Trunk Injection of Citrus Trees with a Polymeric Nanobactericide Reduces Huanglongbing Severity Caused by Candidatus Liberibacter asiaticus
Article information
Abstract
Huanglongbing (HLB) is a disease caused by the phloem-limited Candidatus Liberibacter asiaticus (CLas) that affects the citrus industry worldwide. To date, only indirect strategies have been implemented to eradicate HLB. Included among these is the population control of the psyllid vector (Diaphorina citri), which usually provides inconsistent results. Even though strategies for direct CLas suppression seem a priori more promising, only a handful of reports have been focused on a confrontation of the pathogen. Recent developments in polymer chemistry have allowed the design of polycationic self-assembled block copolymers with outstanding antibacterial capabilities. Here, we report the use of polymeric nano-sized bactericide particles (PNB) to control CLas directly in the phloem vasculature. The field experiments were performed in Rioverde, San Luis Potosí, and is one of the most important citrus-producing regions in Mexico. An average 52% reduction in the bacterial population was produced when PNB was injected directly into the trunk of 20 infected trees, although, in some cases, reduction levels reached 97%. These results position PNB as a novel and promising nanotechnological tool for citrus crop protection against CLas and other related pathogens.
Huanglongbing (HLB) is the most destructive citrus disease worldwide. It is caused by Candidatus Liberibacter spp. bacteria, which include Candidatus Liberibacter asiaticus (CLas) and Ca. Liberibacter americanus (CLam). These microorganisms are transmitted by the Asian citrus psyllid (ACP, Diaphorina citri Kuwayama), whereas Ca. Liberibacter africanus (CLaf) is transmitted by African citrus psyllid (Trioza erytreaea) (Bové, 2006; Bové and Ayres, 2007; Jagoueix et al., 1994; Li et al., 2006), which can harbor the phytopathogens for up to 12 weeks (Hung et al., 2004). Candidatus Liberibacter spp. infection causes fruit malformation, blotchy mottle leaves, branch dieback, and reduced quality and yield of fruits (Bassanezi et al., 2009, 2011; da Graça and Korsten, 2004; do Carmo Teixeira et al., 2005; Jagoueix et al., 1996). Candidatus Liberibacter spp. presence has been reported in over 40 countries (Croxton and Stansly, 2014), with CLas being the most widespread species in Asia and America, having almost completely replaced CLam in Brazil (Lopes et al., 2009). The only remaining HLB-free citrus areas in the world are the Mediterranean basin, Australia, and New Zealand (Alquézar et al., 2022). All commercially cultivated citrus varieties are susceptible to HLB infection, although at different levels (Folimonova et al., 2009; Gottwald et al., 2012). HLB is currently considered the most destructive disease in highly productive citrus regions.
In July 2009, HLB was detected for the first time in citrus trees in Mexico and has been localized in two regions with varying HLB incidence: (1) the Pacific coast, with high intensity, and (2) the Yucatan peninsula, with lower intensity (Mora-Aguilera et al., 2014). The major economic impact of HLB in Mexico is associated with the harvested fruit volume. For example, HLB-related losses of Persian lemon trees (C. latifolia), were approximately 18% in the state of Yucatan (Flores-Sánchez et al., 2015). Also, the state of Colima reported a drastic 64.7% and 60.8% reduction in orange and lemon fruit yields, respectively, in the 2014–2015 season, compared to those obtained the year 2011 (Servicio de Información Agroalimentaria y Pesquera, 2022). At present, HLB has been detected in 352 municipalities in 23 states of Mexico, corresponding to an affected area of 307,805 ha, equivalent to 50% of the total citrus-producing regions (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria, 2023).
It is worth mentioning that diverse control strategies, efforts, and significant financial investments have been made to find a long-term management approach to reduce losses caused by this pathogen. Unfortunately, they have been unable to find a relevant solution to this damaging sanitary problem. The traditional strategies employed, which include control of the ACP with insecticide applications in addition to vector exclusion practices, biological control, enhanced tree nutrition, among others, have proven to be insufficient (Gaire et al., 2022; Stansly et al., 2014). Therefore, a more effective strategy should involve the direct elimination of the CLas phytopathogens lodged in the phloem, where they proliferate and block the transport of nutrients and photosynthates (Koh et al., 2012). Unfortunately, no cure or effective treatment has been successfully implemented to mitigate HLB under field conditions. For instance, the use of phytosanitary strategies based on the use of antibiotics such as streptomycin sulfate, oxytetracycline hydrochloride, oxytetracycline calcium, and penicillin, applied via foliar sprays, root drenches, or trunk injections has been explored in HLB-positive trees, with low rates of pathogen elimination (Li et al., 2020; Wang et al., 2017; Zhang et al., 2011). However, oxytetracycline was effective at certain seasonal stages of citrus crops on a short-term basis, producing beneficial effects, such as fruit drop reductions and increases in fruit yields and fruit size, without greatly reducing CLas titers (Archer et al., 2023).
Recently, nanotechnology has emerged as an alternative approach to counteract the HLB disease by focusing on designing novel nanomaterials with bactericidal properties. For example, Stephano-Hornedo et al. (2020) reported a significant reduction of CLas titers in the phloem of HLB-infected lime trees (C. aurantifolia) treated with silver nanoparticles applied via foliar spraying and trunk injection. This report was the first evidence of the possibility of successfully targeting an antibacterial factor directly to the phloem of citrus trees.
Since 2017, our research group has performed several bioassays with a polymeric nano-bactericide (PNB), previously developed by Yáñez-Macías et al. (2017), in citrus trees and Solanaceae plants. PNB is an amphiphilic diblock copolymer composed of quaternized DMAEMA(Q-DMAEMA) and methylmethacrylate as hydrophilic and hydrophobic blocks. Typically, this diblock has the structure poly(QDMAEMA)16-b-(MMA)37. The copolymer was formed in water using the polymerization-induced self-assembly of diblock copolymers via RAFT polymerization to obtain nanoparticles. Thus, The PNB is an aqueous suspension of core-shell particles (ø~ 90 nm). The shell comprises quaternized polyDMAEMA, and the core contains polymethyl methacrylate. The PNB was highly effective in tomato plants (Solanum lycopersicum L.) infected with Candidatus Liberibacter solanacearum (García-Sánchez et al., 2021). Based on the success of these trials, the main objective of the present study was to determine the effect of this PNB on the CLas titers of leaves of PNB-treated citrus trees by foliar spraying and trunk injections under greenhouse and field conditions, respectively.
Materials and Methods
First experiment with HLB-positive trees
Fifteen trees with suspected symptoms of HLB-caused citrus greening, including blotchy mottle, chlorosis, and asymmetrical fruits, were selected from a non-commercial orchard localized in Paredón (latitude 25.936899°, longitude −100.914219°) in the state of Coahuila, México. Therein, the three youngest trees, identified as sweet orange (Citrus sinensis), Mexican lemon (Citrus aurantifolia), and mandarin (Citrus reticulata), were confirmed as CLas-positive specimens by PCR; their leaves were collected and frozen in liquid nitrogen and stored at −80°C until further use. The first exploratory experiment was performed in May–August 2017 to evaluate the possible effects of the PNB treatments in these three HLB-positive trees. All were injected in the trunk with 10 ml of a 20 ppm PNB-containing suspension (T1), followed by three booster injections at 30 (T2), 60 (T3), and 90 (T4) days after the initial injection (DAI). Trunk injections were performed with a tree injector (CHEMJET, Kerrville, TX, USA), which was used to introduce the PNB in a 5 cm-deep hole drilled in the trunk of the trees 30 cm above the soil line. The injection was made at low pressures (<60 psi). After 90 DAI, eight asymptomatic leaves were collected under the same conditions described above and stored until further use.
Second experiment using HLB-positive citrus plants grown in pots
Ten CLas-positive sweet orange (Citrus sinensis) trees were acquired from a commercial open citrus nursery. They were selected from a total population of 22 trees, all of which were grown in pots (5 liter) and placed in a greenhouse to perform a controlled experiment with a randomized complete block design, with two treatments and three repetitions. This second experiment was conducted in July–October 2017 to evaluate PNB effects in citrus plants grown in low-technology greenhouse conditions. The greenhouses are localized at the Centro de Investigación en Química Aplicada (CIQA) in Saltillo, Coahuila, México (25°27N, 101°02′O). All citrus pots were maintained inside a tunnel structure (5 m × 1.5 m × 2 m) covered with an anti-aphid mesh to avoid re-infestation by ACP. The foliar tissue was sprayed thrice with a 5 ml suspension containing 20 ppm of PNB (T1), followed by three applications at 30 (T2), 60 (T3), and 90 (T4) days after the initial spraying. At the end of the treatment, the four youngest leaves of each tree were collected individually, flash-frozen, and stored at −80°C until required.
Third experiment with HLB-positive trees from commercial orchards
The third and main trial was performed with sweet orange (C. sinensis) trees cultured in a commercial orchard to evaluate PNB effectivity under open-field conditions. This experiment was performed in September–December 2019 in Rioverde, San Luis Potosí, México (latitude 22.017904°, longitude −100.123101). For HLB screening, twenty-five 3-year-old trees were evaluated for CLas, resulting in 20 CLas-positive trees, all of which were selected for further experimentation. Similar to the previous experiments, the selected orange trees were injected thrice at monthly intervals for three months (T2 to T4), after the initial injection (T1) with 10 ml of a 20 ppm PNB suspension. Ten leaves from each tree were subsequently collected at T1 and T4, flash frozen, and subsequently stored at −80°C until required.
DNA isolation
For DNA isolation, all samples were processed using the DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions, with the use of added DNAase-free RNAase. The samples were separated on 1.2% agarose gels (Bio-Rad Laboratories, Hercules, CA, USA) to verify the quality of the isolated DNA. All DNA samples were quantified using a Nano-Drop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Later, all samples were tested through endpoint PCR to verify the presence of CLas.
End-point and real-time quantitative PCR
End-point PCR was used initially to verify the presence of Ca. Liberibacter species according to Orce et al. (2014). The primers used were p3G-F 5′-CTTATCACCGGCAGTCCCTATAAAG-3′ and p3G-R 5′-CAGCTCGTGTCGTGAGATGTTG-3′, designed to produce a 122 bp amplicon from DNA isolated from leaves of infected citrus trees. Specific primers were designed from sequences retrieved from the GenBank accessions for CLas (DQ431999.1), CLaf (EU921621.1), and CLam (AY742824.1) and were the following: CLib-F 5′-GCAGAACCTTACCAGCCCTT-3′, CLib-R 5′-CAACATCTCACGACACGAGC-3′ (115 bp amplicon). Cytochrome oxidase from C. sinensis (GenBank: KF933043.1) was used as an endogenous control. The resulting primers designed using this information were as follows: COX-F 5′-GTATGCCACGTCGCATTCCAGA-3′ and COX-R 5′-GCCAAAACTGCTAAGGGCATTC-3′, which are predicted to generate a 68 bp amplicon (Li et al., 2006). All PCR amplifications were performed using a Taq PCR Master Mix Kit (QIAGEN) under the following conditions: one cycle at 94°C, for 3 min; 35 cycles at 94°C, for 45 s, 60°C, for 45 s, and 72°C, for 45 s, followed by a final cycle at 72°C, for 5 min. The amplicons were obtained from the DNA sampled from leaves of infected plants and from insect vectors, previously separated on 2% agarose gels, and purified using a QIAquick Gel Extraction Kit (QIAGEN). Later, these fragments were quantified and sequenced (ELIM Biopharmaceuticals, Inc., Hayward, CA, USA). The sequences obtained were compared to those available in the NCBI database.
The quantitative PCR (qPCR) analysis was made using iQ SYBR Green Supermix chemistry (Bio-Rad), together with the p3G, Clib, or COX1 primers at 300 nM, and 25 ng of genomic DNA within Hard-Shell PCR Plates (Bio-Rad) according to the manufacturer’s protocol. The qPCR protocol was performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) to analyze the following samples: (1) mature leaves from the same branch of the three citrus trees that were trunk-injected with PNB as part of the first test experiment and collected at 0 days (T1) and after 90 days (T4). Three technical replicates per sample were employed for the analysis; (2) mature leaves from the same branch, sampled from the 10 infected trees included in the second test experiment at 0 days (T1) and after 90 days (T4) with three technical replicates per sample, and (3) mature leaves, collected from the same branch at 0 days (T1) and after 90 days (T4), from the 21 citrus trees that were PNB-treated in the third field experiment. In this particular case, four technical replicates of T1 and T4 samples were analyzed. The qPCR was performed under the following parameters: 1 cycle at 95°C, for 15 min, 40 cycles of denaturation at 95°C, for 15 s, annealing at 60°C for 60 s, and extension at 72°C for 30 s, and final extension step at 60°C, for 60 s. Data were obtained using the CFX Manager version 3.1 software (Bio-Rad). Quantification of CLas in the infected citrus plants versus control plants was calculated relative to the endogenous COX1 gene according to the comparative Ct method (2−ΔΔCt) (Livak and Schmittgen, 2001). As a negative control, DNA obtained from healthy citrus trees (C. sinensis) grown in pots in a certified nursery was used to compare Ct values with those of potted citrus trees showing no HLB symptoms and with CLas-free trees. The 2−ΔΔCt method was used to calculate CLas titer in citrus trees as follows: ΔΔCt = (Ct CLib − Ct COX1)Infected − (Ct CLib − Ct COX1)Healthy.
Data analysis
All biological samples were analyzed in triplicate, and average values (2−ΔΔCt) were calculated for each sample. Results of all bioassays were analyzed via one-way ANOVA, and Tukey’s Multiple Comparison Test was performed to test all possible pair-wise differences of means obtained. Statistical analyses were performed using GraphPad Prism version 8 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
First test experiment with PNB in HLB-positive citrus trees
A first assay with HLB-positive citrus trees was performed to test whether a PNB could effectively reduce the abundance of CLas. For this experiment, three different citrus tree species growing in the wild, that were also the youngest trees in the experimental site, were selected for analysis, based on the presence of the typical HLB blotchy mottle symptom produced by CLas infection (Fig. 1A, Supplementary Fig. 1A). The trunk injection of 20 ppm of PNB was applied twice in June, July, and August 2017. At the end of the 90-day treatment, the frequency of blotchy mottle symptoms was reduced in the leaves of the sweet orange (Fig. 1A), lemon (Supplementary Fig. 1A), and mandarin trees (Supplementary Fig. 2A) analyzed. However, the relative CLas titer abundance was only significantly diminished, at T4, in PNB-treated sweet orange tree (P = 0.0351) (Fig. 1B), in which the infection severity was 84.38% lower, assuming a 100% severity value in T0 samples. Conversely, the lemon and mandarin tree leaves presented 62.86% and 27.27% reductions in CLas titer, respectively, that were not significantly different from the titer detected in the T0 controls (P = 0.0602 and P = 0.1598, respectively) (Supplementary Figs. 1B and 2B).
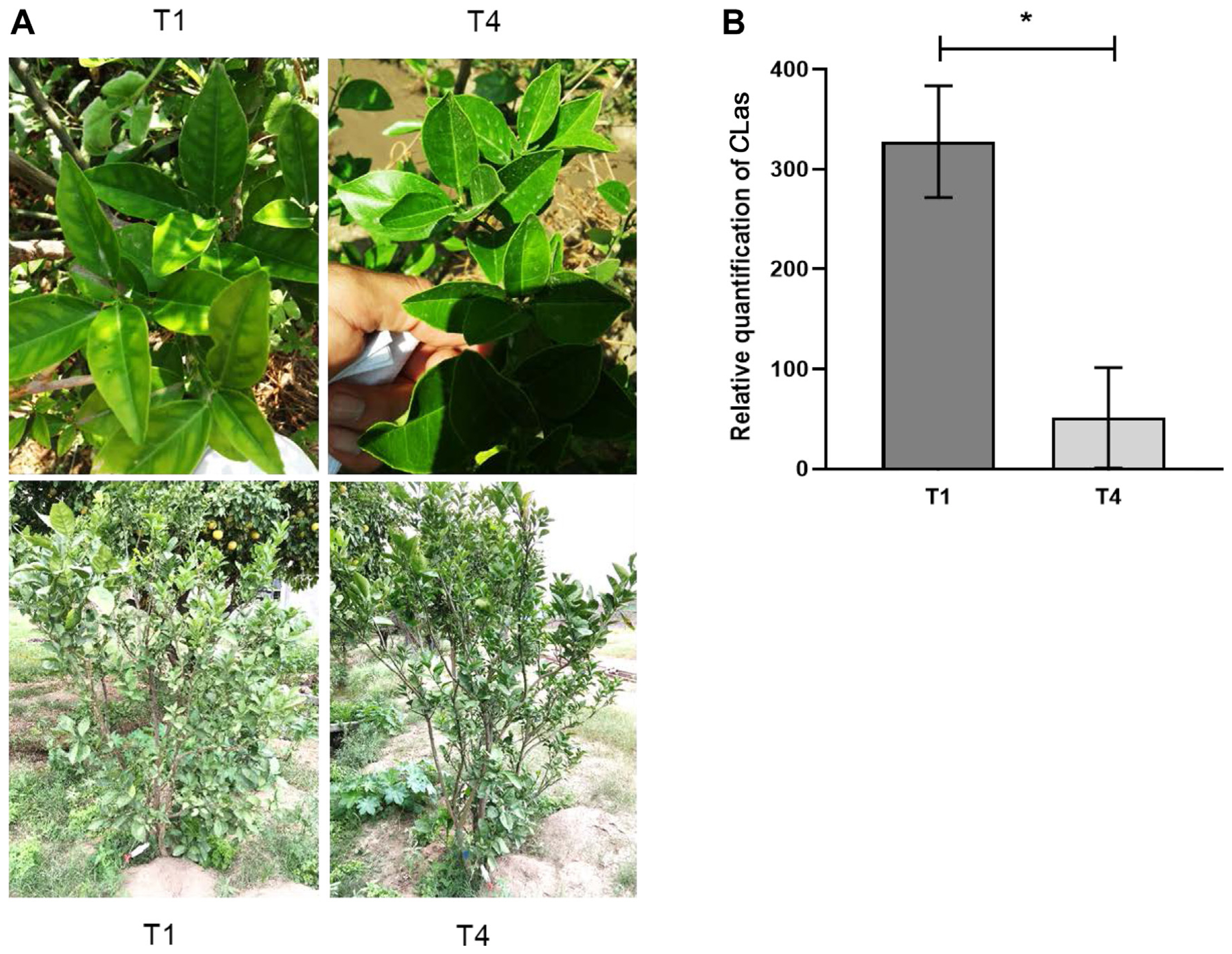
Sweet orange tree (Citrus sinensis) injected with a polymeric nano-bactericide suspension. (A) Leaves of trees showing blotchy mottle huanglongbing symptoms at the beginning (T1) and end of the treatment (T4). (B) Relative quantification of Candidatus Liberibacter asiaticus (CLas) titer in leaves sampled at 0 days (T1) and after 90 days (T4). The asterisk (*) denotes significant differences between treatments (P < 0.05).
The PNB effect on CLas in citrus trees grown under greenhouse conditions
A second test experiment was performed with commercial sweet orange trees (C. sinensis) maintained in nursery pots in order to compare the effects against CLas produced by either foliar spraying or trunk injection of the PNB suspension. Although all trees selected for analysis showed weak HLB symptoms of blotchy mottle, only ten were detected to be CLas-positive. In order to avoid a possible re-infestation by the ACP vector, all psyllid insects were removed from the branches of the experimental citrus trees employed. This was done, by gentle aspiration with an insect collector. Subsequently, all trees were placed in the greenhouse. The citrus pots were monitored weekly for HLB symptoms until the end of the experiment. No increase in symptomatology was observed in the citrus tree leaves after 90 days of treatment (Supplementary Fig. 3). However, from the ten citrus trees that were sprayed with PNB, only seven showed a CLas titer reduction, only two of which resulted to be statistically significant: tree AI-4, with a 99% CLas titer reduction (P = 0.0341) and tree AI-8, with a 59.71% CLas titer reduction (P = 0.0076). Meanwhile, tree AI-6 yielded an unexpected result, since it showed a 2,331% increase in CLas titer (P = 0.0224) (Fig. 2). The CLas titer in the other seven sweet orange trees evaluated was not significantly different from the T0 controls, i.e., in trees AI-1 (P = 0.4872), AI-3 (P = 0.0616), AI-5 (P = 0.1660), AI-7 (P = 0.2175), AI-9 (P = 0.1163), AI-10, (P = 0.2376), and AI-11 (P = 0.0565) (Fig. 2).
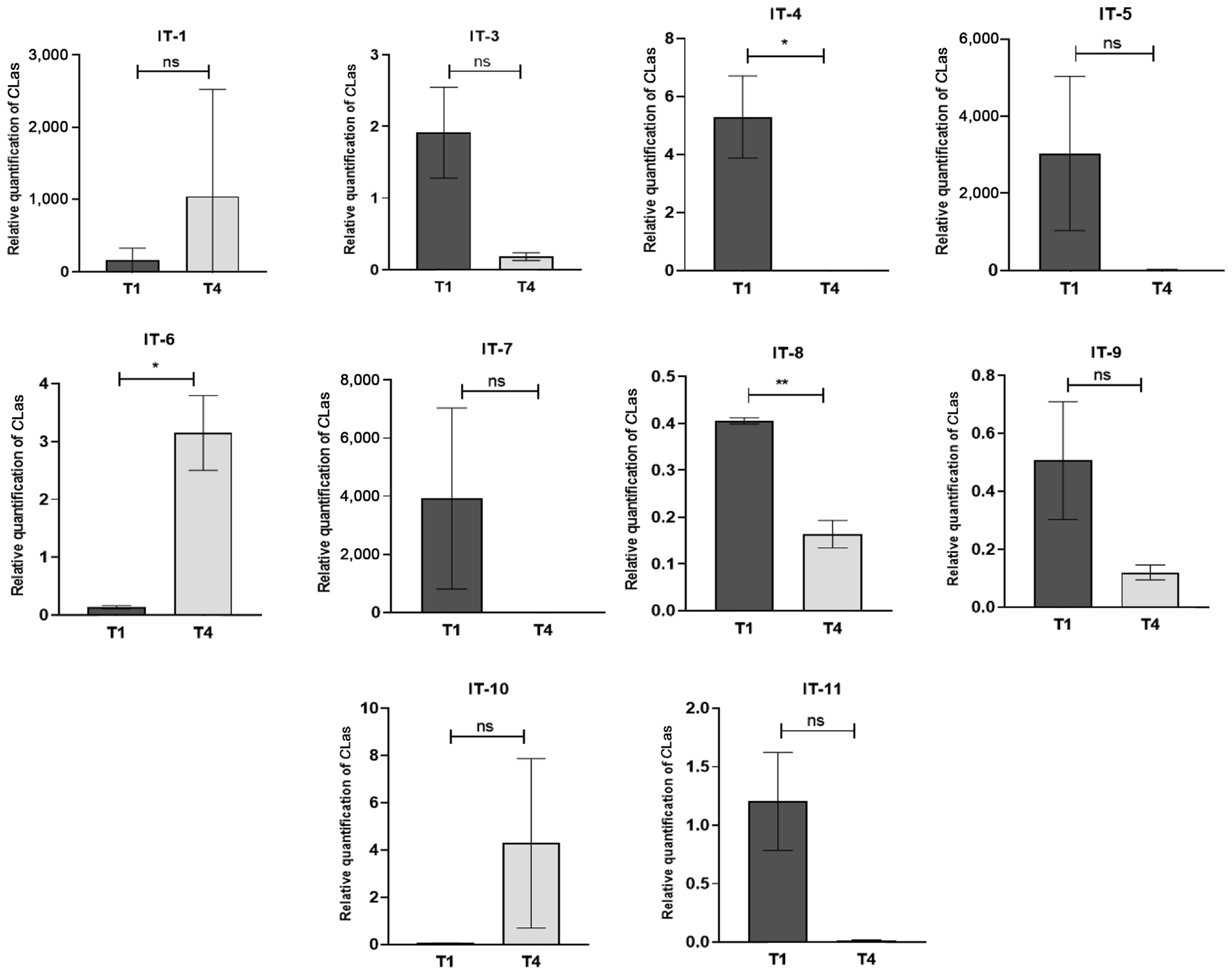
Relative quantification of Candidatus Liberibacter asiaticus (CLas) titer in leaves of 10 sweet orange trees (Citrus sinensis) cultivated in pots under greenhouse conditions. The trees were sprayed with a polymeric nano-bactericide (PNB) suspension sampled at 0 days (T1) and 90 days after the PNB treatment was started (T4). The asterisks ** and * indicate significant differences between treatments at P < 0.01 and P < 0.05, respectively. No significant differences between treatments at P > 0.05 are represented as “ns.”
Field experiment using PNB applications on sweet orange trees cultivated in a commercial orchard
An additional field experiment with 3-year-old C. sinensis sweet orange trees was performed after the two previous test experiments. This experiment was designed to confirm the effect of PNB against CLas via trunk injection under open sky field conditions. From 25 trees showing the HLB blotchy mottle symptoms, only 20 were detected as being CLas-positive and were selected for further analysis (Fig. 3A and B). Also, all psyllid vectors found in the trees selected were collected (Fig. 3C). The further analysis of a combined mix of 20 insects was confirmed to be CLas-positive (data not shown). The trunk injection using 20 ppm PNB was performed in September, October, and November of 2019 (Fig. 3D). After the triple injection, 12 sweet orange trees showed a considerable reduction of the blotchy mottle symptoms, compared to untreated trees, both in the newly emerged leaves, shortly after T1, and in the expanded leaves, at T4. However, from the 21 orange trees evaluated, only 11 trees presented a reduced CLas titer (i.e., in RV2, RV4, RV5, RV6, RV7, RV8, RV9, RV17, RV18, and RV25 trees), with the RV2, RV9, and RV25 trees presenting the highest and statistically significant differences from the T0 controls, i.e., RV2, 75.69% CLas titer reduction (P < 0.0001); RV9, 82.81% reduction (P < 0.0001), and RV25, 78.9% reduction (P < 0.0001) (Figs. 4 and 5). Unexpectedly, the RV12 sample showed a 498% increase in CLas titer at T4 (P < 0.0001) (Fig. 4).
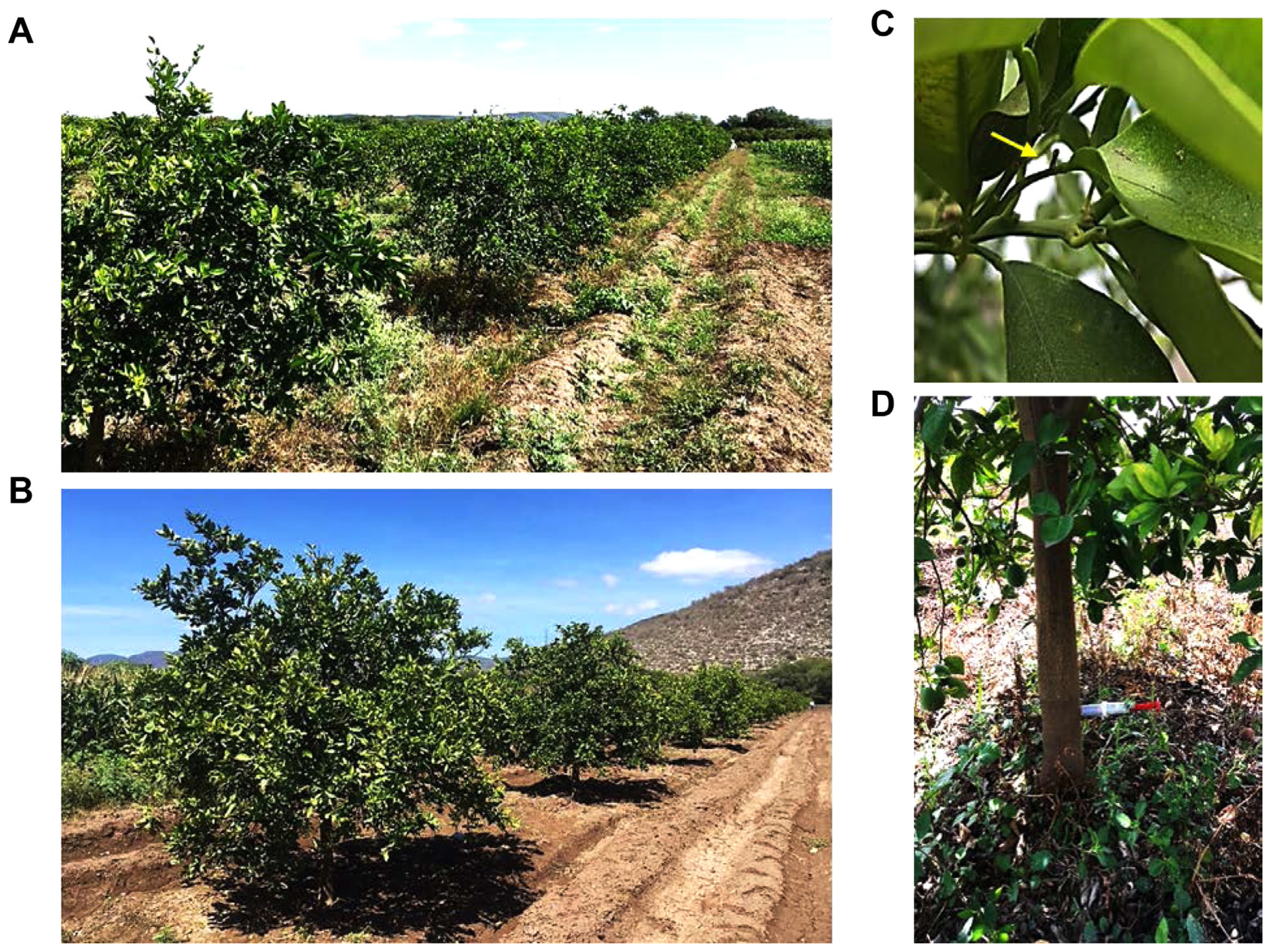
Images of the experimental sweet orange trees (Citrus sinensis) localized in a commercial orchard in the Rioverde community, San Luis Potosí, México. (A) Twenty-five orange trees showing blotchy mottle hanglongbing symptoms (0 days) were selected for the polymeric nano-bactericide (PNB) treatment and subsequent analysis of Candidatus Liberibacter asiaticus (CLas) titer by PCR. (B) Orange trees after PNB treatment (90 days). (C) The yellow arrow indicates the presence in the orchard of at least one psyllid vector (Diaphorina citri), which was confirmed to be CLas-positive. (D) Trunk injection, with the 20 ppm PNB suspension.
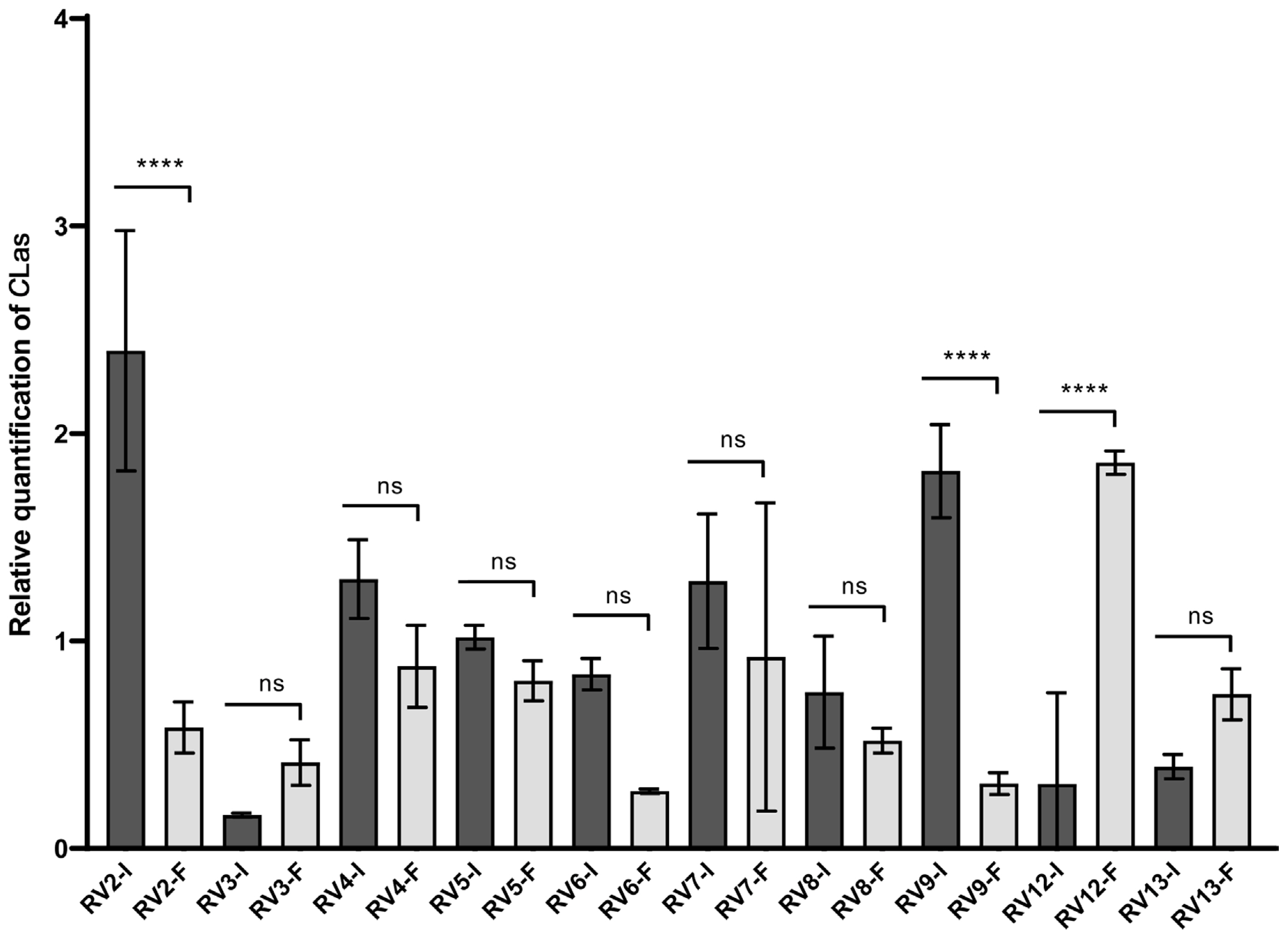
Sweet orange trees (Citrus sinensis) cultivated in field conditions (RV, Rioverde) were trunk-injected with polymeric nano-bactericide (PNB) suspensions to test their efficiency for the control of huanglongbing (I). The bars represent the relative quantification of C. Liberibacter asiaticus (CLas) titer in leaves of trees that were trunk-injected with 20 ppm PNB suspensions at 0 days (RV-I) and 90 days after the initial injection (RV-F). Asterisks, ****, represent significant differences between treatments at P < 0.0001. The “ns” initials show no significant differences between treatments at P > 0.05.
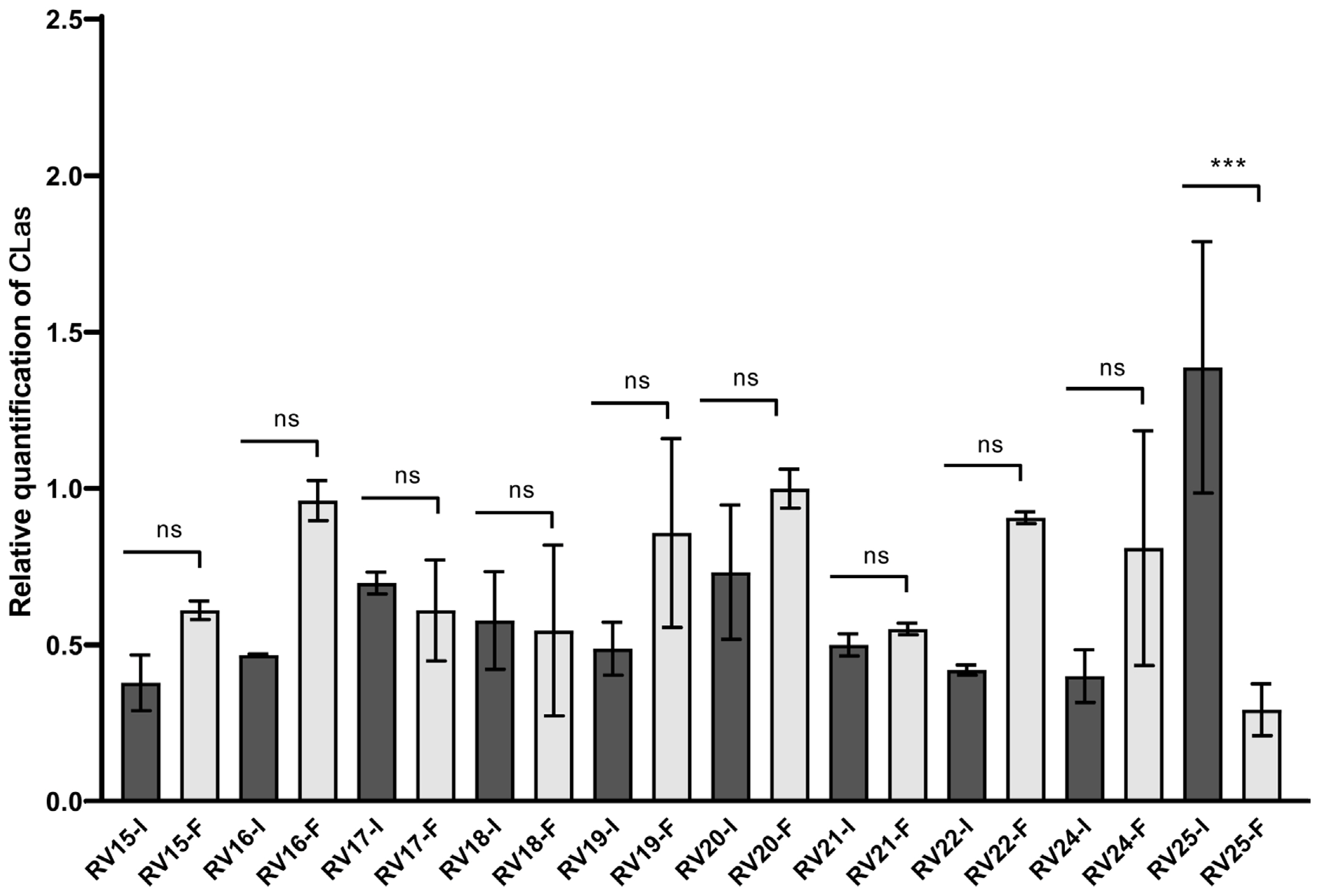
Sweet orange trees (Citrus sinensis) cultivated in field conditions (RV, Rioverde) were trunk-injected with polymeric nano-bactericide (PNB) suspensions to test their efficiency for the control of huanglongbing (II). The bars represent the relative quantification of C. Liberibacter asiaticus (CLas) titer in leaves of trees that were trunk-injected with 20 ppm PNB suspensions at 0 days (RV-I) and 90 days after the initial injection (RV-F). Asterisks, ***, represent significant differences between treatments at P < 0.001. The “ns” initials show no significant differences between treatments at P > 0.05.
Discussion
The elimination of HLB in citrus plants worldwide represents a formidable challenge. One frequently used but ineffective strategy has been to reduce the psyllid vector population drastically. Thus, the relatively poor effectiveness that this strategy has shown so far, is a strong indicator that a better understanding of the interactions occurring between citrus trees, psyllid vectors, and the HLB pathogen is needed to establish better control of this highly damaging disease. In this context, the main objective of this study was to evaluate the bactericide effects of a PNB against CLas in HLB-positive citrus trees. This report provides evidence that this particular PNB can effectively reduce the population of phloem-limited CLas, the causal agent of HLB in citrus plants. Recently, García-Sánchez et al. (2021) demonstrated that this PNB was effective against phloem-limited Ca. Liberibacter solanacearum in tomato plants by showing that it reduced the symptoms associated with this pathogen and caused a significant decrease in the bacterial titers. In the present work, a preliminary study was initially performed with citrus trees from non-commercial orchards in order to assess the effectivity of this PNB. It also offered the possibility of testing whether the trunk injection of the PNB suspension could be effective against CLas (Fig. 1, Supplementary Figs. 1 and 2). The choice of the trunk injection method employed in this study was based on the low volumes of nanomaterial required and also on the fact that the trunk injection could improve the distribution of the PNB through the vascular system, xylem and phloem, of citrus trees, including the transpiration stream (Archer et al., 2021). An additional advantage was that the trunk injection procedure reduced unintended PNB contamination that could damage other non-target organisms in the environment (Wise et al., 2014). The preliminary results obtained in the first experimental phase were partially successful since CLas titer reduction was significantly reduced only in the sweet orange tree examined. In contrast, the effect in the treated lemon and mandarin trees proved ineffective. However, the typical blotchy mottle symptoms were reduced in both of them. In this regard, the environmental conditions in which this particular experiment was performed might have affected the inconsistent results obtained. For instance, the presence of D. citri psyllids vectors was detected in the leaves of the lemon and mandarin trees analyzed 50 days after the treatment was initiated. This unwanted event could have led to CLas re-infection of these trees and a consequent increase in the bacterial titers analyzed at the end of the experiment. The second test experiment was performed under much more controlled conditions to avoid repeating this incident. First, ten CLas-positive orange trees grown in pots were selected and treated by foliar spraying of the PNB suspension. No differences were observed between treatments when the pot-grown citrus trees were visually evaluated for HLB symptoms throughout the experiment. Also, the relative quantification of CLas titer was highly variable, with non-significant differences detected in the majority of the citrus trees tested (Fig. 2). We propose that the low permeability of the citrus leaf surfaces negatively affected the effectiveness of the PNB treatment by foliar aspersion. This factor could have also adversely affected the outcome of several other reports describing the poor performance of foliar spraying for the application of chemical compounds designed to control the HLB disease, including antibiotics such as streptomycin sulfate, oxytetracycline hydrochloride, and oxytetracycline calcium. These antibiotics were approved, in 2016, for foliar spraying of HLB-positive trees in Florida (Wang et al., 2017). Additionally, the environmental risks associated with spraying antibiotics on HLB-positive citrus trees are considerable, taking into account that they may lead to antibiotic resistance and/or eliminate non-target bacteria. Thus, many more studies are needed to evaluate the fate and effect of the unrestricted use of antibiotics in nature. An investigation by Li et al. (2020) reported, for instance, that streptomycin (STR) sprayed on HLB trees in the field was unable to reduce CLas titers. However, STR remained detectable in citrus leaves for six additional weeks post-application. In addition, when a single trunk injection was applied, STR at 2.0 g/tree led to the accumulation and persistence of STR in the leaves for more than five months, which was the time found to be necessary to reduce the CLas titers (Li et al., 2020).
Within this scenario, the trunk injection represents an alternative for an efficient delivery and distribution of chemical compounds having the potential to control HLB in citrus trees. Therefore, we performed a third experiment with 20 CLas-positive orange trees under field conditions. Here, a PNB dose of 20 ppm was injected four times into each tree to test whether it could exert a bactericidal effect after a 90-day period. The relative quantification of CLas titers was reduced in 11 trees, and the reduction was found to be highly significant in three of them. The reduced efficiency of the PNB treatment in this particular field experiment could be attributed to the highly possible exposure to psyllid vectors and consequent CLas re-infection. Such eventuality was supported by the detection of D. citri adults and nymphs in the youngest leaves of the citrus trees under analysis, which could have altered the quantification of CLas titers at the end of the assay.
The PNB evaluations in citrus plants were performed using 20 ppm as an effective dose. However, higher doses, i.e., 40 and 70 ppm of PNB, were required in other experiments performed to evaluate their bactericidal effect against Ca. Liberibacter solanacearum in tomato plants (García-Sánchez et al., 2021). In this study, the PNB was effective via foliar spraying of a 70 ppm suspension, performed three times during successive weeks, since the HLB symptom attenuation observed was accompanied by a ca. 90% relative reduction of CLas titers (García-Sánchez et al., 2021). It could be argued that the effective responses of herbaceous plants to the PNB treatment were much more evident due to the much higher permeability of tomato leaves compared to citrus leaves. Supporting reasoning for this proposal is based on a previous observation made to indicate that the thick epicuticular wax characteristic of citrus leaves, in combination with weather conditions, retarded their uptake of sprayed STR (Li et al., 2020).
Regarding the antibacterial effect of the PNB against CLas, it could also be anticipated that it was most probably caused by the quaternary ammonium groups present on the particle’s surface. In addition, the size of the PNB (ca. 50 nm) could have permitted their unimpeded translocation through the vascular system and a widespread distribution in the plant sites where the pathogen is usually localized. This property is considered to be a critical factor affecting the capacity of this PNB to suppress phloem-limited pathogens (e.g., Ca. Liberibacter spp.), which are usually difficult to target with precision using other methods. Moreover, the lack of evident phytotoxicity symptoms due to foliar spraying in both tomato and citrus trees was corroborated by the lack of induced superoxide dismutase expression levels and accumulation of damaging reactive oxygen species (data not shown). In contrast to PNBs, tetracyclines are reported to be bacteriostatic, not bactericidal, a characteristic that could cause the re-growth of CLas populations in citrus plants (Zhang et al., 2013). Moreover, several antibiotics, such as penicillin G, streptomycin, kasugamycin, and oxytetracycline applied via trunk injection in citrus trees, led to seasonal fluctuations in CLas titers, thereby suggesting that CLas is capable of moving to different sections of the plant during the growth season (Wang et al., 2017; Zhang et al., 2013). The CLas mobility in citrus tissues suggests that long-term tests, longer than 1 year, may be required to explore the true antimicrobial effects of trunk-injection protocols (Blaustein et al., 2018).
Therefore, in future studies with HLB-positive citrus trees, it will be necessary to consider the use of more elevated doses (e.g., 70 ppm of PNB) and an increased number of periodical applications for at least 1 year to permit a more precise monitoring of the fluctuations of CLas populations, and their plant-localization through time. However, the PNB translocation in the vascular system of citrus trees and solanaceous plants remains to be demonstrated. Experimental strategies are being considered to follow the movement of PNBs in planta in order to obtain more evidence of the final fate of these nanomaterials. Also considered is the use of the PNB in combination with several reported inductors of plant priming, such as ascorbic acid, β-aminobutyric acid, 2,1,3-benzothiadiazole, 2-deoxy-D-glucose, and 2,6-dichloroisonicotinic acid (INA), which were previously shown to be effective for the suppression of CLas titers by up to 30% (Li et al., 2016). Also, systemic acquired resistance inducers (INA, acibenzolar-S-methyl, salicylic acid, and imidacloprid) applied via trunk injection exhibited a significant reduction in HLB disease (Li et al., 2021). Other studies performed with Azospirillum brasilense, a plant growth-promoting rhizobacteria in Mexican lemon plants (C. aurantifolia), showed that it effectively reduced CLas titers, as well (Trinidad-Cruz et al., 2019). However, even though A. brasilense represents a beneficial alternative against HLB, it might not be effective when combined with the PNB due to the possible translocation of the PNB to roots and the consequent bactericidal effect on A. brasilense.
In summary, the results of this work revealed the potential effects of this water-dispersible PNB on relative CLas titer reduction in citrus trees. Despite environmental conditions that might have negatively influenced CLas titers by psyllid vector re-infestation, the PNB hereby described has the potential to be an effective agent for the control of these notoriously difficult-to-control phytopathogens under field conditions. Finally, the confrontation of this PNB against citrus trees having high HLB incidence represents an important challenge to consider in future studies.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors acknowledge the financial support provided by the Centro de Investigación en Química Aplicada (CIQA), through grant N° 6495/6679/2022. This study was partially supported by the Program “Investigadoras e Investigadores por México” from the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCyT) (Project No. 1333 to JHVS). The authors also acknowledge Iliana Marcela Rodríguez Salinas for her valuable technical support during earlier experiments with PNB.
Electronic Supplementary Material
Supplementary materials are available at Plant Pathology Journal website (http://www.ppjonline.org/).
