum remains unexplored. With the assumption that aerial hyphae collapse acts as a prerequisite for inducing perithecia formation, the primary objective of this study is to conduct a functional characterization of a set of genes regulated during aerial hyphae collapse. Additionally, our aim is to elucidate the relationship between aerial hyphae collapse and sexual development in F. graminearum. By unraveling the molecular mechanisms underlying aerial hyphae collapse and its significance in sexual development, this study contributes valuable insights towards the development of targeted strategies for managing FHB.
The F. graminearum Z3643 strain, obtained from Dr. Robert L. Bowden of the United States Department of Agriculture-Agricultural Research Service (USDA-ARS) was used as a wild-type (WT) strain for this study. For fungal genomic DNA extraction, the WT strain and gene deletion strains were grown in CM agar medium (0.2% NaNO3, 0. 25% peptone, 0.1% yeast extract, 3% sucrose, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.05% KCl, 0.2% trace element solution, 1% vitamin stock solution, 1.5% agar powder). Fungal conidia or perithecia production was induced using CMC medium (1.5% carboxymethyl cellulose sodium salt, 0.1% yeast extract, 0.05% MgSO4·7H2O, 0.1% NH4NO3, 0.1% KH2PO4) and carrot agar medium (Leslie and Summerell, 2006), respectively. For regeneration of fungal protoplasts, a regeneration medium (1 M sucrose, 0.1% yeast extract, 0.1% casein enzymatic hydrolysate, 1.5% agar powder) was employed. All fungal cultures grown on agar media were maintained at 25°C, while the liquid cultures were incubated at 140 rpm in a shaking incubator. Perithecia formation was induced under two culture conditions: either incubating the fungal inoculum on carrot agar medium for 7 days, followed by the removal of aerial hyphae using 2.5% Tween 60, and subsequent incubation under a black light blue lamp for 7-10 days as previously described (Lee et al., 2003), or incubating on the same carrot agar medium for 3-4 weeks without any treatment.
For the expression analysis of specific genes, total RNA was extracted from areas where aerial hyphae were formed, naturally collapsed, or artificially removed in 55 mm or 150 mm Petri dishes using Ribospin II from GeneALL (Seoul, Korea). The extracted total RNA was then reverse-transcribed into cDNA using ReverTra qPCR RT Master Mix from TOYOBO (Osaka, Japan). The synthesized cDNA served as the template for qPCR, conducted with 2× Real-Time PCR Master Mix from BIOFACT (Daejeon, Korea). To ensure data normalization, the EF1α gene (FGSG_08811) was employed as a control, as previously described (Shin et al., 2022). The primers used for assessing the expression patterns of the selected genes are detailed in Supplementary Table 1. For RNA-seq analysis, total RNA was extracted from two distinct regions of the F. graminearum WT strain culture grown on carrot medium at 25°C for 3 days, specifically during the occurrence of aerial hyphae collapse. The RNeasy mini kit from Qiagen (Hilden, Germany) was employed for RNA extraction from both the collapsed and non-collapsed regions. Subsequently, the extracted RNA samples were sent to Theragen Co., Ltd. (Seongnam, Korea) for library construction and RNA-seq analysis using the Illumina HiSeq2500 platform. Differentially expressed gene (DEG) analyses were conducted by considering genes that exhibited at least a twofold (|log2 of the FPKM value|≥1) increase or decrease in expression between the two regions.
To generate deletion strains for the selected 26 genes, we employed a slightly modified fusion PCR method based on a split-marker recombination procedure (Catlett et al., 2003). The DNA regions 5′ and 3′ of each target gene were amplified using specific primer pairs (Supplementary Table 2). Selectable markers conferring resistance to geneticin (gen) were amplified from the plasmid pII99 (Namiki et al., 2001). Subsequently, three PCR fragments—5′ flanking region, gen, and 3′ flanking region—were used to generate two fused PCR products, including a split marker, in a second PCR using nested primers (Supplementary Table 1). These final PCR products, followed by purification with DNA Clean & Concentrator-5 from Zymo Research (Irvine, CA, USA), were introduced into protoplasts of the WT strain. Protoplasts were generated by treating the young mycelia with Driselase (Sigma-Aldrich, St. Louis, MO, USA), as previously described (Shin et al., 2022). The occurrence of gene deletion events, resulting from homologous recombination between the split marker and the genome in fungal transformants was validated through PCR analysis (Supplementary Figs. 1-5). In this experiment, a minimum of three independent deletion strains were obtained for each targeted gene.
To induce the perithecim formation in F. graminearum under laboratory conditions, an agar block (1 mm in diameter) of hyphae of the F. graminearum Z3643 WT strain were inoculated into the center of carrot agar medium and cultured for 7 days for the formation of aerial hyphae. When the aerial hyphae were artificially removed, they could not be re-formed; instead, perithecia were formed in the area where they were removed (Fig. 2A), which is a commonly used method for inducing perithecium formation in F. graminearum (Kim et al., 2008). Conversely, when the fungus was grown on carrot agar medium without artificially removing the aerial hyphae, we observed a phenomenon termed “aerial hyphae collapse” characterized by the falling of hyphae to the medium’s bottom as described by Hein (1928) (Fig. 2B). Clusters of perithecia formed in the collapsed region over 3-4 weeks of continuous hyphal culture at 25°C (Fig. 2C). In contrast, perithecia formed individually throughout the carrot agar medium when induced through artificial hyphal removal (Fig. 2A).
For RNA-seq analysis, total RNAs were extracted from regions with aerial hyphae collapse (inside) and regions with ongoing aerial hyphae growth (outside) on a carrot agar medium plate cultured for 3 days at 25°C (Fig. 3). We identified 699 DEGs with 334 genes showing increased expression in the inside compared to those in the outside, and 365 genes showing increased expression in the outside (Supplementary Table 3). Out of the 699 DEGs, excluding 479 annotated as hypothetical proteins, 120 (17.2%) were associated with various transporters, 33 were related to tailoring enzymes such as oxidoreductase, cytochrome P450, methyltransferase, and so on, and 29 belonged to transcription factors carrying a DNA-binding domain (Supplementary Table 3). We compared these DEGs with the MAT target genes (Kim et al., 2015), which are transcriptionally regulated by mating-type loci (MAT loci) collectively controlling the sexual reproductive process of F. graminearum. Among the 699 DEGs, only 55 and 42 genes, whose expression levels specifically increased internally and externally, respectively, were overlapped with the MAT target genes (Supplementary Table 4). Interestingly, within the group of genes overlapping with the MAT target genes, the frequency of genes regulated in the post-meiotic stage (i.e., those simultaneously regulated by both MAT1-1 and MAT1-2 after fertilization) during the sexual reproduction of F. graminearum (Supplementary Table 4) (Kim et al., 2015) was very low—4 out of the 55 inside-up regulated genes (7.3%) and 8 out of 42 outside-up genes (19.0%). All other overlapped genes were regulated only by either MAT1-1 or MAT1-2 (Supplementary Table 4). In addition, four MAT genes (MAT1-1-1, MAT1-1-2, MAT1-1-3, and MAT1-2-1) located at both MAT1-1 and MAT1-2 loci, respectively, were not found among the 699 DEGs. Subsequently, we selected 26 genes from the total 699 DEGs, which showed at least 10-fold difference in FPKM values between the inside and the outside region for further functional analysis. Eleven genes exhibited higher expression in the inside (aerial hyphae collapse region), while 15 genes demonstrated higher expression in the outside region (aerial hyphae growth region) (Table 1).
To observe phenotype of the gene deletion strains, changes in perithecium/ascus/ascospore formation and maturation after the artificial removal of aerial hyphae, as well as those in hyphal growth during the vegetative growth stage on carrot medium, were compared with the WT Z3643 strain. Among the deletion strains of five genes (FGSG_09210, FGSG_09896, FGSG_03638, FGSG_08210, and FGSG_13952) out of the 26-selected genes (hereafter denoted as ΔFGSG_09210, ΔFGSG_09896, ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952, respectively), phenotypic changes in either vegetative growth or sexual reproduction were observed (Figs. 4 and 5). While no dramatic changes in the ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952 strains in the shape, color of the aerial hyphae, and the pattern of aerial hyphae collapse were observed compared to the WT strain, in the ΔFGSG_09210 strain, whether collapse occurred or not seemed unclear. The amount of aerial hyphae in the center of the medium was relatively small, and the color was mixed yellow and white. In the ΔFGSG_09896 strain, aerial hyphae collapse was confirmed to have occurred, but the height of the aerial hyphae was low, preventing them from reaching the ceiling of the petri dish. The ΔFGSG_09210, ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952 strains grew their aerial hyphae high enough to reach the ceiling of the petri dish (Fig. 4).
In the case of sexual reproduction, the WT strain forms large quantities of typical perithecia on the surface of the carrot agar medium. In contrast, no perithecia were formed in the culture of the ΔFGSG_09210 strain, and the numbers of perithecia formed in the ΔFGSG_09896, ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952 strains were reduced by approximately 8-49% compared to the WT level, determined by the areas of the medium where the perithecia were distributed (Figs. 5 and 6). The size of perithecium also differed in the gene deletion strains. In the WT strain, majority (87%) of the perithecia produced on the agar surface of the plate had a diameter greater than 100 μm, whereas in the four gene deletion strains, the proportion of perithecia larger than 100 μm was significantly reduced to 47.1-74.7% of the WT level (Fig. 7). Additionally, except for the ΔFGSG_09210 strain that produced no asci and ascospores due to the absence of perithecia, the remaining gene deletion strains showed differences in the number and maturation status of asci/ascospores within a perithecium. In the ΔFGSG_09896 strain, ascospores were either immature or absent. However, eight ascospores within an ascus, similar to those in the WT strain, were observed in the ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952 strains, respectively, although the number of mature asci seemed to be lower than in the WT strain (Fig. 5B).
When grown on carrot agar medium without artificial removal of aerial hyphae, changes in vegetative growth or sexual reproduction were observed in the ΔFGSG_09210, ΔFGSG_09896, ΔFGSG_03638, ΔFGSG_08210, and ΔFGSG_13952 strains (Fig. 8). In the WT strain, white hyphae were produced until 3 to 4 days after inoculation. Then, they turned yellow when the hyphae touched the plate wall and could no longer grow laterally. The color of the bottom of the medium changed from orange to red. After the color of the aerial hyphae changed at day 14, the overall color became whitish and cloudy, and hyphae at the edge of the medium turned distinctly white. In the ΔFGSG_09210 strain, the amount of aerial hyphae was relatively low, making it unclear whether aerial hyphae collapse occurred. In the ΔFGSG_09896 strain, the color of the medium turned red, but the color of the aerial hyphae remained white continuously, and the center of the medium turned a cloudy yellow at day 14. In the ΔFGSG_03638 strain, unlike the other strains, a large amount of aerial hyphae grew beyond the plate. For the ΔFGSG_08210 and ΔFGSG_13952 strains, the phenotype was similar to the WT during vegetative growth (Fig. 8). Perithecia were formed clustered at the region of aerial hyphae collapse, and the maturation of asci and ascospores within the perithecia proceeded normally in the WT strain. In contrast, we found that no perithecia formed in the ΔFGSG_09210, ΔFGSG_03638, and ΔFGSG_13952 strains, and only a fewer number of perithecia were formed in the remaining gene deletion strains (Fig. 8), similar to the case of perithecia formation induced by the artificial hyphal collapse described above.
Expression patterns of five selected genes (FGSG_09210, FGSG_09896, FGSG_03638, FGSG_08210, and FGSG_13952), which showed phenotypic changes in gene deletion strains, were analyzed using qPCR in the WT strain grown on carrot agar (Fig. 9). For total RNA extraction, fungal samples were collected from the inside and the outside regions of hyphal growth, respectively, on carrot medium in the WT strain every 3 days for a total of 24 days. The expression patterns of each gene were quantitatively analyzed using primers derived from the exon parts of the five genes. To normalize the gene expression data, comparisons were made based on the expression in the inside at day 3 of fungal culture. FGSG_09210 showed continuously increased expression throughout the culture period, peaking at day 21 in the inside and day 15 in the outside; the expression level in the inside at day 21 was 4.8-fold higher than that in the outside (Fig. 9A). In contrast to FGSG_09210, the FGSG_09896 gene exhibited lower expression levels with no significant fluctuations during most time periods in both conditions. The gene expression at day 3 in the outside reached the peak and 2.3-fold higher than that in the inside (Fig. 9B). The FGSG_03638 gene showed a similar pattern to the FGSG_09896 gene (Fig. 9C): the gene expression in the outside reached the peak level at day 3, abruptly reduced at day 6, and remained at basal levels. The remaining two genes FGSG_08210 and FGSG_13952 exhibited dramatically increased expression patterns until day 24 in the outside with much higher levels in in the inside during the same time points, indicating that these genes are specific to aerial hyphae growth. In particular, FGSG_08210 showed no difference in expression between the inside and outside until day 9, but the expression in the outside began to increase compared to inside from day 12, reaching the peak at day 24, where it was 15.2-fold higher than in the inside (Fig. 9D). Similarly, the FGSG_13952 gene showed that the expression level began to increase from day 6, and reached to 370-fold higher level compared to that at day 3; the gene expression in the inside remained only at basal levels during most incubation time points (Fig. 9E).
We conducted a second set of gene expression analyses to compare the expression levels among the genes functionally examined above, except for FGSG_08210, in two different carrot agar cultures of the WT strain (Fig. 10). One culture was maintained for natural aerial hyphae collapse, as described earlier, while the other undergoing artificial removal of aerial hyphae. Additionally, two major MAT genes (MAT1-1-1 and MAT1-2-1), located at MAT1-1 and MAT1-2 loci, respectively were included in this analysis. It is important to note that, in the first condition, total RNA was extracted from the entire culture in a petri plate, rather than from two separate regions such as the inside and outside, as described earlier. Overall, the expression patterns of the four genes in the first culture condition were similar to those investigated in the initial gene expression analysis, where total RNAs were extracted from the inside and outside regions, respectively (Fig. 10A). The FGSG_09210 gene exhibited a dramatically increased expression pattern up to the last day of culture (i.e., day 19), with expression levels were much higher compared to those of the other genes during most incubation time points. In contrast, the expression level of FGSG_09896 peaked at day 3, then continuously decreased until day 19 (Fig. 10A). The patterns of the remaining two genes (FGSG_03638 and FGSG_13952), shown in the enlarged image in Fig. 10A, were similar to those from the outside region of fungal culture in the initial analysis (Fig. 9). In addition, the two MAT transcripts were gradually increased, reaching the peak at day 11 (with ~20-fold higher than at day 1), and remained at similar levels until day 19. Their expression levels were much lower than those of FGSG_09210 during the entire incubation period (Fig. 10A).
Interestingly, under the second culture condition, which is the conventional condition for inducing perithecim formation, the expression patterns of these genes differed from those under the first condition (Fig. 10B). First of all, the two MAT genes began to be dramatically induced and showed the peak expression level at day 8, one day after aerial hyphae were artificially removed, then abruptly reduced (Fig. 10B). The expression of FGSG_09210, which was already confirmed to be sexual stage-specific, also reached the peak at day 8, and abruptly decreased, as did the MAT genes; its expression level at day 8 was similar to those of the MAT genes (Fig. 10B). Similarly, the expressions of FGSG_03638 and FGSG_13952 increased abruptly and reached the peak with levels similar to or even higher than those in FGSG_09210, as well as two MAT genes at day 12 and 16, respectively. These patterns were different from those shown under first culture condition (Fig. 10A). FGSG_09896 showed an early vegetative growth-specific expression pattern, as in the first condition (Fig. 10B).
Unlike many other heterothallic species, F. graminearum, a homothallic species, does not require hyphal contact between two opposite sexual partners for the formation of sexual fruiting bodies (perithecia). Instead, this fungus is capable of producing large quantities of peirtheicia on carrot agar medium by simply removing the aerial hyphae that had previously grown on the medium. The artificial removal of aerial hyphae, a well-established method for studying sexual reproduction in F. graminearum (Kim et al., 2008), is an irreversible phenomenon, typically occurring in highly self-fertile F. graminearum strains. New aerial hyphae do not appear in the region where old aerial hyphae were artificially removed. In contrast, other members of the F. graminearum species complex, such as F. asiatiucm, which usually have a very low capacity for self-fertility, frequently exhibit the reappearance of new aerial hyphae even after several rounds of removing old aerial hyphae on carrot agar (Jang et al., 2019; Kim et al., 2012). We wondered if a similar phenomenon occurs for perithecia formation on host plants in nature. In this context, we observed that F. graminearum retains the capacity to produce perithecia on carrot agar medium without any treatment, albeit taking a longer time and resulting in fewer perithecia compared to the artificial hyphae removal method. A new phenomenon termed “aerial hyphae collapse”, where perithecia formation occurred naturally without the artificial removal of aerial hyphae. This finding is consistent with Hein’s earlier work (1928). Aerial hyphae collapse, characterized by the falling of hyphae to the bottom of the medium, leads to the subsequent formation of clustered perithecia in the collapsed region. This contrasts with the evenly distributed perithecia formed through artificial hyphal removal. This implies that alterations in aerial hyphae structure, whether artificially removed or naturally collapsed, may serve as a prerequisite for transitioning from vegetative growth to sexual development in F. graminearum.
Based on this assumption, we investigated several questions regarding the relationship between aerial hyphae collapse and sexual development in F. graminearum. First, to explore whether aerial hyphae collapse leads to induction of genes involved in sexual developmental process, we identified 699 genes specifically up- or down-regulated in the culture region where early aerial hyphae collapse occurred. Among the DEGs with functional categories including transporters, tailoring enzymes, and transcription factors, only 13.9% were overlapped with those identified in the F. graminearum strains deleted for the MAT genes, known to be master regulators controlling the entire sexual developmental process. Moreover, the majority of DEGs that overlapped with the MAT target genes were found to be regulated only by individual MAT1-1 or MAT1-2 genes (Kim et al., 2015), suggesting that most DEGs during the early aerial hyphae collapse are not directly controlled by MAT-mediated regulatory system, although a subset of DEGs are associated with sexual development based on gene functional analysis. Even MAT-regulated genes seem to play some roles in the early stages of sexual development, probably before cytoplasmic and/or nuclear fusion between cells. Considering the other fact that aerial hyphae collapse is irreversible in the F. graminearum WT strain, natural aerial hyphae collapse may lead to alterations in fungal physiological status by regulating genes required for blocking the subsequent growth of aerial hyphae and switching the fungal vegetative growth mode to the early sexual developmental stage, which could be prerequisite for inducing the formation of fertile perithecia. Once these alterations occurred by aerial hyphae collapse, it can be inferred that the expression of genes involved in the main stages of sexual reproduction such as cytoplasmic/nuclear fusion and meiosis, is regulated by MAT genes later.
Second, to investigate whether natural aerial hyphae collapse is a physical cue for inducing perithecium formation, it is necessary to compare the two different procedures for the induction of perithecia formation on carrot agar. The artificial method is so efficient that it produces a much larger quantity of fertile perithecia at least two three weeks earlier than the natural method. A comparison of gene expression patterns between natural aerial hyphae collapse and artificial removal conditions revealed distinct temporal profiles. In particular, the sharp induction of MAT genes and selected DEGs in response to artificial hyphal removal clearly demonstrates that it is a physical stimulus for undergoing sexual development mainly controlled by the MAT genes in F. graminearum. In contrast, the effect of natural hyphae collapse on the induction of MAT gene expression was not as dramatic as that of artificial hyphae removal. Nevertheless, it appears to gradually induce MAT gene expression and maintain expression levels, albeit much lower than in the case of the artificial method, consistently until the last incubation day. This is also different from the artificial method, where MAT gene expressions, once dramatically induced by artificial removal, were abruptly reduced (Fig. 10B). In addition, the five selected DEGs, which are functionally required for perithecia formation (Fig. 5), clearly exhibited differential expression patterns, particularly in the inside (collapsed) region, either sustaining upregulation (FGSG_09210) or downregulation (FGSG_09896, FGSG_03638, FGSG_08210, FGSG_13952) throughout the culture periods (Figs. 9 and 10A), although two genes (FGSG_03638 and FGSG_13952) were up-regulated at the later stage of sexual development under the artificial condition (Fig. 10B). Notably, both FGSG_09210 and FGSG_09896 showed a consistent expression pattern in both culture conditions—the former is specific to sexual development induced by both artificial and natural methods, and the latter specific to early vegetative (aerial hyphae) growth before the induction of MAT genes (Fig. 10). Based on the functional requirement of these two genes, determined by gene deletion analysis, they are specifically operated during aerial hyphae collapse (or removal). Taken all together, it is clear that natural aerial hyphal collapse plays a physical cue for inducing perithecia formation as the artificial hyphal removal, although its effect is not as dramatic as in the artificial method.
Third, targeted gene deletion analysis revealed specific functions of the selected DEGs during both natural hyphae collapse and perithecium formation in F. graminearum. In particular, all five genes described in this study were confirmed to be involved in natural aerial hyphae collapse, with their deletion strains forming smaller or no hyphae collapsed region and fewer perithecia compared to the WT strain. (Figs. 4 and 5). FGSG_09210, which encodes a putative phospholipid-translocating ATPase and is sexual stage-specific in gene expression, may be involved in various membrane biology essential for proper growth and pigmentation of aerial hyphae growth, as well as sexual development in F. graminearum. FGSG_09896, encoding an isocitrate lyase, down-regulated in the MAT1-2-1-overexpressing strain (Kim et al., 2015), was vegetative growth-specific at the transcriptional level (Figs. 9 and 10) and functionally essential for pigmentation of aerial hyphae, perithecia formation and ascus/ascospore maturation, as previously confirmed (Lee et al., 2009). This indicates that the tight regulation of glyoxylate cycle, activated during aerial hyphae growth, while repressed during natural aerial hyphae collapse or after artificial hyphae removal, is required for sexual development in F. graminearum. FGSG_03638, which encode a putative choline dehydrogenase, shows early hyphal growth-specific expression (Figs. 9 and 10) and transcriptionally regulated by MAT genes (Kim et al., 2015), has been associated with the production of secondary metabolites under nutrient-limited conditions, indicating that secondary metabolism related to this gene is necessary for aerial hyphae growth, but not during aerial hyphae collapse (Fernando et al., 2019). The FGSG_08210, a hypothetical protein, is known to be a member of the PKS6 gene cluster required for the biosynthesis of the lipopepdie fusaristatin A in F. graminearum (Sørensen et al., 2014). Fusaristatin A is known to have a negative effect on fungal growth and aggressiveness in the wheat pathogen F. pseudograminearum (Khudhair et al., 2020). Although it is unknown if FGSG_08210 is essential for fusaristatin A production in F. graminearum, it is likely that fusaristatin A biosynthetic pathway is associated with the maintenance of natural hyphal collapse. FGSG_13952, a small secreted cysteine-rich proteins-encoding gene, is known to be very highly expressed under saprophytic condition on dead wheat (Boedi et al., 2016) but down-regulated during wheat head infection (Hao et al., 2020). Interestingly, both FGSG_08210 and FGSG_13952 were identified as putative effector-coding genes, indicating that these genes are necessary for aerial hyphae growth, but not for sexual development or in planta growth (Hao et al., 2020).
In conclusion, our findings provide valuable insights into the intricate relationship between aerial hyphae collapse and sexual development in F. graminearum, offering a novel perspective on the regulatory mechanisms underlying these processes. The natural aerial hyphal collapse on carrot agar, akin to the artificial hyphal removal on the same medium, emerges as a significant physical cue for inducing peritheciua formation, even on host plants or plant debris in nature, which serves as a survival strategy of the fungus during winter. Therefore, further investigations into molecular mechanisms controlling aerial hyphae collapse can provide clues for a new disease control strategy for FHB caused by F. graminearum.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Information, Communication & Technology (NRF-2017R1A2B4011541), and also supported by the Soonchunhyang University Research Fund.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig. 1
Diagram illustrating the growth of hyphae, asexual spores (conidia), and sexual fruiting bodies (perithecia) in Fusarium graminearum on carrot agar medium, modified from Balmant et al. (2015) according to Creative Commons license.
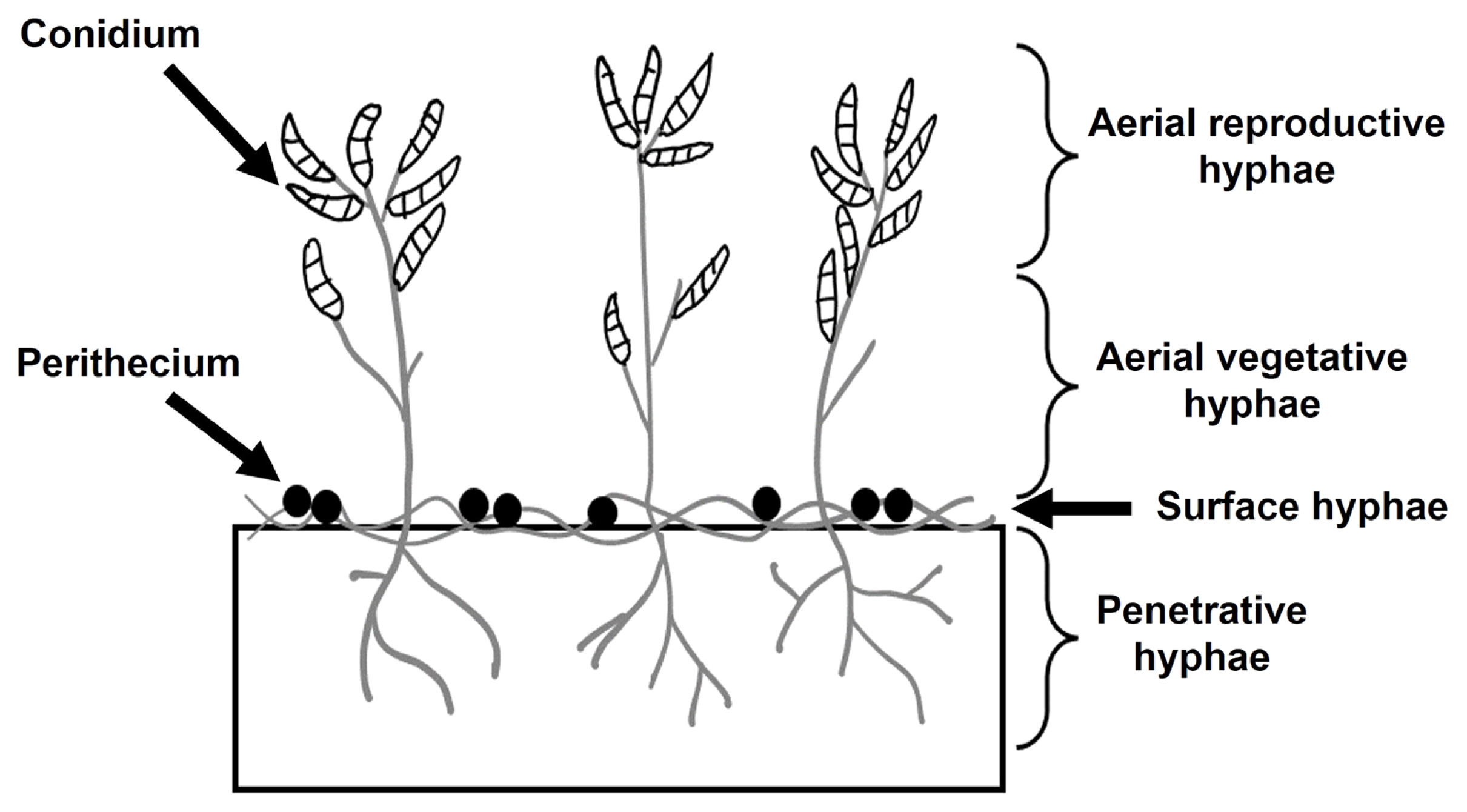
Fig. 2
Induction of perithecium formation by the artificial removal of aerial hyphae (A) or in the region of natural collapse of aerial hyphae (B, C). (A) After incubating a fungal inoculum on carrot agar medium for 7 days at 25°C, the aerial hyphae were removed with 2.5% Tween 60 and re-incubated for 5 days. (B, C) Conidia of Fusarium graminearum were inoculated onto carrot agar, incubated under the same conditions as (A) for 25 days, and the aerial hyphae were removed for clear visualization of clusters of perithecia formed on the agar surface. The regions of aerial hyphae collapse was indicated by arrows.
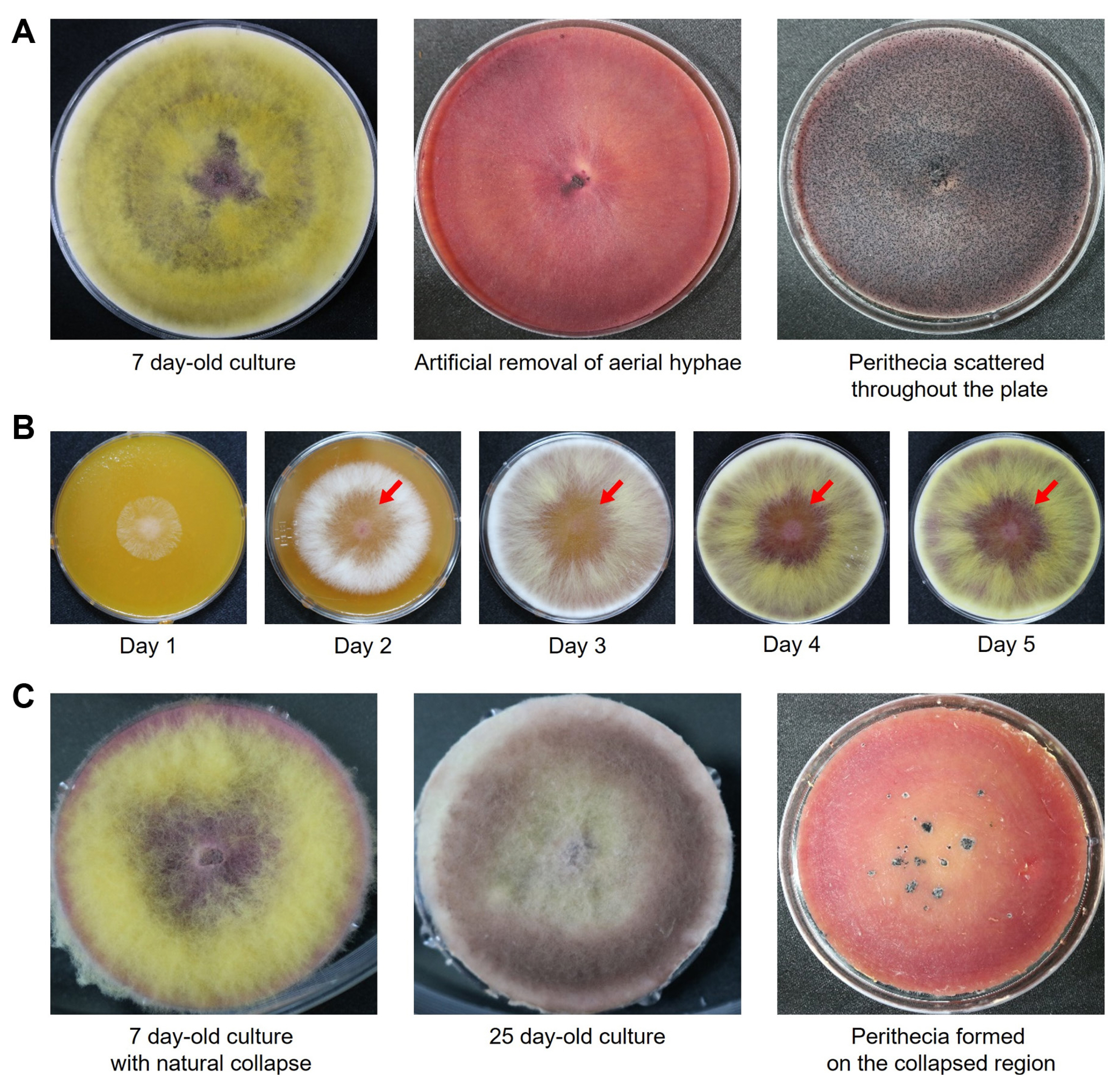
Fig. 3
The fungal culture areas on carrot agar medium, corresponding to the regions of aerial hyphae collapse (designated inside) and aerial hyphae growth (outside), respectively, were used for total RNA extraction for an RNA-seq analysis.
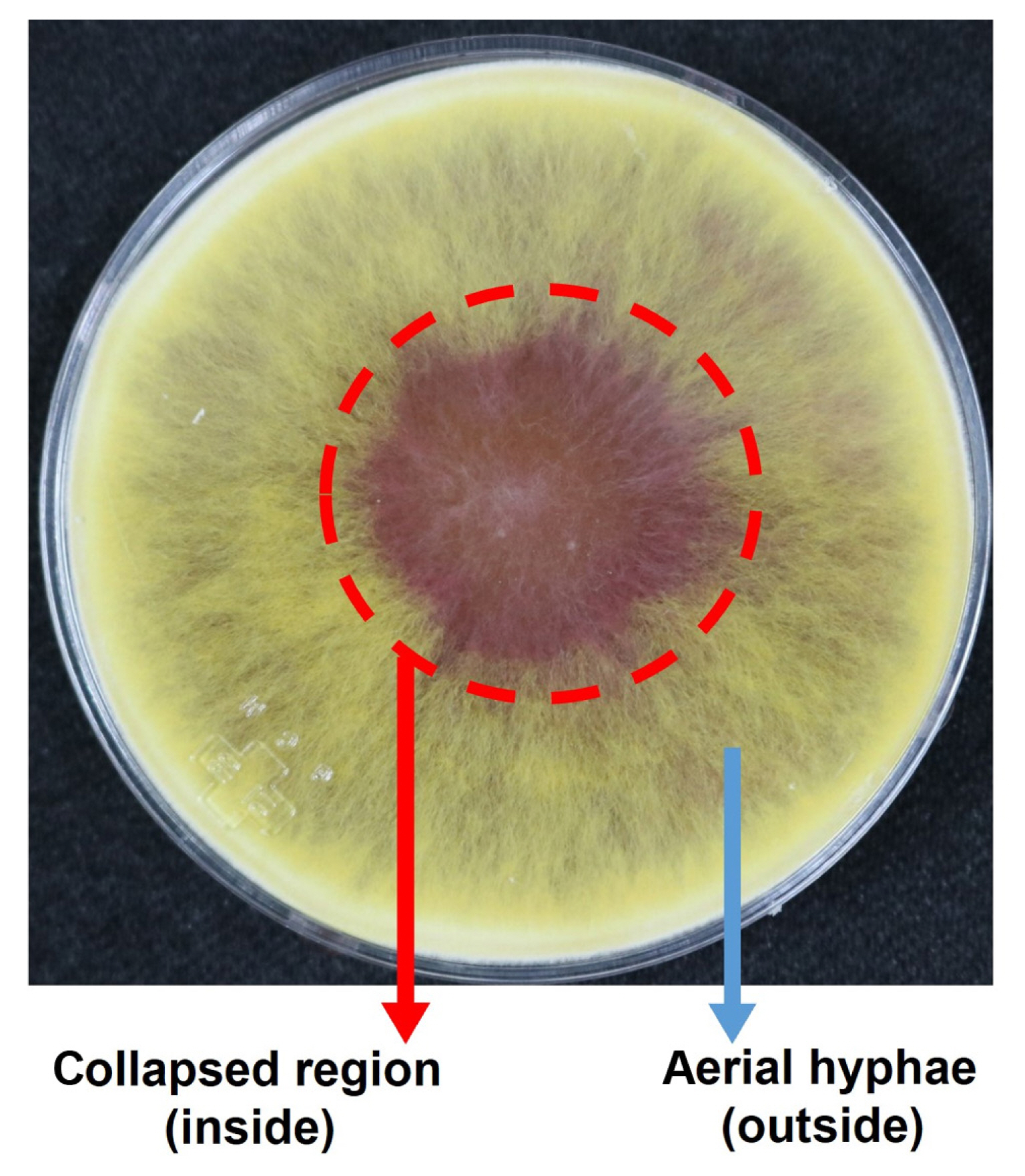
Fig. 4
Hyphal growth in the gene deletion strains on carrot agar medium. (A) Wild type Z3643 strain. (B) ΔFGSG_09210. (C) ΔFGSG_09896. (D) ΔFGSG_03638. (E) ΔFGSG_08210. (F) ΔFGSG_13952. After 7 days of incubation on carrot medium at 25°C, vegetative growth of aerial hyphae was observed.
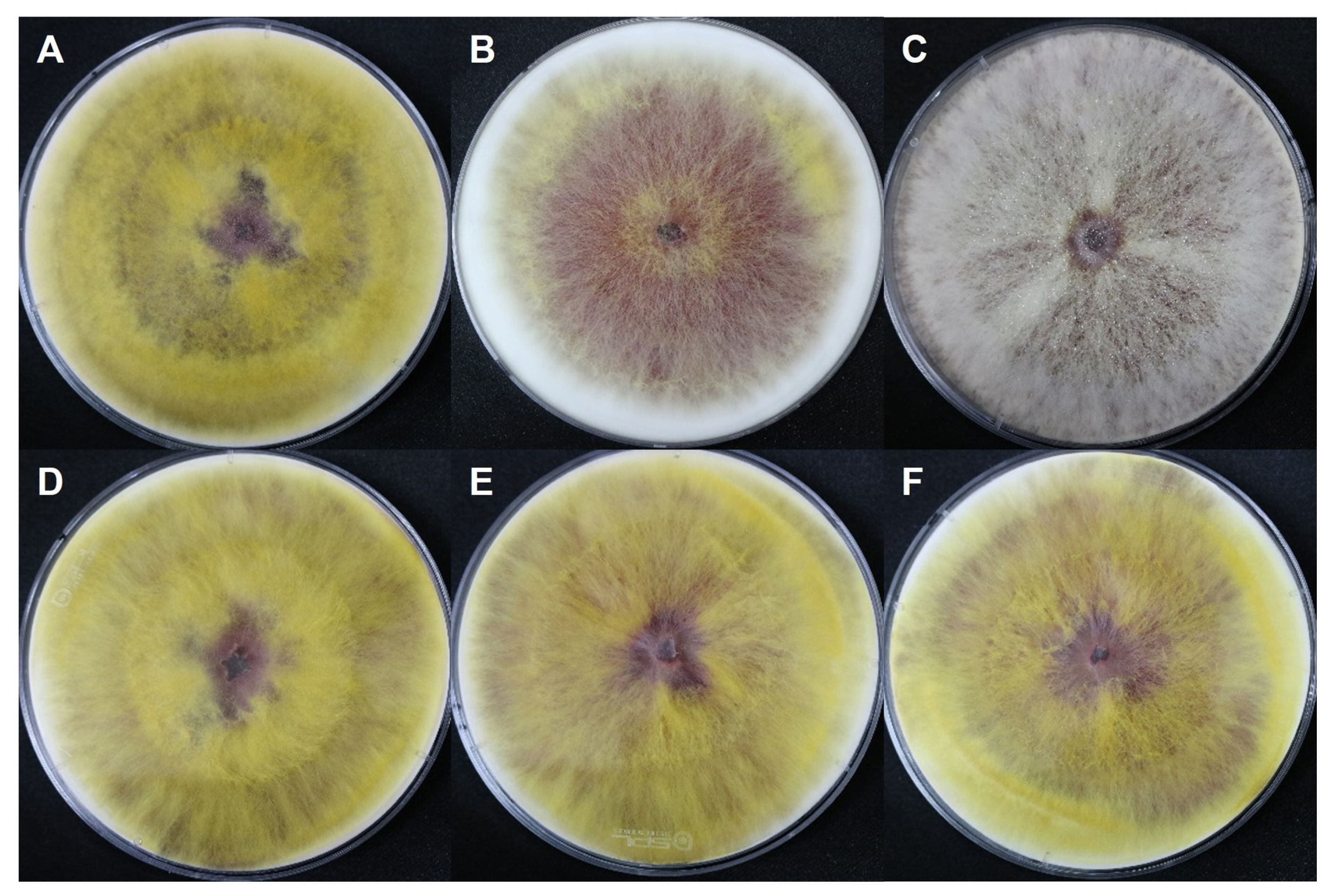
Fig. 5
Perithecium formation in the gene deletion strains after artificial removal of aerial hyphae. a, wild-type strain; b, ΔFGSG_09210; c, ΔFGSG_09896; d, ΔFGSG_03638; e, ΔFGSG_08210; f, ΔFGSG_13952. (A) After 7 days of incubation on carrot medium shown in Fig. 4, the aerial hyphae were removed and incubated for 7 days (×40, scale bars = 200 μm). (B) Asci and ascospores formation within a perithecium in the gene deletion strains (×200, scale bars = 40 μm).

Fig. 6
Relative perithecia formation areas on carrot agar culture of the gene deletion strains. The areas of perithecia formation on the carrot agar medium for each strain were determined using ImageJ software. To clearly distinguish between the colors of the agar medium and perithecia, respectively, the images were binarized, and the areas were measured by calculating the number of pixels.
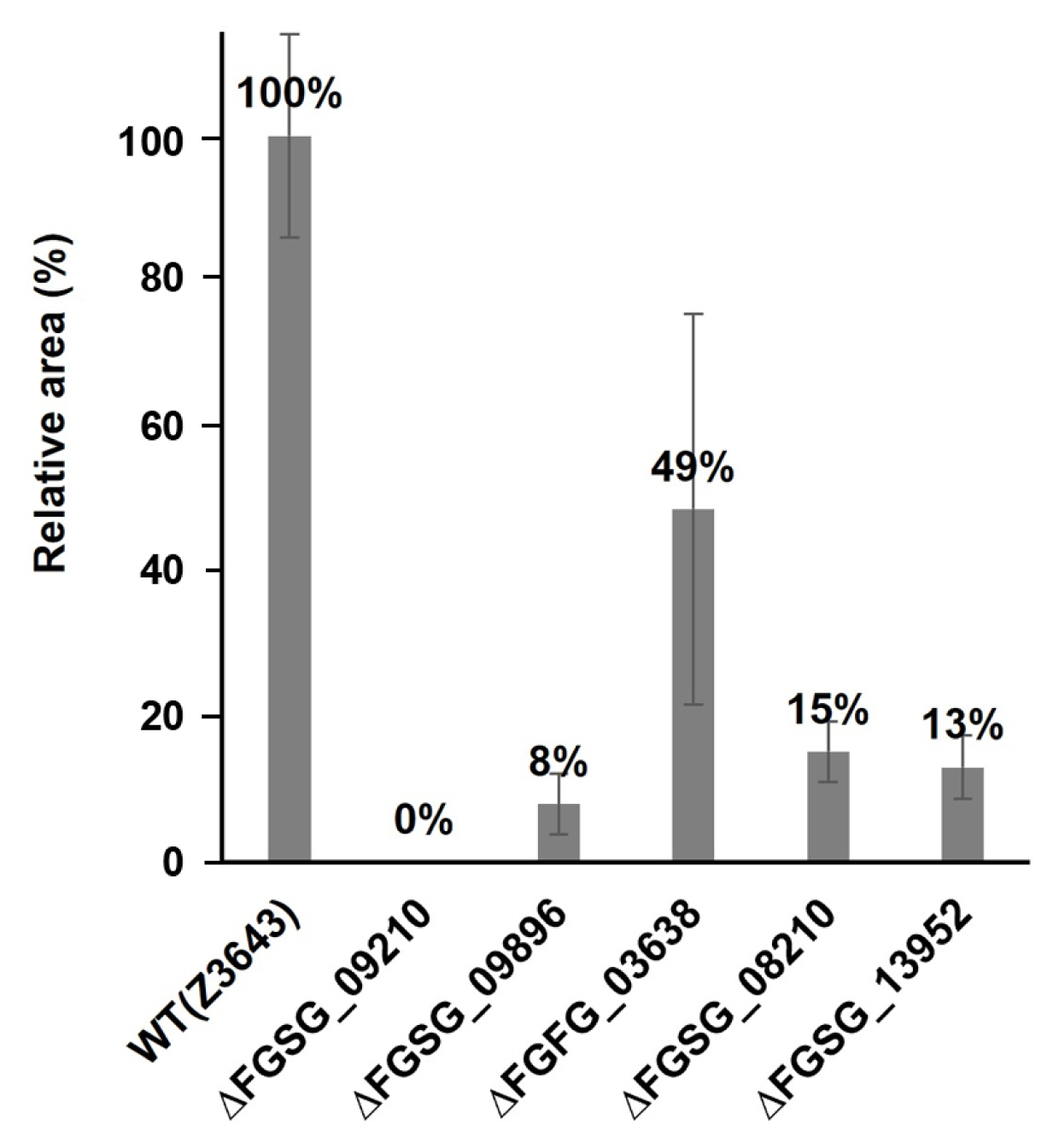
Fig. 7
Relative ratios of perithecia with diameters larger or smaller than 100 μm in the gene deletion strains.
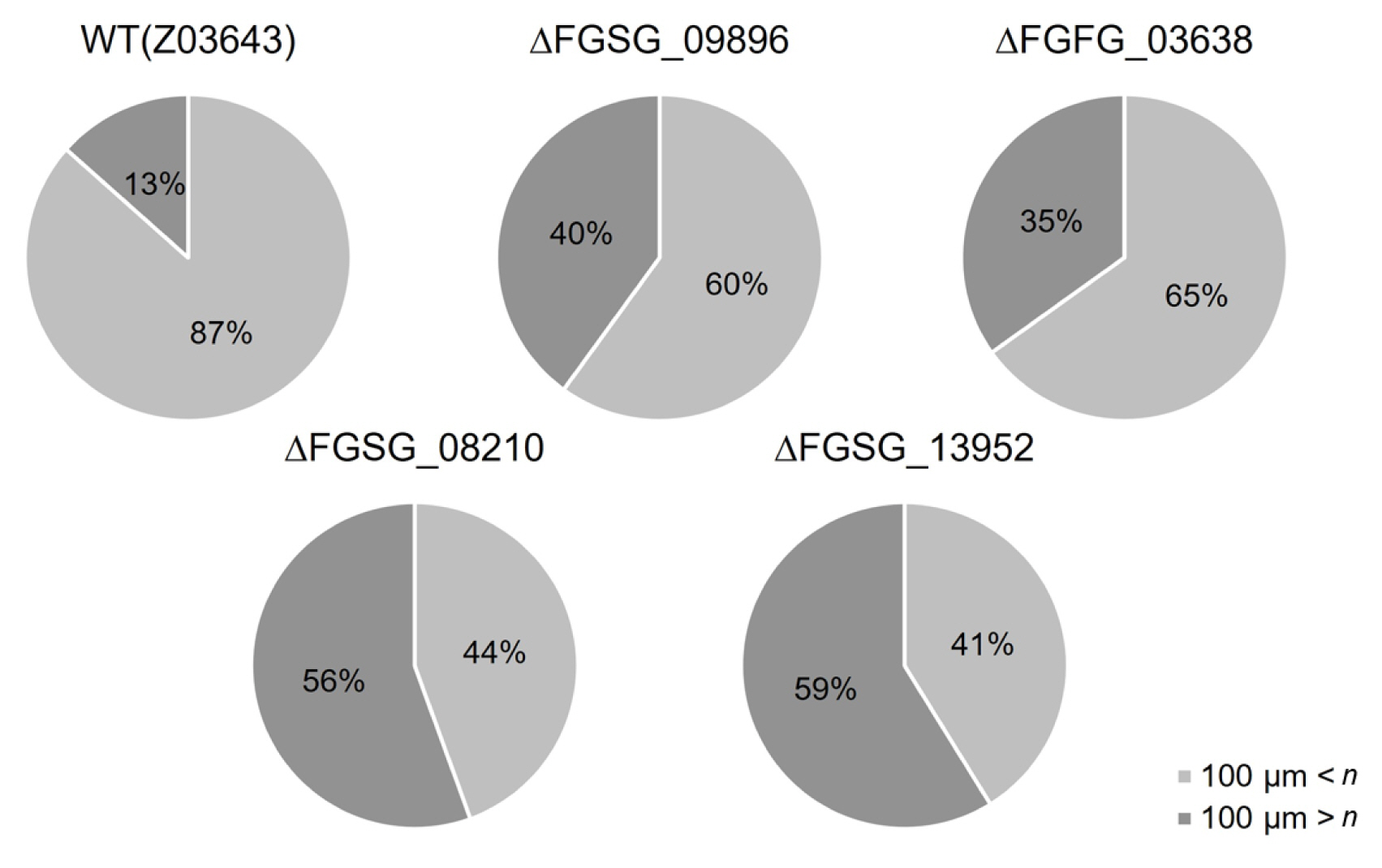
Fig. 8
Hyphal growth in the gene deletion strains without artificial removal of aerial hyphae. (A) Wild type strain. (B) ΔFGSG_09210. (C) ΔFGSG_09896. (D) ΔFGSG_03638. (E) ΔFGSG_08210. (F) ΔFGSG_13952. At day 28, aerial hyphae were removed for clear visualization of perithecia as in Fig. 2, and the perithecia clusters formed were indicated by a white arrow.
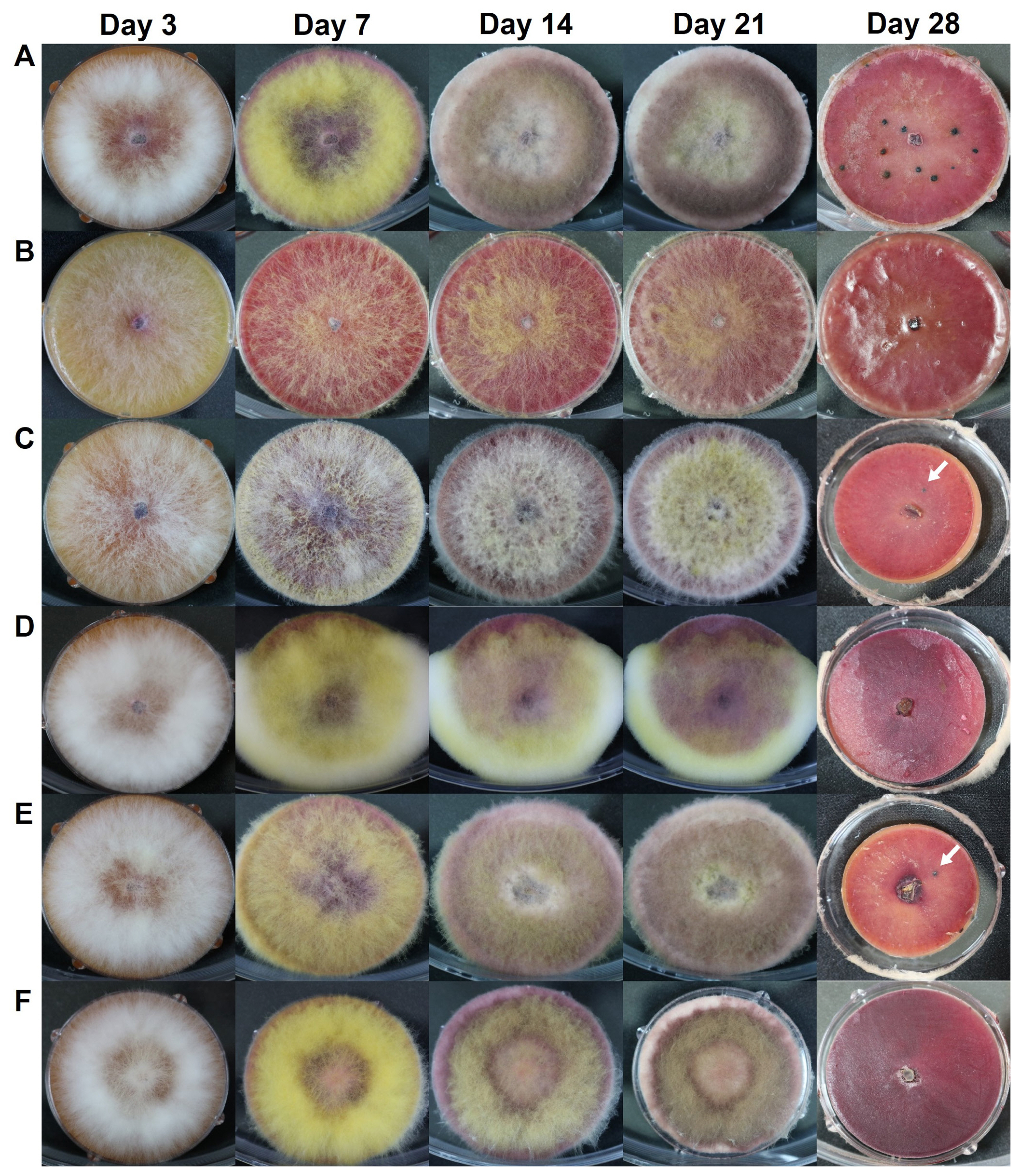
Fig. 9
Time-course expression pattern of the selected genes in the wild-type Z3643 strain grown on carrot agar medium. (A) FGSG_09210. (B) FGSG_09896. (C) FGSG_03638. (D) FGSG_08210. (E) FGSG_13952.
Fig. 10
Time-course expression patterns of the selected genes and two MAT genes in the wild-type Z3643 strain on carrot agar medium under conditions for natural aerial hyphae collapse (A) or for artificial hyphae removal (B).
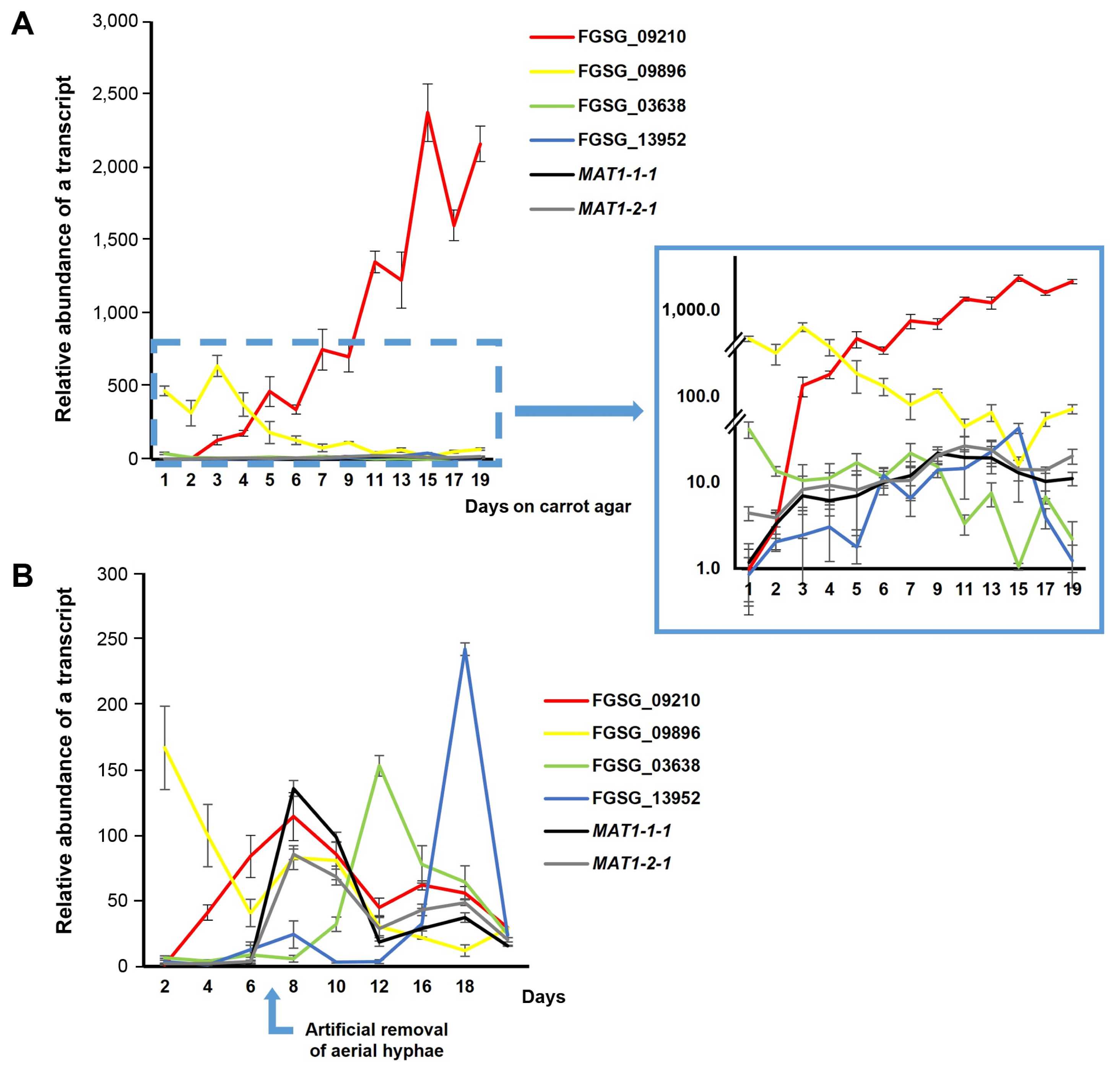
Table 1
List of DEGs for targeted gene deletions
References
Baek, S. G., Kim, S., Jang, J. Y., Kim, J. and Lee, T. 2020. Ferulic acid content of barley and wheat grains and head blight resistance. Res. Plant Dis. 26:250-255.


Balmant, W., Sugai-Guérios, M. H., Coradin, J. H., Krieger, N., Furigo, A. Junior and Mitchell, D. A. 2015. A model for growth of a single fungal hypha based on well-mixed tanks in series: simulation of nutrient and vesicle transport in aerial reproductive hyphae. PLoS ONE 10:e0120307.



Boedi, S., Berger, H., Sieber, C., Münsterkötter, M., Maloku, I., Warth, B., Sulyok, M., Lemmns, M., Schumacher, R., Güldner, U. and Strauss, J. 2016. Comparison of Fusarium graminearum transcriptomes on living or dead wheat differentiates substrate-responsive and defense-responsive genes. Front Microbiol. 7:1113.



Catlett, N. L., Lee, B.-N., Yoder, O. C. and Turgeon, B. G. 2003. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl 50:9-11.

Chung, H. S. 1975. Cereal scab causing mycotoxicoses in Korea and present status of mycotoxin researches. Korean J. Mycol. 3:31-36.
Fernando, U., Chatur, S., Joshi, M., Bonner, C. T., Fan, T., Hubbard, K., Chabot, D., Rowland, O., Wang, L., Subramaniam, R. and Rampitsch, C. 2019. Redox signalling from NADPH oxidase targets metabolic enzymes and developmental proteins in Fusarium graminearum. Mol. Plant Pathol. 20:92-106.



Goswami, R. S. and Kistler, H. C. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5:515-525.


Hao, G., McCormick, S., Usgaard, T., Tiley, H. and Vaughan, M. M. 2020. Characterization of three Fusarium graminearum effectors and their roles during Fusarium head blight. Front. Plant Sci. 11:579553.



Hein, I. 1928. Studies on morphogenesis in fungus mycelia. Bull. Torrey Bot. Club 55:513-528.
Jang, J. Y., Baek, S. G., Choi, J.-H., Kim, S., Kim, J., Kim, D.-W., Yun, S.-H. and Lee, T. 2019. Characterization of nivalenol-producing Fusarium asiaticum that causes cereal head blight in Korea. Plant Pathol. J. 35:543-552.




Khudhair, M., Kazan, K., Thatcher, L. F., Obanor, F., Rusu, A., Sørensen, J. L., Wollenberg, R. D., McKay, A., Giblot-Ducray, D., Simpfendorfer, S., Aitken, E. and Gardiner, D. M. 2020. Fusaristatin A production negatively affects the growth and aggressiveness of the wheat pathogen Fusarium pseudograminearum. Fungal Genet. Biol. 136:103314.


Kim, H.-K., Cho, E. J., Lee, S., Lee, Y.-S. and Yun, S.-H. 2012. Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol. Lett. 337:89-96.


Kim, H.-K., Jo, S.-M., Kim, G.-Y., Kim, D.-W., Kim, Y.-K. and Yun, S.-H. 2015. A large-scale functional analysis of putative target genes of mating-type loci provides insight into the regulation of sexual development of the cereal pathogen Fusarium graminearum. PLoS Genet 11:e1005486.



Kim, H.-K., Lee, T. and Yun, S.-H. 2008. A putative pheromone signaling pathway is dispensable for self-fertility in the homothallic ascomycete Gibberella zeae. Fungal Genet. Biol. 45:1188-1196.


Kim, J.-I., Ha, A., Park, A. R. and Kim, J.-C. 2017. Isolation and characterization of antifungal metabolites from Pterocarpus santalinus against Fusarium graminearum causing Fusarium head blight on wheat. Res. Plant Dis. 23:268-277 (in Korean).

Lee, J., Lee, T., Lee, Y.-W., Yun, S.-H. and Turgeon, B. G. 2003. Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae. Mol. Microbiol. 50:145-152.


Lee, S.-H., Han, Y.-K., Yun, S.-H. and Lee, Y.-W. 2009. Roles of the glyoxylate and methylcitrate cycles in sexual development and virulence in the cereal pathogen Gibberella zeae. Eukaryot. Cell 8:1155-1164.




Leslie, J. F. and Summerell, B. A. 2006. The Fusarium laboratory manual. Blackwell, Ames, IA, USA. pp. 388.
McMullen, M., Jones, R. and Gallenberg, D. 1997. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 81:1340-1348.


Namiki, F., Matsunaga, M., Okuda, M., Nishi, K., Fujita, Y. and Tsuge, T. 2001. Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis. Mol. Plant-Microbe Interact 14:580-584.


Nganje, W. E., Bangsund, D. A., Leistritz, F. L., Wilson, W. W. and Tiapo, N. M. 2002. Estimating the economic impact of a crop disease: the case of Fusarium head blight in U.S. wheat and barley. In: 2002 National Fusarium Head Blight Forum Proceedings, eds. by S. M. Canty, J. Lewis, L. Siler and R. W. Ward, pp. 275-281.
Shin, Y.-K., Kim, D.-W., Lee, S.-W., Lee, M.-J., Baek, S. G., Lee, T. and Yun, S.-H. 2022. Functional roles of all five putative hydrophobin genes in growth, development, and secondary metabolism in Fusarium graminearum. Fungal Genet. Biol. 160:103683.


Sørensen, J. L., Sondergaard, T. E., Covarelli, L., Fuertes, P. R., Hansen, F. T., Frandsen, R. J. N., Saei, W., Lukassen, M. B., Wimmer, R., Nielsen, K. F., Gardiner, D. M. and Giese, H. 2014. Identification of the biosynthetic gene clusters for the lipopeptides fusaristatin A and W493 B in Fusarium graminearum and F. pseudograminearum. J. Nat. Prod 77:2619-2625.


Trail, F., Xu, H., Loranger, R. and Gadoury, D. 2002. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94:181-189.


Turkington, T. K., Petran, A., Yonow, T. and Kriticos, D. J. 2016.
Fusarium graminearum. HarvestChoice Pest Geography. STePP-HarvestChoice, St. Paul, MN, USA. pp. 9.
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 591 View
- 92 Download
- ORCID iDs
-
Sung-Hwan Yun

https://orcid.org/0000-0003-3438-6637 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



