 |
 |
| Plant Pathol J > Volume 32(3); 2016 > Article |
Abstract
We observed the changes in aggressiveness and fecundity of the anthracnose pathogen Colletotrichum acutatum on hot pepper, under the ambient and the twice-ambient treatments. Artificial infection was repeated over 100 cycles for ambient (25°C/400 ppm CO2) and twice-ambient (30°C/700 ppm CO2) growth chamber conditions, over 3 years. During repeated infection cycles (ICs) on green-pepper fruits, the aggressiveness (incidence [% of diseased fruits among 20 inoculated fruits] and severity [lesion length in mm] of infection) and fecundity (the average number of spores per five lesions) of the pathogen were measured in each cycle and compared between the ambient and twice-ambient treatments, and also between the early (ICs 31-50) and late (ICs 81-100) generations. In summary, the pathogen’s aggressiveness and fecundity were significantly lower in the late generation. It is likely that aggressiveness and fecundity of C. acutatum may be reduced as global CO2 and temperatures increase.
Atmospheric CO2 is projected to double by the end of this century with increases in temperature due to the accumulation of greenhouse gases (IPCC, 2014). The interactions between plants and pathogens will be affected by the elevated CO2 levels (Chakraborty et al., 2008). Within this context, when considering the effects of climate change on the plant disease triangle, it is important to consider not only atmospheric changes but also changes in host resistance and pathogens adaptation.
Pathogen fitness includes both virulence and aggressiveness (Shaner et al., 1992). Virulence is the ability to overcome host resistance. Aggressiveness is the damage it causes to the host, regardless of resistance genes (Akinsanmi et al., 2007; Burdon, 1987). There are many examples of virulence overcoming host resistance in gene-for-gene interactions. For example, Uromyces appendiculatus adapted to some cultivars after five asexual reproduction cycles (Alexander et al., 1985) and Erysiphe graminis overcame its host resistance after 30 asexual cycles (Newton and McGurk, 1991). Pathogen aggressiveness can be enhanced by overcoming the host’s heritable quantitative resistance, as reported for Mycosphaerella graminicola (Mundt et al., 1999).
A pathogen’s aggressiveness reflects its ability to infect and reproduce (Johnson and Taylor, 1972), but can be affected by environmental factors (Newton, 1989). Increasingly aggressive strains may result from favorable weather conditions (Mundt et al., 1999). Under high CO2 conditions, the aggressiveness of anthracnose was found to increase on the tropical legume Stylosanthes scabra cv. Seca, but this was not the case for the susceptible cultivar Fitzroy, over 25 consecutive infection cycles (ICs) (Chakraborty and Datta, 2003).
We previously reported the changes in disease development on hot pepper (Capsicum annum L.) diseases in response to elevated CO2 (700 ppm) and temperature (30°C) in a growth chamber (Shin and Yun, 2010). The four main diseases studied were anthracnose (C. acutatum), Phytophthora blight (Phytophthora capsici), bacterial spot (Xanthomonas campestris pv. vesicatoria), and bacterial wilt (Ralstonia solanacearum). The results showed that climate change affected the interaction between hot pepper and the four pathogens. In the present study, we investigated the impact of anthracnose, highlighting significant aspect of the pathogen and trying to show the gradual changes of the aggressiveness and fecundity of C. acutatum under elevated CO2 and temperature over repeated ICs. Its aggressiveness may or may not be changed by the climate change treatment over sequential ICs. We investigated the impacts of elevated CO2 and temperature on the aggressiveness of anthracnose on hot pepper fruits over 100 ICs.
Green peppers were treated in two growth chambers, which were controlled at 25°C/400 ppm CO2 (ambient) and 30°C/700 ppm CO2 (twice-ambient). The chambers were monitored for CO2 concentration, temperature, and light (Shin and Yun, 2010).
The pathogenic fungal isolate C. acutatum SM017 was collected from a field in Asan, Korea, in 2007 (Shin and Yun, 2010). The isolate is generally aggressive to most hot pepper varieties. A fully grown green-pepper from organic markets was wounded with a needle lancet (autolancet™; Ambimedinc, Captola, CA, USA) and sprayed with conidial suspensions. A total of 20 inoculated peppers were treated in each growth chamber. Each pepper was placed in a 4 cm diameter test tube, with a water-saturated Kimwipe (Kimberly-Clark, Irving, TX, USA) at the bottom, for 5-7 days to maintain saturated humidity. The lesions on each fruit were assessed and expressed as incidence (the % of diseased fruits among the 20 green-pepper fruits) and severity (the sum of lesion lengths on 20 green-pepper fruits inoculated) 7 days after inoculation. Five lesions per treatment were chosen at random and assessed for pathogen fecundity. The average number of the conidia was recorded. Spores were removed by vortexing in 20 ml sterile distilled water for 10 seconds, and spores in suspensions were counted using a hemacytometer. The number of spores per lesion was used as a measure of fecundity (Chakraborty and Datta, 2003).
Single spores were isolated for every IC and each treatment. To obtain a pure culture for the next generation, single spores were isolated and plated on potato dextrose agar. The pure cultures for each IC were stored in a deep freezer for future use. A total 103 ICs were used in each treatment. The inoculations were repeated for twice or three times until the conidia were available for the next generation. Each IC took a week or more, and more than 3 years were required to finish the experiment. Data were not available for cycles 1-14, 22-25, and 53-61.
Early and late ICs were designated as generations 31-50 and 81-100, respectively. Comparisons between the ambient (25°C/400 ppm CO2) and twice-ambient (30°C/700 ppm CO2) were conducted for the early and late generations. Comparisons between the early and late generations were also made within each treatment. The unpaired Student’s t-test was performed for the comparisons between two populations of the early (ICs 31-50) and the late (ICs 81-100) generations from the two treatments. The alternative hypothesis of the comparison was the two populations were different (two tailed tests) by S-link (ver. 2.2; S-Link, Seoul, Korea) of the statistical analysis software program.
Elevation of CO2 and temperature led to different incidences of infection after about 50 generations (Fig. 1A). It was obvious from ICs 81-100, except for IC 83, that incidences in the late generation were 85-100% and 40-60% under the ambient and the twice-ambient treatments, respectively.
The severity of infection differed less between the treatments, but were particularly obvious in ICs 81-100, except for ICs 94 and 100 (Fig. 1B). Differences in fecundity between the treatments were not obvious, particularly compared to aggressiveness (incidence and severity). The spore counts from the late generation under the ambient treatment were around 1,000 conidia, whereas they were less than 200 conidia under the twice-ambient treatment (Fig. 1C).
When considering only the early (ICs 31-50) generations, the incidences were 97.8% and 79.3% for the ambient and twice-ambient treatments (Table 1), respectively; their difference was statistically significant (P = 0.006; Table 2). Mean severity scores were 343.5 mm and 289.1 mm (Table 1), respectively; their difference was not statistically significant (P = 0.074; Table 2). Fecundities were 1,866 and 986.7 spores/lesion, respectively; their difference was statistically significant (P = 0.005; Table 2).
During the late (ICs 81-100) generations, the incidences were 84.3% and 50.0% (P < 0.000; Table 1, 2), severities were 382.6 and 144.6 mm (P < 0.000; Table 1, 2), and fecundity values were 997 and 173 spores/5 lesions, respectively (P < 0.000; Table 1, 2). Differences in pathogenicity and fecundity between treatments after IC 65 were statistically significant (P < 0.001; Table 2, Fig. 1).
The incidence was maintained at 80-100% throughout the 100 generations under ambient conditons, but decreased to around 50%, starting at IC 50, under the twice-ambient treatment, with few exceptions (Fig. 1A). Between ICs 60-80, incidences were undulating and it may affect severity and fecundity. Severity was similarly maintained at 300-400 mm at the early, but changed in the later, starting at IC 45; it was around 150 mm at IC 75 (Fig. 1B). Comparisons between the early and the late generations were shown that incidence and severity were statistically significant at the twice-ambient treatment with P < 0.001 on aggressiveness, but not at the ambient treatment with P = 0.051 and P = 0.258, respectively (Table 2). Fecundity was higher under the ambient treatment than the twice-ambient treatment (Fig. 1C). The differences on fecundity between the early and the late generations were both significant at ambient and twice-ambient treatments (P < 0.001; Table 2).
Taken together, these results suggest that the pathogenicity of this fungus did not change under ambient conditions, but the number of conidia decreased over time. Pathogenicity and fecundity were significantly different between early and late generations in the twice-ambient treatment (Table 1).
Our results showed that aggressiveness of the anthracnose pathogen was significantly reduced at 700 ppm CO2 and in response to a temperature increase of 5°C after 100 ICs. Whereas, aggressiveness was maintained for over 100 ICs at an incidence of 80-100%. According to Chakraborty and Datta (2003) with anthracnose on S. scabra under twice-ambient CO2, aggressiveness increased during the first 10 ICs, regardless of resistant and susceptible cultivars. Because their results of anthracnose aggressiveness declined after cycle 22 (Chakraborty and Datta, 2003), we think ICs used in their study were too short to show the changes on the pathogen under no dramatic changes in temperature.
Aggressiveness is very sensitive to temperature (Newton, 1989). Aggressiveness increased under similar higher temperature conditions in asexually reproducing fungal pathogens (Ahmed et al, 1996; Cowger and Mundt, 2002; Newton and McGurk, 1991). In most of aggressiveness studies, future warmer temperatures were not considered and too short ICs were examined, compared to our study. According to our previous chamber study (Shin and Yun, 2010), incidence of anthracnose was slightly decreased, but the decrease was not statistically significant. The results were from several ICs under the same at 700 ppm CO2. However, we increased 5°C warmer than the ambient treatment. In this regard, future anthracnose on pepper under climate change would decrease according to the results of host-pathogen interaction (Shin and Yun, 2010) and pathogen’s pathogenicity as well as fecundity in this study.
A simulation study based on an infection model predicted possible increase in infection risk of pepper anthracnose up to 2,100 when temperature and precipitation were projected under the A1B scenario of greenhouse gas emission (Shin and Yun, 2011). When we simulated the model to show impact of global warming on anthracnose in the future, we did not consider gradual adaptation of C. acutatum to environmental changes. According to this study, global warming would threaten C. acutatum survival, but coevolutionary processes with superior varieties, fungicides, and agricultural practice in the future are still potentials to occur disease epidemics by well-adapted the pathogen.
We found that elevated CO2 levels and temperature decreased the fecundity of anthracnose, in contrast to previous studies on other pathogens (Chakraborty et al., 2000; Chakraborty and Datta, 2003; Hibberd et al., 1996). Most fecundity studies involving climate change have been conducted on plants in an altered canopy microclimate, which is more conducive to anthracnose development. In addition, increased fecundity and a favorable microclimate in a global warming scenario could accelerate pathogen evolution. On the other hands, our study involved artificial infection of fruits, but changes in canopy microclimate were not considered. According to several previous studies (Chakraborty et al., 1998, 2008; Manning and Tiedemann, 1995), significant changes in plant growth induced by elevated CO2 levels are likely to influence severity. Although our chamber study was simple and not to design to find an altered canopy changes, it was much longer periods of observation over 100 ICs to show the changes of the pathogen’s ability under climate changes than the previous studies with 22 ICs (Chakraborty and Datta, 2003). Aggressiveness and fecundity are not correlated in the M. graminicola-wheat cropping system (Zhan et al., 2002). Moreover, there is a trade-off between aggressiveness and transmissibility, leading to lower aggressiveness and slower evolution to an optimal level of aggressiveness (Jarosz and Davelos, 2005; Lenski and May, 1994). In this study, we found that there was a high correlation between incidence and severity (r = 0.81), but either incidence and fecundity or severity and fecundity were little correlated with r = 0.53 and r = 0.57, respectively (data not shown).
Molecular analyses of aggressiveness can provide concrete evidence of genetic changes in response to selection under changing climates. We have not attempted to identify pathogen changes on a molecular level. From Chakraborty and Datta (2003) study of the changes in the random amplified polymorphic DNA fingerprint of anthracnose on S. scabra, they could not provide molecular evidence for the association of genetics and aggressiveness. In the future, molecular evidence may become available once the pathogenic genes have been confirmed. Our preserved anthracnose pathogens, from up to 100 or more ICs, will be helpful to elucidate genetic adaptations to the altered pathosystem.
Fig. 1
Changes in aggressiveness (A + B, incidence + severity) and fecundity (C, spores/lesion) of Colletotrichum accutatum after 103 sequential infection cycles (ICs) for ambient (25°C/400 ppm CO2, ■) and twice-ambient (30°C/700 ppm CO2,○) treatments. Incidence is the percentage of diseased fruits among the 20 green-pepper fruits inoculated after wounding in each IC; severity is the sum of lesion lengths on the 20 green-pepper fruits inoculated after wounding in each IC; and fecundity is the average number of spores collected from five lesions selected at random from the 20 green-pepper fruits inoculated after wounding in each IC.
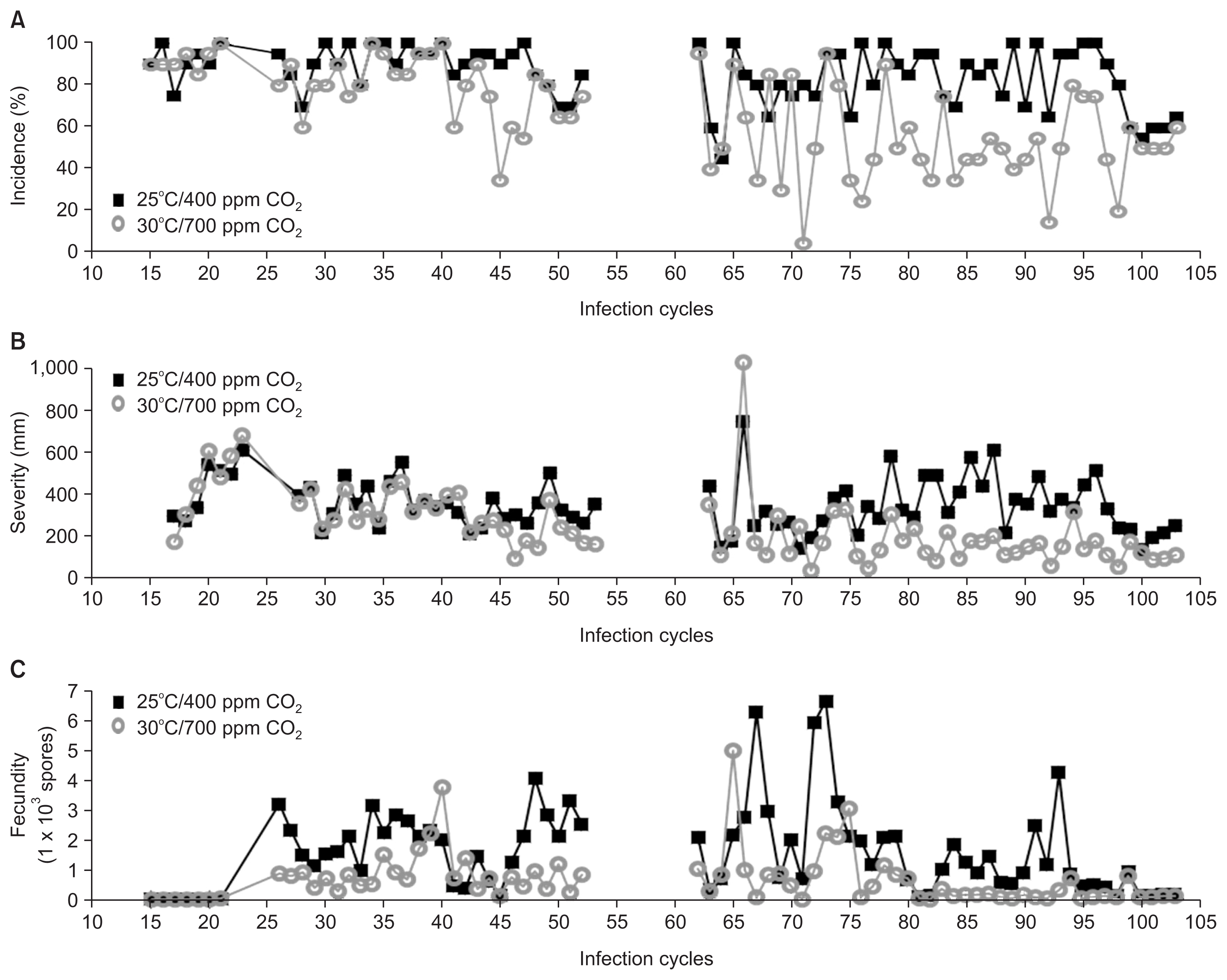
Table 1
Incidence, severity, and fecundity of Colletotrichum acutatum for the early (ICs 31-50) and the late (ICs 81-100) generations under ambient (25°C/400 ppm CO2) and the twice-ambient (30°C/700 ppm CO2) treatments
| Treatment | ICs | Incidence (%)* | Severity (mm)† | Fecundity (1 × 103 spores)‡ |
|---|---|---|---|---|
| Ambient | Early | 91.75 ± 8.31 | 343.5 ± 88.8 | 1866.3 ± 1025.0 |
| Late | 84.25 ± 14.4 | 382.6 ± 123.8 | 996.9 ± 978.9 | |
| Twice-ambient | Early | 79.25 ± 16.9 | 289.1 ± 98.2 | 986.6 ± 843.2 |
| Late | 50.00 ± 17.8 | 144.6 ± 60.2 | 173.3 ± 213.4 |
Table 2
Differences in incidence, severity, and fecundity of the isolate between the early (ICs 31-50) and the late (ICs 81-100) generations as well as between ambient (25°C/400 ppm CO2) and the twice-ambient (30°C/700 ppm CO2) treatments
| Comparison | P-value | ||
|---|---|---|---|
|
|
|||
| Incidence* | Severity† | Fecundity‡ | |
| (Ambient vs. twice-ambient) early | 0.006 | 0.074 | 0.005 |
| (Ambient vs. twice-ambient) late | < 0.001 | < 0.001 | 0.001 |
| (Early vs. late) ambient | 0.051 | 0.258 | 0.009 |
| (Early vs. late) twice-ambient | < 0.001 | < 0.001 | < 0.001 |
References
Ahmed, HU, Mundt, CC, Hoffer, ME and Coakley, SM 1996. Selective influence of wheat cultivars on aggressiveness of Mycospaerella graminicola (Anamorph Septoria tritici). Phytopathology. 86:454-458.

Akinsanmi, OA, Chakraborty, S, Backhouse, D and Simpfendorfer, S 2007. Passage through alternative hosts changes the fitness of Fusarium graminearum and Fusarium pseudograminearum. Environ Microbiol. 9:512-520.


Alexander, HM, Groth, JV and Roelfs, AP 1985. Virulence changes in Uromyces appendiculatus after five asexual generations on a partially resistant cultivar of Phaseolus vulgaris. Phytopathology. 75:449-453.

Burdon, JJ 1987. Diseases and plant population biology. Cambridge University Press, Cambridge, UK. 217.
Chakraborty, S and Datta, S 2003. How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytol. 159:733-742.



Chakraborty, S, Luck, J, Freeman, A, Norton, RM, Garrett, KA, Percy, KE, Hopkin, AA, Davis, C and Karnosky, DF 2008. Impacts of global change on diseases of agricultural crops and forest types. CAB Rev Perspect Agric Vet Sci Nutr Nat Res. 3:1-15.
Chakraborty, S, Murray, GM, Magarey, PA, Yonow, T, O’Brien, R, Croft, BJ, Barbetti, MJ, Sivasithamparam, K, Old, KM, Dudzinski, MJ, Sutherst, RW, Penrose, LJ, Archer, C and Emmentt, RW 1998. Potential impact of climate change on plant diseases of economic significance to Australia. Australas Plant Path. 27:15-35.

Chakraborty, S, Pangga, IB, Lupton, J, Hart, L, Room, PM and Yates, D 2000. Production and dispersal of Colletotrichum gloeosporioides spores on Stylosanthes scabra under elevated CO2. Environ Pollut. 108:381-387.


Cowger, C and Mundt, CC 2002. Aggressiveness of Mycosphaerella graminicola isolates from susceptible and partially resistant wheat cultivars. Phytopathology. 92:624-630.


Hibberd, JM, Whitbread, R and Farrar, JF 1996. Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis. Physiol Mol Plant Pathol. 48:37-53.

IPCC. 2014. Summary for policymakers. In: Climate change 2013: the physical science basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds. by TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex and PM Midgley, Cambridge University Press, Cambridge, UK.
Jarosz, AM and Davelos, AL 1995. Effects of disease in wild plant populations and the evolution of pathogen aggressiveness. New Phytol. 129:371-387.


Johnson, R and Taylor, AJ 1972. Isolation of Puccinia striiformis collected in England from wheat varieties Maris Beacon and Joss Cambier. Nature. 238:105-106.


Lenski, RE and May, RM 1994. The evolution of virulence in parasites and pathogens: reconciliation between two competing hypotheses. J Theor Biol. 169:253-265.


Manning, WJ and Tiedemann, AV 1995. Climate change: potential effects of increased atmospheric carbon dioxide (CO2), ozone (O3), and ultraviolet-B (UV-B) radiation on plant diseases. Environ Pollut. 88:219-245.


Mundt, CC, Hoffer, ME, Ahmed, HU, Coakley, SM, DiLeone, JA and Cowger, C 1999. Population genetics and host resistance. In: Septoria on cereals: a study of pathosystems, eds. by JA Lucas, P Bowyer and HM Anderson, 115-130. CAB International, Wallingford, UK.
Newton, AC 1989. Genetic adaptation of Erysiphe graminis f. sp. hordei to barley with partial resistance. J Phytopathol. 126:133-148.

Newton, AC and McGurk, L 1991. Recurrent selection for adaptation of Erysiphe graminis f. sp. hordei to partial resistance of barley. J Phytopathol. 132:328-338.

Shaner, G, Stromberg, EL, Lacy, GH, Barker, KR and Pirone, TP 1992. Nomenclature and concepts of aggressiveness and virulence. Annu Rev Phytopathol. 30:47-66.


Shin, JW and Yun, SC 2010. Elevated CO2 and temperature effects on the incidence of four major chili pepper diseases. Plant Pathol J. 26:178-184.

Shin, JW and Yun, SC 2011. Impact of climate change in fungicide spraying for anthracnose on hot pepper in Korea during 2011-2100. Korean J Agri Forest Meteorol. 13:10-19.
Zhan, J, Mundt, CC, Hoffer, ME and McDonald, BA 2002. Local adaptation and effect of host genotype on the rate of pathogen evolution: an experimental tests in a plant pathosystem. J Evol Biol. 15:634-647.
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




