Biological Control of Rice Bakanae by an Endophytic Bacillus oryzicola YC7007
Article information
Abstract
In our previous study, we reported that a novel endophytic bacterium Bacillus oryzicola YC7007 has suppressed bacterial diseases of rice via induced systemic resistance and antibiotic production. This endophytic strain, B. oryzicola YC7007 was used as a biological control agent against bakanae disease of rice caused by Fusarium fujikuroi, and its mechanism of interaction with the pathogen and the rice was further elucidated. Root drenching with B. oryzicola YC7007 suspension reduced the disease severity of bakanae significantly when compared with the untreated controls. The treatments of B. oryzicola YC7007 suspension (2.0 × 107 cfu/ml) to the rice rhizosphere reduced bakanae severity by 46–78% in pots and nursery box tests containing autoclaved and non-autoclaved soils. Moreover, in the detached rice leaves bioassay, the development of necrotic lesion and mycelial expansion of F. fujikuroi were inhibited significantly by spraying the culture filtrate of B. oryzicola YC7007. Drenching of ethyl acetate extracts of the culture filtrate to the rhizosphere of rice seedlings also reduced the bakanae disease severity in the plant culture dish tests. With the root drenching of B. oryzicola YC7007 suspension, the accumulation of hydrogen peroxide was observed at an early stage of rice seedlings, and a hormonal defense was elicited with and without pathogen inoculation. Our results showed that the strain B. oryzicola YC7007 had a good biocontrol activity against the bakanae disease of rice by direct inhibition, and was also capable of inducing systemic resistance against the pathogen via primed induction of the jasmonic acid pathway.
Introduction
Bakanae disease of rice caused by Fusarium fujikuroi Nirenberg (anamorph) is destructive especially in Asian countries, where rice is an economically important crop. Its incidence ranges from 3% to 80% in major rice growing areas, including China, India, Bangladesh, and Korea, and yield losses due to the bakanae were estimated at approximately 10% to 50% (Bonman, 1992; Mew and Gonzales, 2002). This disease has recently been found in California, United States, causing 30% losses in those areas (Carter et al., 2008). The pathogen F. fujikuroi is known to be seed and soil-borne, and can survive effectively on diseased crop residues. The spores or conidia of the pathogen are dispersed widely to spread and develop the disease in rice nursery beds and paddy fields with biotrophic and necrotrophic life styles (Goswami and Kistler, 2004; Ou, 1985). The symptoms of this disease vary depending on its severity; these include abnormally elongated seedlings, foot and seedling rot, pale green flag leaves, yellowish leaves bearing empty panicles, discoloration of grains, and in severe cases, sterile seeds and dried dead seedlings (Bonman, 1992). Despite the considerable economic impact of the rice bakanae, a few efficient and effective control methods are available, except the treatment of seeds with chemical fungicides. Seed treatments with the fungicides have been used widely in most rice growing areas during the last decades. However, due to the occurrence of increasing fungal resistance to these chemicals, the efficacy of these chemicals has been greatly reduced (Park et al., 2009; Yang et al., 2012). Seed soaking in hot water (50–55°C) or salty hot water has been used to control seed-borne diseases including bakanae in some areas, but it is not commonly practiced (Bonman, 1992; Tung and Serrano, 2011). Because of the above limitations, more effective and environmentally sound control measures using antagonistic microorganisms have been alternatively explored to control F. fujikuroi. Biological control of rice diseases such as rice sheath blight, rice blast and brown spot with some rice associated antagonistic bacteria including Bacillus species, showed somewhat effective results in rice growing areas (Mew et al., 2004; Park et al., 2006; Sung and Chung, 1997; Zhiyi et al., 2001). A variety of Bacillus species has been isolated from the rhizosphere to find biological control activity against rice fungal pathogens (Gnanamanickam, 2009). However, no bacterial species has yet been developed for practical use at commercial farms to control rice bakanae and seed-borne diseases, although some Pseudomonas and Bacillus species have been used for controlling bakanae disease of rice (Rosales and Mew, 1997; Rosales et al., 1986). A limited number of studies are available to elucidate the mechanism of resistance induction by these Bacillus species in the rice immune system.
The immune system of the plants can be enhanced locally or systemically by biological agents or abiotic inducers, with or without pathogen infection (Pieterse et al., 2009; Sang and Kim, 2011; van Loon et al., 2006; Walters et al., 2005). The innate immunity for general and basic protection system of plants can be regulated by the cellular defensive mechanism through reactive oxygen species (ROS) production, phytoalexin, camalexin and callose formation. When the plant is attacked by pathogens, the ROS is produced immediately after recognition of pathogen associated molecular pattern by pattern recognition receptors of the host. The produced ROS generates innate immune resistance, which can be also regulated by the bacterial agents against pathogens (Alquéres et al., 2013; De Vleesschauwer et al., 2008; Shimizu et al., 2010). On the contrary, the hormonal defense is triggered by salicylic acid (SA), jasmonic acid (JA) and ethylene (ET), which activate the expression of defense related genes (Sanchez et al., 2012; Vallad and Goodman, 2004; Zhang et al., 2012). A number of defense related genes have been reported in various defense signaling pathways: OsLOX2, OsAOC, JIOsPR10 for JA; OsPRIb, PBZ1, OsESS1, OsPAD4 for SA; and EBP89 for ET (De Vleesschauwer et al., 2010, 2012; Qiu et al., 2007; Riemann et al., 2013). These hormonal defenses can be elicited by continuous stimulation of the rhizosphere bacteria as an induced systemic resistance (ISR), systemic acquired resistance (SAR) or primed induced resistance, depending on the lifestyle of the pathogens and non-pathogenic rhizobacteria (Ahn et al., 2007; Ton et al., 1999). Some non-pathogenic rhizobacteria are also able to elicit an ISR response either through SA or JA/ET pathway, or simultaneously through both the pathways, and are dependent on NPR1 (non-expressor of pathogenesis-related proteins1) (De Vleesschauwer et al., 2008; Niu et al., 2011; van de Mortel et al., 2012; Weller et al., 2012).
In our previous study, a novel endophytic strain, Bacillus oryzicola YC7007 has been reported to suppress bacterial blight and grain rot of rice via resistance induction (Chung et al., 2015). In this study, the cellular and hormonal defense mechanisms into the suppression of bakanae disease by B. oryzicola YC7007 have been highlighted, and its colonization on different locations of the rice was also investigated. To gain more insights into the hormonal networking, the expression of defense related genes of rice, OsPR1-a, OsLOX-L2, and OsAOC, were examined with or without infection of the pathogen.
Materials and Methods
Plant materials and growth conditions
Seeds of rice (Oryza sativa L. ssp. japonica cv. Dongjin) were surface-sterilized with 1.2% sodium hypochlorite (NaOCl) solution for 5 minutes, followed by 70% ethanol for 5 minutes, and finally washed with sterile distilled water several times. For germination, the seeds were then immersed in water at 30°C for three days in dark, with daily change of water. The germinated seeds were sown in commercial nursery potting soils (Dasuran Sangto, Jinju, Korea) and kept in a growth chamber at 30°C (light; 200 μmole/m2/sec, and a 16-hour-light/8-hour-dark regime) for growing the seedlings. For evaluating the efficacy of B. oryzicola YC7007 in natural conditions, 2-week-old seedlings were then transplanted into plastic pots (9.5 × 8 × 7 cm3) containing about 150 g nursery soil, which was either non-autoclaved intact soil, or soil that had been autoclaved for 20 minutes at 121°C twice on two consecutive days.
Preparation of pathogen inoculum and measurement of Fusarium concentrations
The bakanae pathogen, F. fujikuroi 44022, was obtained from the Korean Agricultural Culture Collection (Jeonju, Korea) and cultivated at 28°C on potato dextrose agar (PDA) media. Soil inoculum of the pathogen was prepared, with slight modification of the method described previously (Singh et al., 1999). Briefly, 50 g chopped potato pieces were mixed with 500 g autoclaved field soil containing 30% (v/w) moisture, in a 2-liter Erlenmeyer flask. These were autoclaved for 1 hour, after which 5 mycelial disks (0.5 cm in diameter) of F. fujikuroi from 8-day-old PDA were inoculated into the mixture. The mixture was incubated for four weeks at 28°C, with intermittent shaking once per week, for generating good mycelial growth. After four weeks incubation, the mixture was dried and lightly macerated using a pestle and mortar. The macerated inoculum was passed through a sieve to yield pieces of inoculum about 2 mm in size, to be used as soil inoculum. The concentration of inoculum was measured by preparing serial dilutions, and the colony forming units (cfu/g) were evaluated on Fusarium selective media, prepared with 2.5 mg malachite green, 10 g potato dextrose broth and 15 g agar per liter of deionized water, supplemented with 30 mg streptomycin (after autoclaving) (Castella et al., 1997). To measure the concentrations of Fusarium species in the root and aerial components, samples were collected from 2 cm above the base of the rice seedlings at 10 days after inoculation (DAI) and surface-sterilized with 70% ethanol for 30 seconds, and then washed three times with sterile distilled water. The sterilized samples were macerated in a sterile pestle and mortar in buffer solution (10 mM MgSO4), and appropriately diluted aliquots were plated onto the selective media. The colonies of Fusarium species were counted after 3 days incubation at 28°C.
Bioassay for biocontrol activity of B. oryzicola YC7007
In our previous study, B. oryzicola YC7007 was isolated from the rice root, and had demonstrated the inhibition of mycelial growth of F. fujikuroi (Chung et al., 2015). The strain B. oryzicola YC7007 was evaluated for its biocontrol activity against bakanae disease of rice, in pots and nursery boxes. Bacterial suspension of B. oryzicola YC7007 was prepared with cells cultivated in one-tenth strength tryptic soy broth (1/10 TSB, Bacto™TSB; BD, Sparks, MD, USA) for 2 days on a rotary shaker (160 rpm, 28°C), and harvested by centrifugation (5,000g, 15 minutes); the resulting suspension was adjusted to different concentrations, 5.6 × 105, 3.6 × 106 and 2.0 × 107 cfu/ml, in a buffer solution (10 mM MgSO4). In the pot tests, 15 ml of the cell suspension of B. oryzicola YC7007 was drenched into the plastic pots (9.5 × 8 × 7 cm3) containing either autoclaved or non-autoclaved intact nursery soil, in which 2-week-old seedlings were transplanted. Control plants were treated with the buffer solution only. After 3 days of bacterial treatment, the rice seedlings were challenged with soil inocula of fungal pathogen (10 g/kg, 6.0 × 105 cfu/g).
For the nursery box test, naturally infected rice seeds collected from commercial farms were used to study the control efficacy of B. oryzicola YC7007 against the bakanae. Rice seeds were washed several times with tap water and immersed for 20 minutes; seeds which sank to the bottom were collected for the tests. For the comparison of efficacy with the chemical seed disinfectants, two chemical fungicides commonly used by Korean farmers, prochloraz (a.i., 25%; Kyung Nong Corporation, Seoul, Korea) and fludioxonil (a.i., 20%; Syngenta Korea Ltd., Iksan, Korea) were treated, as per the manufacturer’s instructions. For prochloraz treatment, the seeds were immersed in a water solution containing 125 μl chemical in 250 ml water, for 24 hours at 30°C, and then incubated for another 2 to 3 days under dark conditions, for germination. During this treatment, water was changed every day. After 3 days treatment with prochloraz, the germinated seeds were soaked for 10 minutes in the chemical solution of fludioxonil, according to the manufacturer’s instructions; finally, the treated seeds were sown in the nursery boxes (57.2 × 27.2 × 0.6 cm3; National Agrobiodiversity Center, Seoul, Korea) for rice cultivation. Germinated rice seeds (about 120 g) sown in a nursery box were covered gently (approximately 1 cm layer) with 500 g nursery potting soils per box (Dasuran Sangto).
For the bacterial treatment, 250 ml suspension of B. oryzicola YC7007 (3.6 × 106 or 2.0 × 107 cfu/ml) were gently and equally poured in one nursery box after 4 days cultivation of seeds at 30°C, that were not treated with chemical fungicides. Simultaneously, tap water was used as a negative control. All the nursery boxes were covered with transparent plastic film for 3 days to retain the moisture, and water was sprayed every day, depending on the moisture condition after uncovering the plastic film. In the nursery box tests, non-autoclaved intact soil collected from university paddy fields were used. Disease severity of bakanae was evaluated based on disease rating scales (0–5) after 30 days of treatment (Amatulli et al., 2010). Disease reduction was calculated using the following formula:
Chlorophyll measurement
Using a spectrophotometer (X-ma 1200 Spectrophotometer; Human Corp., Seoul, Korea), the content of chlorophyll was determined using detached rice leaves after 20 DAI of pathogen. In a glass grinder, approximately 100 mg tissue was pulverized with 10 ml 80% acetone, followed by centrifugation at 13,000g for 5 minutes. The absorbance of the resulting supernatant was measured at wavelengths of 646.8 nm and 663.2 nm. The total chlorophyll content was calculated using the following formula (Xie et al., 2009):
Suppression of bakanae by culture filtrate and extract of B. oryzicola YC7007
For checking whether the culture filtrate and extract of B. oryzicola YC7007 has activity to induce resistance against F. fujikuroi, the bioassay using detached leaves and seedlings was conducted in petri plates and plant culture dishes, respectively. For detached leaves assay, rice leaves collected from three-week-old seedlings were surface-sterilized with 1.2% NaOCl for 1 minute and washed three times in distilled water. The culture filtrate of B. oryzicola YC7007 was prepared from the 10 times diluted 60 hours culture broth with the buffer solution (10 mM MgSO4), which was filtered through a millipore filter (0.45 μm). The diluted culture filtrate was sprayed as droplets onto the small pieces of rice leaves, and the pieces were set in petri plates for inoculation of pathogen after 2 days of spraying. Conversely, for the cell suspension treatment, the lower portion of rice leaves (1 cm) was dipped in the cell suspension of B. oryzicola YC7007 (2.0 × 107, cfu/ml) for 30 seconds and kept for 48 hours at 28°C in closed petri plates humidified using wet filter papers. After treatment of cell suspension or spraying of the culture filtrates, the detached leaves from both methods were inoculated with two mycelial disks of 8-day-old F. fujikuroi grown on PDA plate. The disks were removed after 24 hours, and the treated leaves were incubated for 5 days at 28°C in an incubation chamber. Control plants were treated with only the buffer solution. Disease severity was rated according to the size of the necrotic lesion. For each treatment, 20 samples of leaves were used with three replicates.
As strain B. oryzicola YC7007 has resistance inducing activity, the test was also conducted to ascertain whether the culture extract with a solvent has suppressive activity against bakanae in plastic plant culture dishes (100 × 40 mm; SPL Life Sciences Co., Ltd., Pocheon, Korea) using rice seedlings. The cell free culture filtrate (3 liters) obtained from 2-day-old culture broth of 1/10 TSB at 28°C, was twice extracted, using equal volume of ethyl acetate (EtOAc). The collected extract was concentrated using a vacuum rotary evaporator at 40°C to about 500 mg, which was then dissolved in dimethyl sulfoxide (DMSO) at various concentrations (50, 100, and 200 μg/ml). The EtOAc suspension (5 ml) of B. oryzicola YC7007 was poured into 10 plant culture dishes, containing 10-day-old seedlings, along with one halfstrength Murashige & Skoog (1/2 MS) agar media. After 3 days, the conidial suspension (5 ml for each culture) of F. fujikuroi (1 × 106 conidia/ml) was inoculated into these seedlings treated with EtOAc extracts. The conidial suspension was prepared by scratching the PDA plates with distilled water. Disease rating was evaluated at 7 DAI of the pathogen, following the rating scales (0–5). Ten seedlings were used for each treatment with three replicates.
Colonization of B. oryzicola YC7007
The location of B. oryzicola YC7007 in rice tissues during colonization was observed using B. oryzicola YC7007 at a concentration of 2 × 107 cfu/ml. The cell suspension (15 ml) was drenched into 150 g soil in pots containing rice seedlings. Root and stem pieces (1 cm above base) were collected at 0, 3, 5, and 8 DAI to measure the population. Samples were surface-sterilized by 70% ethanol for 30 seconds, and then washed several times with distilled water. The sterilized samples were homogenized in the buffer solution (10 mM MgSO4) using a sterile pestle and mortar, and the aliquots were diluted appropriately before plating onto LB agar (Difco™ LB broth; Sparks, MD, USA) media supplemented with chloramphenicol (40 μg/ml) the plates were then incubated at 28°C. After incubation, the number of colonies was determined as cfu/g of leaf fresh weight.
Detection of hydrogen peroxide accumulation
The accumulation of hydrogen peroxide (H2O2) was investigated in leaf sheath areas from the samples of treated and untreated controls. First, the bacterial suspension was drenched in the soil, and then 3 days after bacterial treatment, the pathogen was challenged, as described previously. Samples were collected at 6 and 12 hours before and after pathogen inoculation. Leaf sheaths were stained with diaminobenzidine (DAB, 1 mg/ml and pH 3.8) for 8 hours under light conditions, then wrapped in foil and incubated at 37°C (Niu et al., 2011). The samples were examined under a light microscope, and the presence of hydrogen peroxide in the tissues was indicated by a reddish-brown precipitate.
Expression of defense genes by RT-PCR
Effect of B. oryzicola YC7007 treatment to the root and the subsequent expression of rice defense genes, OsPR1-a, OsLOX-L2, and OsAOC in rice, were analyzed by RT-PCR. First, the bacterial suspension was drenched in the soil; 3 days after the bacterial treatment, the pathogen was challenged, as described previously. The leaves of B. oryzicola YC7007 treated and non-treated control seedlings were sampled before and after the pathogen F. fujikuroi challenge at 0, 6, 12, 24, 48, and 72 hours intervals. Total RNA was extracted by using RNA extraction kit (Qiagen RNeasy Plant Mini Kit; Qiagen, Hilden, Germany) and complementary DNA was synthesized (1 μg from total RNA of the sample) using the RT-PCR Kit (QuantiTect Reverse Transcription Kit; Qiagen). Briefly, PCR (20 μl) using aliquots (1 μl) of cDNA samples were denatured at 94°C for 2 minutes, and then conducted for 30 cycles with intermittent steps of 94°C for 20 seconds, 57°C for 30 seconds and 72°C for 40 seconds for all genes, except the OsAOC gene for 30 seconds. Finally, the reaction was fixed up by an extended temperature of 72°C for 5 minutes. The gene specific primers used for OsPR1-a, OsLOX-L2, and OsAOC are listed in Table S1 (Fanata et al., 2013; Peng et al., 2012; Qin et al., 2013; Riemann et al., 2013). Accumulation levels were normalized using OSActin1 as an internal reference.
Statistical analysis
All data were analyzed using the variance technique, and mean differences were estimated by Tukey’s honestly significant difference (HSD) and Duncan’s multiple range test, using statistical software, SPSS 17 (SPSS Inc., Chicago, IL, USA) and Sigma plot ver. 10.
Results
Suppression of rice bakanae by root drenching of B. oryzicola YC7007 in pots
Disease suppression and change in Fusarium population were investigated after drenching of B. oryzicola YC7007 suspension in pots. The control efficacy of B. oryzicola YC7007 against bakanae was determined by root drenching at different concentrations of the cell suspension, in pots containing autoclaved and non-autoclaved soils (Table 1). Treatments of three different concentrations of B. oryzicola YC7007 revealed significantly lower disease severity as compared to the control in the autoclaved soil. In the non-autoclaved soils, the disease severity index in bacterial treated seedlings at different concentrations was also lower than the control. However, in autoclaved soil conditions, there was no significant difference in the disease severity between the concentrations of 3.6 × 106 and 2.0 × 107 cfu/ml, which showed the consistent disease suppression of bakanae at 10 DAI of pathogen. Also, the disease suppression by treatments of B. oryzicola YC7007 in autoclaved soils was much higher (51%, 74%, and 78%, respectively), than in non-autoclaved soils (22%, 34%, and 46%, respectively) at all three concentrations evaluated.

Suppression of rice bakanae caused by Fusarium fujikuroi, by root drenching of Bacillus oryzicola YC7007 in pots
After evaluating the disease severity the control efficacy of B. oryzicola YC7007 was determined up to 30 DAI of pathogen only, in the autoclaved soil samples. The lowest disease severity was observed at the three different concentration of B. oryzicola YC7007 at 20 DAI of pathogen as compared to control. The disease severity in all B. oryzicola YC7007 treated seedlings was significantly lower as compared to control at 30 DAI of pathogens. However, there was no significant difference in % disease reduction between the concentrations 3.6 × 106 and 2.0 × 107 cfu/ml of B. oryzicola YC7007 at 30 DAI (Fig. 1A, B).
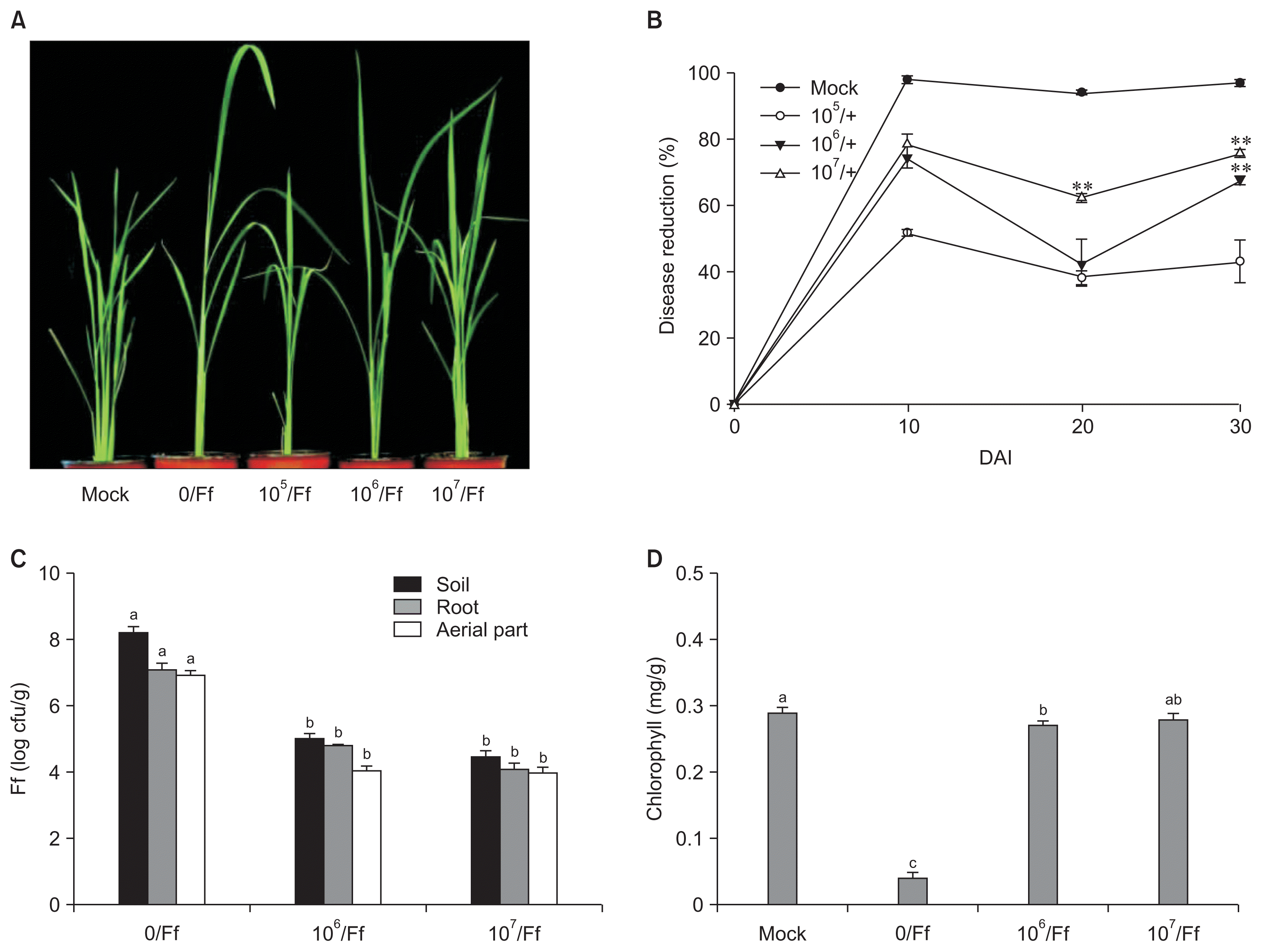
Suppression of bakanae disease and Fusarium population by drenching of Bacillus oryzicola YC7007 in pots with cultivation time. Cell suspension (15 ml) of B. oryzicola YC7007 (3.6 × 106 cfu/ml and 2.0 × 107 cfu/ml) was drenched into pots containing autoclaved soils (150 g/pot), 3 days before pathogen inoculation (10 g/kg). (A) Photographs depicting representative symptoms were taken at 20 days after inoculation (DAI) of pathogen. (B) Disease reduction (%) was determined from 10 to 30 DAI of pathogen using the formula: [(disease severity of the control – disease severity of a treatment) / disease severity of the control] × 100. (C) Population of Fusarium at the rhizosphere soil, root and aerial parts of the rice plant was measured at 10 DAI of pathogen, expressed in log cfu/g, by using the selective media. (D) Chlorophyll contents in the treated leaves collected at 20 DAI of pathogen were determined. Values are presented as mean ± standard error of three replicates, each consisting of 10 plants. Different letters indicate statistically significant differences, as evaluated by Tukey’s honestly significant difference (HSD) test (P < 0.01) and asterisks indicate statistically significant differences (Tukey’s HSD test by using SPSS 17 version, P < 0.01 for **). Ff means inoculation with the soil inoculum of F. fujikuroi (10 gm/kg) and mock means without pathogen inoculation. Mock plants were treated with the buffer solution only.
Further, from the same samples of B. oryzicola YC7007 treated and non-treated rice seedlings in autoclaved soils, the population of Fusarium in the rhizosphere soil, root and aerial parts was also determined. The treatments of B. oryzicola YC7007 (3.6 × 106 and 2.0 × 107 cfu/ml) revealed significantly (P < 0.01) lower concentration of Fusarium as compared to the untreated controls, in the soil, root and aerial parts of rice at 10 DAI of pathogen (Fig. 1C). The Fusarium population was reduced by 45.1%, 42.0%, and 42.3% in the soil, root and aerial parts of B. oryzicola YC7007 (2.0 × 107 cfu/ml) treated seedlings, as compared with the untreated control seedlings. However, no significant difference was found in the Fusarium population between the concentrations 3.6 × 106 and 2.0 × 107 cfu/ml. Bakanae symptoms of yellowing and chlorosis were observed and assessed in terms of chlorophyll amounts in treated and untreated control rice leaves. The chlorophyll content in B. oryzicola YC7007 suspension (2.0 × 107 cfu/ml) treated rice leaves was 0.28 ± 0.01 mg/g leaves, which was similar to mock plants having a chlorophyll content of 0.29 ± 0.01 mg/g leaves. In only Fusarium inoculated plants, the chlorophyll content was much lower (0.04 ± 0.01 mg/g) (Fig. 1D).
Comparison of the efficacy of B. oryzicola YC7007 and chemical fungicides, against bakanae in rice nursery boxes
The control efficacy of B. oryzicola YC7007 against bakanae was compared with that of seed treatment using chemical fungicides, in rice nursery boxes at the greenhouse. Bacterial treatments reduced the bakanae disease severity significantly as compared with the untreated control. The chemical seed treatment with only prochloraz had no control efficacy against bakanae. However, the seed treatment with dual chemical fungicides, prochloraz and fludioxonil, significantly reduced the disease with an index 0.81 ± 0.13, as compared with the control. There was no significant difference in the control efficacy between dual chemical treatment and bacterial root drenching in the nursery boxes, with 55.6% to 66.7% disease reduction respectively (Table 2).
Colonization of B. oryzicola YC7007
The colonization of endophytic strain, B. oryzicola YC7007 in the rice root and stem was determined at 0, 3, 5, and 8 DAI of B. oryzicola YC7007 suspension in the rhizosphere (Fig. 2). However, bacterial population was detected only in the root and basal 1 cm area of the stem from 0 to 8 DAI of bacterial suspension (Fig. 2). The population of B. oryzicola YC7007 was 6.53 ± 0.04 cfu/g and 5.7 ± 0.14 log cfu/g in the root and stem respectively, at 8 DAI of B. oryzicola YC7007 suspension.
Suppression of bakanae by culture filtrate and EtOAc extract of B. oryzicola YC7007
The effects of cell suspension, culture filtrates and EtOAc extracts of B. oryzicola YC7007 were evaluated to check for suppression of bakanae in petri plates and plant culture dishes (Fig. 3). In petri plate settings, the detached leaves bioassay was conducted by dipping the leaves in the cell suspension and the culture filtrate of B. oryzicola YC7007, after which the treated leaves were challenged with the pathogen. The development of necrotic lesion was suppressed significantly by treatment of both cell suspension and culture filtrate as compared to the control at 5 DAI of pathogen. Prolonged necrotic and blighted lesions with dense mycelial growth were observed in the untreated control leaves, when compared with the treated healthy leaves. The control efficacy of the cell suspension and culture filtrate was 86% and 90% respectively, as compared with the untreated control. However, no significant difference was seen between the two treatments, namely cell suspension and culture filtrate treated leaves. Untreated, mock leaves remained fresh and green without any symptoms of blight (Fig. 3A).

Suppression of bakanae by culture filtrate and ethyl acetate (EtOAc) extracts of Bacillus oryzicola YC7007. (A) Development of necrotic lesion of detached leaves after dipping in the cell suspension of B. oryzicola YC7007 (2.0 × 107 cfu/ml) for 30 seconds, and then kept for 48 hours at 28°C. Leaves were challenged by two fungal disk of Fusarium fujikuroi (Ff) that were removed after 24 hours, after which all samples were incubated for 5 days at 28°C for disease development. The culture filtrate (Cf) was sprayed to the detached leaves till to droplet and after 2 days, the leaves were subjected to the same bioassay above. (B) Representative pictures of the effect of EtOAc extract of B. oryzicola YC7007 in dimethyl sulfoxide (DMSO), at concentrations of 50, 100, 200 (μg/ml) followed by the treatment of conidial suspension of F. fujikuroi (1 × 106 conidia/ml) 3 days after treatment of EtOAc extracts. (C) The EtOAc extracts (5 ml) of B. oryzicola YC7007 in DMSO, at concentrations of 50, 100, 200 (μg/ml), was poured in 10-day-old seedlings in the plant culture dish. Seedlings were inoculated with the conidial suspension of F. fujikuroi (1 × 106 conidia/ml) 3 days after treatment of EtOAc extracts. Values are presented as mean ± standard error of three replicates, each consisting of 10 plants. Different letters indicate statistically significant differences by Tukey’s honestly significant difference (HSD) test at P < 0.01. Mock plants were treated with only buffer solution (10 mM MgSO4).
In plant culture dish settings, 10-day-old rice seedlings were drenched with a mixture of EtOAc extract suspension (5 ml) of B. oryzicola YC7007 (three concentrations) in 1/2 MS media. The treatment of EtOAc extracts at 50, 100, and 200 μg/ml in DMSO showed significantly lower disease severity of bakanae as compared to the untreated control. At 100 μg/ml of EtOAc extract, disease suppression was highest at 69.77%, compared with the untreated control; however, it was not statistically different at 200 μg/ml (Fig. 3B, C). Mock plants not treated to either pathogens or B. oryzicola YC7007, were observed to show the bakanae severity at 0.30 ± 0.06.
Accumulation of hydrogen peroxide by B. oryzicola YC7007
The effect of root drenching by B. oryzicola YC7007 on the accumulation of H2O2, was observed after staining the detached leaves at base foot (first internodes and leaf sheath areas) of culm rice seedlings (Fig. 4). To determine whether the bacterial treatment of B. oryzicola YC7007 switched on the activation of H2O2 accumulation in the base foot, sampling was done at 6 and 12 hours in B. oryzicola YC7007 treated leaves before and after inoculation of pathogen F. fujikuroi. The elicitation of H2O2 accumulation in B. oryzicola YC7007 treated leaves was observed to be low at 6 and 12 hours before inoculation of pathogen. The dark browning was more distinct at 6 hours, when B. oryzicola YC7007 was the only treatment, as compared with the untreated control. In only F. fujikuroi inoculated leaves, the accumulation of H2O2 was typically distinct at 6 and 12 hours, but did not show any difference in B. oryzicola YC7007 followed by F. fujikuroi treated leaves (Fig. 4). Surprisingly, the accumulation of H2O2 was attenuated at 12 hours as compared to 6 hours of bacterial treatment only.
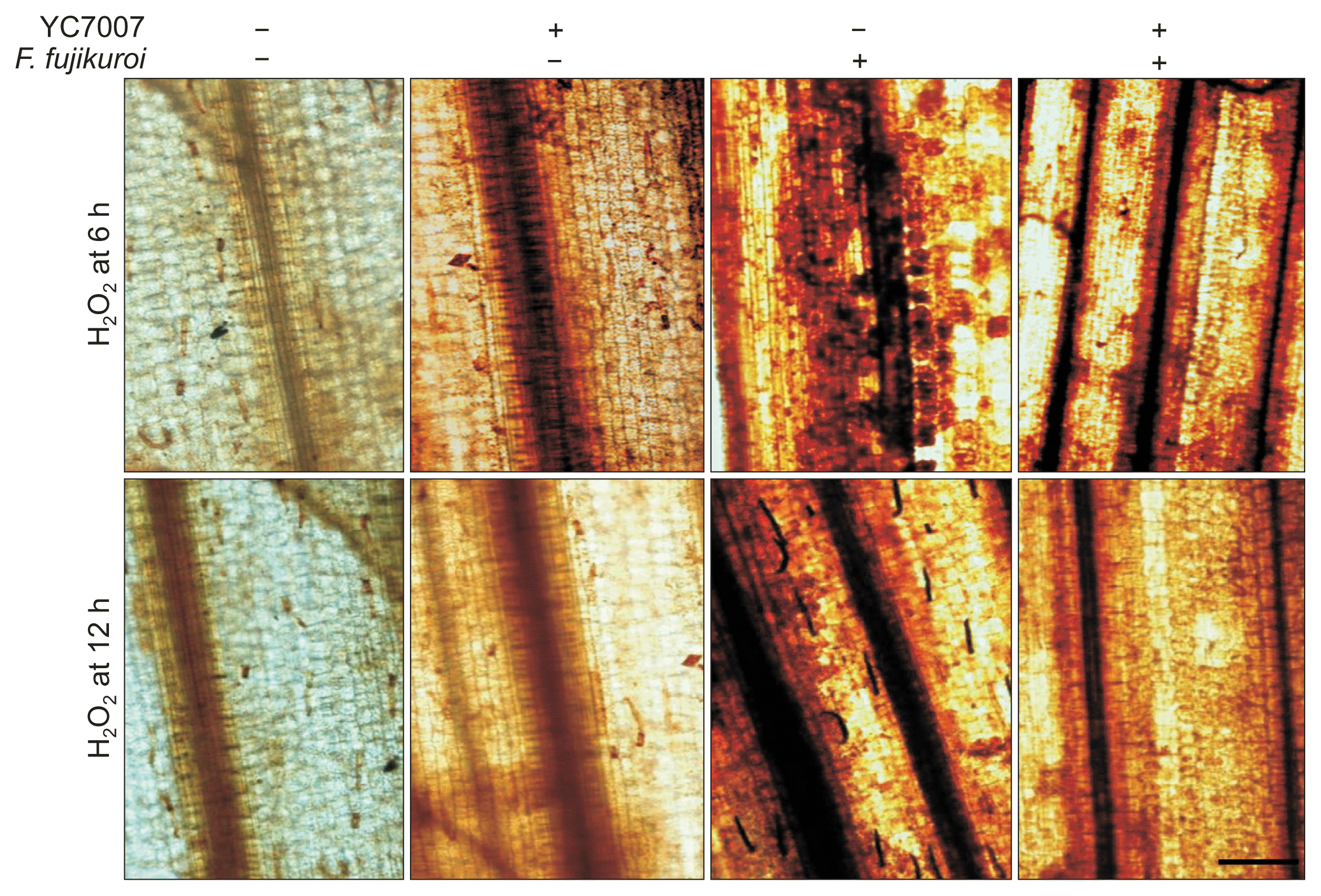
Effect of Bacillus oryzicola YC7007 on the accumulation of hydrogen peroxide in the base foot (first internodes and leaf sheath areas) of rice seedlings upon infection by Fusarium fujikuroi. +, inoculation with pathogen or B. oryzicola YC7007; -, no inoculation. Scale bar = 100 μm by 10× Olympus confocal microscope.
Primed induction of systemic resistance by B. oryzicola YC7007
To scrutinize the defensive mechanism for disease suppression by treatment of B. oryzicola YC7007, the expression of genes related to pathogenesis, OsPR1-a, OsLOX-L2, and OsAOC, were examined before and after the pathogen inoculation. Transcript levels of these genes in the leaves of rice seedlings were determined by semi-quantitative RT-PCR. We found that B. oryzicola YC7007 elicited and primed the hormonal response after inoculation of the pathogen (Fig. 5). Without pathogen inoculation, the expression levels of these genes were negligible in the leaves pretreated with the suspension of B. oryzicola YC7007 by soil drenching, and absent in the mock leaves, until 72 hours. The expression of OsPRI-a at 12 to 24 hours, and OsLOX-L2 at 12 to 72 hours, was observed only in the B. oryzicola YC7007 treated rice, but OsAOC was not expressed in B. oryzicola YC7007 treated plant (Fig. 5A). Contrarily, with the inoculation of the pathogens, the expression of OsAOC and OsLOX-L2 increased in B. oryzicola YC7007 treated leaves at 12 and 24 hours, when compared with only the pathogen inoculated seedling. In only pathogen inoculated plants, the level of expression was minimal lately at 12 to 72 hours for OsLOX-L2, 6 to 72 hours for OsPRI-a, and 12 to 72 hours after pathogen inoculation for OsAOC. During interaction between B. oryzicola YC7007 and the pathogen, the expression of defense genes was stronger and faster as compared with only pathogen inoculated plants. We found that the interaction of B. oryzicola YC7007 and the pathogen primed the expression of OsAOC gene, as compared to only pathogen inoculated leaves (Fig. 5B), thus proving that B. oryzicola YC7007 upregulated the genes related to pathogenesis, which would help to suppress the disease caused by pathogenic invasion.

Effect of Bacillus oryzicola YC7007 on the expression of defense related marker genes in rice. (A) Expression of defense genes, OsPR1-a and OsLOX-L2 in B. oryzicola YC7007 treated rice leaves without pathogen inoculation. (B) Expression of defensive genes by treatment of B. oryzicola YC7007 after inoculation of pathogen (Fusarium fujikuroi) was determined by qualitative RT-PCR in leaves inoculated with only pathogen (left), and combination of B. oryzicola YC7007 and pathogen (right). Transcript accumulation levels were normalized using OsActin1 as an internal reference and expressed relative to the normalized expression levels in control and inoculated plants at 0, 6, 12, 24, 48, and 72 hours after inoculation.
Discussion
Biological control using antagonistic bacteria and fungi would be an environmentally sound option, and can be an alternative to agrochemicals in the management of plant diseases. During the last few decades, many Bacillus species have been used for controlling plant diseases with some success, but only a few have been developed for the practical use in commercial farms (McSpadden Gardener, 2004, 2010; Paulitz and Bélanger, 2001). Several Bacillus species have also been tried in some Asian rice growing areas for controlling rice diseases (Gnanamanickam, 2009; Park et al., 2006). Recently, a novel endophytic bacterial strain B. oryzicola YC7007 isolated from rice roots with plant growth promoting activity, has been reported to control bacterial blight and panicle blight of rice through the induction of systemic resistance (Chung et al., 2015). In this study, root drenching with the suspension of B. oryzicola YC7007 displayed strong suppression of rice bakanae in autoclaved and nonautoclaved soils at 10 DAI of the pathgen. The control of soil-borne diseases through ISR by the split root treatment with fluorescent Pseudomonas species, and the pathogen within different time intervals, was also found similarly in a previous study. Through the ISR approach, all treatments with three different concentrations of B. oryzicola YC7007 reduced the bakanae severity, and the two higher concentrations (over 3.6 × 106 cfu/ml) significantly suppressed the bakanae more than 50%, when compared to the controls. This suggests at least 106 cfu/ml is required to control the disease effectively (Table 1). In non-autoclaved soils, the disease reduction by B. oryzicola YC7007 was less than in autoclaved soils, indicating that the activity of B. oryzicola YC7007 might be adversely affected by the other microbial consortium residing in the rhizosphere (Aslam et al., 2013; Mendes et al., 2011). In nursery box settings without pathogen inoculation, the reduction of bakanae severity at the higher treatment concentrations was confirmed with control values of 60.0–66.7%, which was not significantly different from that of the chemical seed treatment with both prochloraz and fludioxonil (Table 2). Seed treatment with only prochloraz almost did not induce the suppression of bakanae; this appeared to be due to the fungicide resistance developed in F. fujikuroi (Yang et al., 2012). In control seedlings, high disease severity was shown in both pot and nursery box tests, which indicated severe infection of rice seeds by the pathogen during cultivation in fields (Kazempour and Elahinia, 2007; Kim, 1981). Overall strain B. oryzicola YC7007 showed good suppression of bakanae with control efficacy similar to those other biotic (polyglycerol polyricinoleate, non-pathogens, cell wall fragments) or abiotic (chemical fungicides, Jingangmycin, Validamycin) elicitors against rice sheath blight, ranging from 50% to 90%, as reported previously (Mew et al., 2004).
We further assessed the quantification of F. fujikuroi in soil, root and aerial parts, in comparison to the treated and untreated control rice seedlings. The lowest concentration of F. fujikuroi may be due to the inhibition of pathogen growth by the bacterial treatment at 2.0 × 107 cfu/ml, in all parts of rice seedlings at 10 days after pathogen inoculation (Fig. 1C). However, the movement of F. fujikuroi was not investigated in this study. Population of F. fujikuroi in the bacterial treated and the control seedling was significantly different (P < 0.01), but was similar between two bacterial concentrations. In the root of barley seedlings treated with mutualistic fungus Piriformospora indica, the fungal DNA of Fusarium culmorum was decreased in the root parts at two weeks after inoculation of the pathogen in soil compared with the untreated control, indicating the pathogen F. culmorum, is systemic (Harrach et al., 2013). In a similar way, the population of F. fujikuroi from soil, rice roots and aerial parts (1 cm from base foot) were observed; we found that bakanae pathogen is also systemic. The content of chlorophyll was much less in abnormally elongated leaves, showing yellowing and chlorosis produced by F. fujikuroi, which may significantly affect the photosynthesis of rice leaves, thus resulting in reduced yields (Fig. 1D).
Since the novel strain, B. oryzicola YC7007 was reported to be endophytic in our previous study, its localization in rice was examined (Fig. 2). B. oryzicola YC7007 was confined in the root and stem tissues at 8 days after bacterial treatment, and its population examined in the root was higher than that in stem tissues. Recently, other endophytic bacteria such as Gluconacetobacter diazotrophicus PAL5 and Burkholderia kururiensis M130 have been also found to colonize rice roots. The defense related gene PR-10 was expressed more strongly compared to PR-1 by G. diazotrophicus PAL5 in bacterial treated rice seedlings (Alquéres et al., 2013; Coutinho et al., 2015). One other colonizing endophytic Pseudomonas poae strain RE*1-1-14 was reported to suppress the growth of fungal pathogen, Rhizoctonia solani. Similarly, plants have been recognized by such types of microbiomes, revealing close symbiotic relationships with their associated microorganisms (Berg et al., 2014; Zachow et al., 2015). From the viewpoint of potential application, the endospore forming endophytic strain B. oryzicola YC7007 has more advantage than these Gram-negative bacteria, which are hardly used practically due to their instability in field conditions.
Many Bacillus species with antagonistic activity against plant diseases are known to produce diverse antibiotic compounds (Raaijmakers et al., 2010). We checked the control efficacy of culture filtrate and EtOAc extracts against bakanae in detached leaves bioassay and plant culture dishes, respectively. The expansion of necrotic lesion by F. fujikuroi and its mycelial growth were strongly inhibited on the detached rice leaves by dipping the end of leaves in cell suspension and spraying of the culture filtrate (Fig. 3). This suggests that the strain may produce compounds which can induce systemic resistance and inhibit the fungal growth simultaneously. We, therefore, investigated whether the EtOAc extract of culture broth showed disease reduction of bakanae through induction of systemic resistance. Drenching of > 50 μg/ml EtOAc extract to the culture dishes showed significantly lower disease severity of bakanae, indicating the induction of systemic resistance by chemical compounds. It is well known that Bacillus species produce many kinds of small peptides and lipopeptides, such as fengycin, iturin and surfactin, having antifungal and ISR activity (Bais et al., 2004; Crane et al., 2013; Dimkic et al., 2013; Ongena et al., 2009; Park et al., 2006).
We checked the innate immunity affected by B. oryzicola YC7007 in the detached leaves bioassay, with DAB staining (Fig. 4). B. oryzicola YC7007 activated the response of rice cells related with the ROS or the accumulation of more H2O2 in leaves in the early stage at 6 hours after B. oryzicola YC7007 inoculation, than the untreated control. This indicates B. oryzicola YC7007 was able to switch on the ROS-mediated defense machinery, one of the earliest events in plant response by rhizobacterial-ISR (Niu et al., 2011). On the contrary, another endophytic bacterium Burkholderia phytofirmans PsJN was reported to induce resistance against the pathogen, Pseudomonas syringae pv. pisi, without generating the ROS (Bordiec et al., 2011). Furthermore, the accumulation of H2O2 was attenuated at 12 hours after inoculation (HAI) of the B. oryzicola YC7007 compared with that at 6 HAI of the strain along with the pathogen, F. fujikuroi, which suggests that B. oryzicola YC7007 might have an additional mechanism to inactivate the production of the host ROS during colonization of the root, especially when associated with superoxide dismutase and glutathione reductase (Alquéres et al., 2013).
In addition to the cellular defense, the hormonal mechanism for inducing resistance against the pathogen by B. oryzicola YC7007 was examined. The expression of some pathogenesis related genes of rice (OsPR1-a, OsLOX-L2, and OsAOC) were examined before and after inoculation of the pathogen (Fig. 5). Without inoculation of the pathogen, the transcript levels of these genes were weakly observed in the leaves pretreated with only B. oryzicola YC7007. On the contrary, B. oryzicola YC7007 elicited a stronger induction of OsLOX-L2 and OsAOC, when challenged along with the pathogen. Priming induction was observed in the case of OsAOC, with no expression seen without the pathogen inoculation. In a previous study (Ahn et al., 2007), PR1 and PDF1.2 were not expressed in Arabidopsis by the treatment of Pseudomonas putida LSW17S without pathogen inoculation, and these genes also underwent primed induction with pathogen inoculation, which was similar to our findings of OsAOC. In only pathogen inoculated plants, the initial expression of these genes was minimal and was induced later. However, with the inoculation of the pathogen, B. oryzicola YC7007 enhanced the early induction of resistance, resulting in the suppression of the bakanae. These data suggest that B. oryzicola YC7007 followed the JA pathway in the wild type background, which is partially similar to the previous studies by rhizobacterial effect in rice, Arabidopsis and ryegrass (Alquéres et al., 2013; De Vleesschauwer et al., 2008; Niu et al., 2011; Rahman et al., 2015). B. oryzicola YC7007 successfully controlled the bakanae via innate and hormonal immune machineries, and also saved energy by priming the induction of resistance. It is generally accepted that plant growth was suppressed during the induction of SAR, probably due to the energy consumption. The commercialized chemical benzothiadiazole (BTH), an analogue of SA, was shown to suppress the growth and biomass of Arabidopsis (Ryu et al., 2004a, 2004b; Walters and Heil, 2007). In this aspect, B. oryzicola YC7007 has no such disadvantage and rather has plant growth promoting activity. The hormonal signaling mechanism during the interaction between F. fujikuroi and B. oryzicola YC7007 needs to be elucidated further through molecular, histochemical and pathological analyses using mutants of rice and Arabidopsis.
B. oryzicola YC7007 has multiple functions, including antibiotic producing, resistance inducing and plant growth promoting activities, the use of B. oryzicola YC7007 would be a good approach for controlling the soil and seed-borne rice diseases. To the best of our knowledge, the present study is the first report on the biological control of rice diseases using endophytic bacteria with multiple mechanisms. In addition, with only one time seed or seedling treatment of endophytic strain B. oryzicola YC7007, the bakanae could be controlled effectively. More studies on the further mechanisms and field tests would explore this novel bacterium B. oryzicola YC7007 to be developed as a biocontrol agent that can be implemented in the sustainable rice production system.
Supplementary Information
Acknowledgments
This work was supported by R&D Program of MOTIE/ KEIT (10044909, Development of the long-lasting and broad-spectrum plant protectants). M.T. Hossain, A. Khan and E.J. Chung were supported by a scholarship from the BK21 Plus Program, the Ministry of Education, Republic of Korea. Our special thanks are extended to Dr N. S. Jwa at Sejong University, Republic of Korea for his kind comments and constructive suggestion to prepare the manuscript.
Notes
Articles can be freely viewed online at www.ppjonline.org.

