 |
 |
| Plant Pathol J > Volume 32(6); 2016 > Article |
Abstract
A bacterial pathogen, Pseudomonas syringae pv. actinidiae (Psa), is a causal agent of kiwifruit bacterial canker worldwide. Psa biovar 3 (Psa3) was first detected in 2011 at an orchard in Dodeok-myeon, Goheunggun, Jeonnam Province in Korea. In this study, we present the results of an epidemiological study regarding Psa3 occurrence on kiwifruit orchards in Korea for the period of 2013 to 2015. Since the first detection of Psa3 in 2011, there was no further case reported by 2013. However, Psa3 was rapidly spreading to 33 orchards in 2014; except for three orchards in Sacheonsi, Gyeongnam Province, most cases were reported in Jeju Island. Entering 2015, bacterial canker by Psa3 became a pandemic in Korea, spreading to 72 orchards in Jeju Island, Jeonnam, and Gyeongnam Provinces. Our epidemiological study indicated that the first Psa3 incidence in 2011 might result from an introduction of Psa3 through imported seedlings from China in 2006. Apart from this, it was estimated that most Psa3 outbreaks from 2014 to 2015 were caused by pollens imported from New Zealand and China for artificial pollination. Most kiwifruit cultivars growing in Korea were infected with Psa3; yellow-fleshed cultivars (Yellow-king, Hort16A, Enza-gold, Zecy-gold, and Haegeum), red-fleshed cultivars (Hongyang and Enza-Red), green-fleshed cultivars (Hayward and Daeheung), and even a kiwiberry (Skinny-green). However, susceptibility to canker differed among cultivars; yellow- and red-fleshed cultivars showed much more severe symptoms compared to the green-fleshed cultivars of kiwifruit and a kiwiberry.
One of the biggest limiting factors in the cultivation of kiwifruit is a bacterial canker disease (hereafter canker) caused by Pseudomonas syringae pv. actinidiae (Psa). Kiwifruit canker is known for its devastating impact on kiwifruit industries worldwide, causing tremendous economic losses by destroying a vine or orchard in one or a few seasons (Scortichini et al., 2012; Vanneste, 2012). Following infection, Psa massively propagates inside the plant, eventually causing severe damage and resulting in death of a whole vine. When a kiwifruit vine is infected by Psa, milky bacterial ooze typically flows out from natural openings or pruning injuries from February or early March. As the disease progresses, the bacterial sap mixes with pigments in the bark tissue, creating dark-red ooze, which gives the appearance that the vine is bleeding. At the end of April, leaves display yellow-green or yellow spots, which eventually create an irregular dark brown pattern 0.5-1 cm in diameter as new shoots grow out. In some cases, the entire leaf dies as a result of severe infection. Typical leaf symptoms usually appear until July and new leaf symptoms do not form as the average daily temperature increases during the Korean summer (Koh et al., 1994). However, leaf symptoms tend to reappear when temperatures begin to decrease around October.
Kiwifruit canker was first occurred in 1983 on a green-fleshed kiwifruit cultivar (Actinidia deliciosa), Hayward, in Japan (Serizawa et al., 1989; Takikawa et al., 1989). In Korea, the disease was first reported in 1988 on Hayward cultivar in Jeju Island (Koh et al., 1994). Many orchards in Japan and Korea were destroyed during that time, resulting in huge economic losses to kiwifruit growers. Around the same time, canker also occurred in Italy (Scortichini, 1994), but did not have as severe an impact as in Japan and Korea. For this reason, kiwifruit canker had not received worldwide attention until the massive outbreaks on the cultivars of yellow-fleshed kiwifruit (A. chinensis) in major kiwifruit-growing countries in 2010.
Global Psa populations were mainly divided into three Psa biovars (biovars 1, 2, and 3) designated Psa1, Psa2, and Psa3 on the basis of genetic diversity and toxin productivity (Chapman et al., 2012). Strains belonging to Psa1 and Psa2 produce phaseolotoxin and coronatine, respectively. In Japan and Korea, canker disease in Hayward was caused by Psa1 and Psa2 in 1980s, respectively (Koh et al., 1994; Serizawa et al., 1989; Takikawa et al., 1989). Strains belonging to Psa3 produce neither phaseolotoxin nor coronatine and are responsible for the global outbreak of bacterial canker of kiwifruit in recent years (Scortichini et al., 2012; Vanneste, 2012). Psa3 began to appear from 2008 in Italy and caused lethal damage to the cultivars of yellow- and red-fleshed kiwifruits (A. chinensis). It then became a pandemic in major producing countries of kiwifruit, such as Italy, France, New Zealand, and Chile, resulting in serious damage to the kiwifruit industries in those counties (Balestra et al., 2009; Everett et al., 2011; Ferrante and Scortichini, 2009; Scortichini et al., 2012). Analysis of the genome of Psa3 strains that caused pandemics in kiwifruit plantations worldwide revealed that all the Psa3 strains belong to the same lineage originating from China (Butler et al., 2013; McCann et al., 2013).
Hort16A was the first yellow-fleshed kiwifruit cultivar introduced to Korea, first cultivated on Jeju Island in 2004. Since then, many domestically-bred cultivars of yellow-fleshed kiwifruit such as Haegeum, Zecy-gold, and Halla-gold, and other imported yellow- and red-fleshed cultivars have been introduced in the country, and their cultivation area is recently skyrocketing due to a strong market demand on the kiwifruits. However, yellow- and red-fleshed kiwifruits are more susceptible to canker than green-fleshed kiwifruits. In fact, the first canker occurrence on the yellow-fleshed kiwifruit cultivar Hort16A by Psa2 was observed in 2006 at a kiwifruit orchard in Pyoseon-myeon, Seogwipo-si, Jeju Island (Koh et al., 2010). Psa3 was first detected in 2011 on the yellow-fleshed kiwifruit cultivar Yellow-king and red-fleshed kiwifruit cultivar Hongyang at an orchard in Dodeok-myeon, Goheung-gun, Jeonnam Province (Koh et al., 2012). Since then, frequent Psa3 incidences have occurred on many yellow- and red-fleshed kiwifruits in major kiwifruit growing areas in Korea.
Because Psa3 causes enormous damage to yellow-fleshed kiwifruits worldwide, there is an urgent need to cut off in advance the possible outbreak of Psa3 in Korea. This is also timely for the Korean kiwifruit industries, considering the cultivation area of yellow-fleshed kiwifruits in Korea is rapidly increasing. An epidemiological study was conducted in order to identify the origin and spread of the Korean Psa3 epidemics by identifying the biovar group of Psa isolated from plant samples collected from infected kiwifruit orchards over three years (2013-2015). We tracked the spatial and temporal spread of Psa3 in Korea, by comprehensively investigating records of Psa3 infection. Finally, in order to identify the likelihood of pollen serving as a vector of Psa3 transmission, we determined whether pollen products used for artificial pollination are infected with Psa3 and whether infected pollen can cause canker to a healthy kiwifruit vine.
From 2013 to 2015, plant samples were collected from kiwifruit orchards that are known to be infected with canker. Samples were collected either directly from the orchards or via shipping from growers. A total of 162 samples were collected from 41 orchards in 2013, and 147 samples from 111 orchards in 2014, and 439 samples from 127 orchards in 2015. Samples were subsequently examined for the identification of the biovar group of Psa strains. Briefly, plant tissue was ground in a sterile mortar, and then spread on a peptone sucrose agar medium to isolate single colonies of bacterium. Each bacterium was propagated in a liquid medium by incubating for 18 h at 25°C and 200 rpm. Multiplex PCRs developed by Koh et al. (2014) and Lee et al. (2016) were then used to determine whether the isolated bacterium is Psa2 or Psa3. Psa3 strains were confirmed to not possess gene fragments of the cfl gene coding for coronatine (Han et al., 2003) nor fragments of the tox-argK gene cluster coding for phaseolotoxin (Sawada et al., 2002).
To investigate the origin and spread of Psa3 in Korea, we carried out an epidemiological study with the Animal and Plant Quarantine Agency. The first investigation was made at the orchard growing Yellow-king and Hongyang, where Psa3 was detected for the first time in Korea in 2011 (Dodeok-myeon, Goheung-gun, Jeonnam Province). The investigation was based on historical records of when and where the orchard was built, when seedlings were purchased, and records of canker symptom development based on inquiries with the grower.
To investigate the main cause of Psa3 epidemics in 2014, we first determined whether the collected samples from fruit-bearing branches, leaves, and pollens used for artificial pollination are infected with Psa3 using the multiplex PCR method developed by Koh et al. (2014). Secondly, we confirmed whether or not the pollens infected with Psa3 can cause canker to a healthy kiwifruit vine. To accomplish this, we inoculated a commercial solution for water pollination (Boseong Kiwifruit Agricultural Association Corporation) with Psa bacterial cells at the concentration of approximately 5 × 106 cfu ml−1, using the method of Stefani and Giovanardi (2012). The Psa3-pollen mixture was sprayed onto healthy fruit-bearing branches of Hort16A in the flowering stage, mimicking the conditions for artificial pollination in the fields. After three weeks, pollinated vines were inspected for the development of canker symptoms. In cases where typical canker symptoms were shown, the causal bacterium was isolated and diagnosed again with the multiplex PCR method to confirm the Koch’s postulates.
Plant samples were collected for three years (2013-2015) from kiwifruit orchards infected with canker and were examined using the multiplex PCR methods by Koh et al. (2014) and Lee et al. (2016) (Fig. 1). A total of 162 samples from 41 kiwifruit orchards collected in 2013, were tested. Among them, only four samples collected from an orchard in Dodeok-myeon, Goheung-gun, Jeonnam Province were Psa3 positive, indicating that the Psa3 detected in 2011 was still present in this orchard. Among the 147 samples from 111 orchards in 2014, 57 samples from 33 orchards were Psa3 positive. Among 439 samples collected from 127 orchards in 2015, Psa3 was detected in 121 samples from 72 orchards (Fig. 1).
Interestingly, most Psa3 samples in 2014 were from orchards in Jeju Island, although there were some Psa3 samples from three orchards in Sacheon-si, Gyeongnam Province. In 2015, however, Psa3 rapidly spread to other regions of Jeonnam and Gyeongnam Provinces, as well as across Jeju Island. Psa2 was detected in 31 orchards in 2013, 42 orchards in 2014, and 59 orchards in 2015. Notably, although Psa2 and Psa3 were present in this time frame, Psa3 became the dominant biovar in 2015. Kiwifruit canker occurred not only on the kiwifruits that are commercially grown, but also on kiwifruit species selected from the wild and grown in orchards (Actinidia arguta, A. kolomikta, etc.) (McCann et al., 2013; Scortichini et al., 2012). Most yellow-, red-, and green-fleshed kiwifruits that have been cultivated in Korea were susceptible to canker by Psa3. More specifically, canker was reported not only on the first reported cultivars, Yellow-king and Hongyang, but also on other yellow- and red-fleshed kiwifruits, such as Hort16A, Zecy-gold, Enza-gold, Enzared, and Haegeum, and even on green-fleshed kiwifruits like Hayward and Daeheung. In addition, a kiwiberry cultivar Skinny-green was reported as a canker host in Korea.
Nevertheless, disease intensity differed between kiwifruit cultivars. For example, in yellow- and red-fleshed kiwifruits, disease symptoms were generally seen on branch and trunk as well as on leaf. Literally all plant parts were affected by Psa3. In green-fleshed kiwifruit, however, leaf symptoms were more common than symptoms on branch or trunk. In fact, it is well known that Hayward is more resistant to Psa3 infection than Hort16A (Vanneste, 2012). Canker has not yet been observed on the yellow-fleshed kiwifruit cultivar Halla-gold, indicating it will be worthwhile to investigate whether Halla-gold is resistant to Psa. It was also noted that Psa2 infection was relatively more prevalent on the cultivars like Hayward, Hort16A, and Hongyang than others, which is one of the characteristics distinguishing Psa2 from Psa3.
In this study, we were not able to examine all kiwifruit orchards in Korea for the occurrence of Psa3. Nevertheless, our results from a limited sampling indicated that Psa3 seemed to spread very rapidly within very short time after its first occurrence in 2011. In particular, the canker in Jeju Island in 2014 showed a clear difference from other canker epidemics in the past. In general, kiwifruit canker starts with typical milky bacterial ooze flowing out from openings on trunk, leader, and twig from February or early March, and further develops typical symptoms of discoloration and diebacks of the infected plant parts (Fig. 2A). While the orchards in Sacheon-si, Gyeongnam Province in 2014 showed the typical symptoms of the bacterial ooze on trunk and leader in early spring (Fig. 2B), there was no canker symptoms observed until flowering stage in the cases of Jeju Island. Instead, from the end of May, after artificial pollination, typical canker symptoms such as bacterial ooze on fruit-bearing branches, dieback of infected branches, and brown leaf spots surrounded with yellowish halo were present (Fig. 2C). In some orchards, symptoms of dieback and bacterial ooze on the fruit-bearing branches appeared even until mid-July (Fig. 2D). The symptoms gradually progressed towards the trunk, producing severe dieback of the fruit-bearing branch, and finally stopped after August. This was quite abnormal symptom development in Korea, where hot summer temperatures suppress the growth of bacterial pathogens and temporarily halt symptom development after June.
In 2015, not only the Psa3 detection was more than doubled nationally, but it also quickly spread from Jeju and Sacheon-si to other regions like Gimhae-si, Goseong-gun, Namhae-gun of Gyeonnam Province and Gwangyang-si, Suncheon-si, Boseong-gun, and Wando-gun of Jeonnam Province (Table 1). Since the first Psa3-detected orchard in Dodeok-myeon, Goheung-gun, Jeonnam Province was completely shut down by the Korean Animal and Plant Quarantine Agency on September 11, 2014, Psa3 has not been detected at any orchards in Goheung-gun since then. This indicates that the Psa3 epidemic in 2015 might not be related to the first Psa3 incidence in Dodeok-myeon. In most Psa3 epidemics in 2015, typical canker symptoms of bacterial ooze running down on trunk and leader were shown in late winter or early spring, indicating that first infection with Psa3 was taken place before 2015. In fact, according to some growers of the Psa3-diseased orchards, some diebacks of fruit-bearing branches occurred in the early summer after pollination in 2014, but they were ignored because of the relatively weak severity at that time. This was quite similar to reports of canker incidences in Jeju Island in 2014.
Based on these findings, artificial pollination with Psa3-contaminated pollens was suspected as the main source of the Psa3 outbreak in 2014 and 2015. Especially, we infer that the Psa3 epidemic in 2015 was not transmitted from other Psa3-infected orchards in 2013 or 2014, but largely due to the use of pollen contaminated with Psa3 in 2014. Slight symptoms may have developed at the time of infection as in Jeju Island, or the Psa3 may have entered into a latency period right after the infection due to unfavorable environmental conditions. Either case could lead to full symptom development in the following year with a favored weather condition, as shown in our observations in 2015.
The orchard in Dodeok-myeon, Goheung-gun, Jeonnam Province, where Psa3 was first discovered in 2011, was growing Yellow-king and Hongyang cultivars. The orchard was built in 2008 in a location not previously used for kiwifruit production. The grower stated that when he planted Yellowking and Hongyang seedlings in 2008, several seedlings died for unknown reasons. After replanting with the same cultivars several times, the grower stated that seedlings kept dying until 2011. Based on the fact that the orchard was the only one infected with Psa3 around the region and very few orchards grew the Yellow-king and Hongyang cultivars at that time, we suspected the imported seedlings were contaminated with Psa3.
To investigate whether the seedlings were a source of Psa3, we conducted an epidemiological study with the Animal and Plant Quarantine Agency for the Yellow-king and Hongyang seedlings. The Yellow-king and Hongyang seedlings were imported from China in 2006 through a company selling seedlings online. Since kiwifruit canker had already existed in Korea from 1998 and there was no concept of Psa biovar defined at that time, Psa3 was not a quarantine pathogen in 2006. Therefore, we assumed that it was impossible to block the introduction of Psa3 into Korea via a quarantine at that time. Another clue to support our assumption was the fact that all Psa3 strains detected worldwide were reported to originate from China (Butler et al., 2013). Our genome analysis of the Psa3 strain isolated from Yellow-king also revealed that the Korean Psa3 strain is in such a very close relationship with the ones collected in China, New Zealand, Italy, and Chile (Butler et al., 2015).
Next, we investigated the cause of Psa3 epidemics on yellow- and red-fleshed kiwifruit cultivars in Jeju Island throughout 2014. The likelihood of contamination of the imported pollen with Psa3 was investigated. Psa3 was first detected from fruit-bearing branches and leaves of Hort16A. In some pollens imported from New Zealand and China, the same Psa3 was detected, while no Psa3 was detected on the pollens used in the orchards in Sacheon-si, Gyeongnam Province. Based on these results, it can be estimated that the imported pollens contaminated with Psa3 were the inoculum source of the Hort16A canker epidemics in Jeju Island. To confirm our hypothesis, pollen was artificially contaminated with Psa3 and then sprayed on the flowers, fruit-bearing branches, and leaves of Hort16A using a water pollination method. Three weeks after inoculation, canker lesions began to appear on the leaves, and some fruit-bearing branches showed typical dieback symptoms of canker as well. Finally, Psa3 was successfully re-isolated from the infected fruit-bearing branches and leaves. The completion of the Koch’s postulates strongly supported our hypothesis that the contaminated pollen to Psa3 caused the 2014 canker epidemics in Jeju Island. Many previous studies in other countries also support our conclusion about the possibility of Psa3-contaminated pollens being an inoculum source for canker (Gallelli et al., 2011; Stefani and Giovanardi, 2012; Vanneste et al., 2011).
The results of this study indicate that the main cause of Psa3 epidemics in Korea might be through the infected kiwifruit seedlings and contaminated pollens from outside. Therefore, in order to prevent additional introduction of Psa3 into Korea, Psa3 must be specified as the quarantine pathogen, through which the import of infected kiwifruit seedlings or contaminated pollens must be prohibited. In addition, there is now the possibility that pollen collected domestically might be infected with Psa3. Therefore, developing technologies for healthy pollen production is essential to block the spread of canker. In addition, there is also a need to develop a method that can sterilize only Psa bacteria without affecting the pollen vitality (Everett et al., 2012).
Overall, Psa2 has been present in Korea since 1988, but Psa3 might be newly introduced into the country through the imported seedlings from China since 2006 and from New Zealand and China through the pollen in around 2014. Recently, Butler et al. (2015) analyzed the characteristics of the Psa3 genomes isolated from Yellowking and showed that Psa3 causing the pandemic in the world is in a genetically-close relation, but also showed some distinctive features within the biovar group. This enables the estimation that there can be various genetic variations in the Psa3 strains currently distributed in Korea. In addition, it should not be ruled out the possibility of the emergence of new biovar through an evolutionary recombination between the Psa2 existing only in Korea and the Psa3 strains introduced from outside. Although we estimate that the origin of 2014 Psa3 epidemics at three orchards in Sacheon-si, Gyeongnam Province is likely to be imported seedlings for now, it is not yet clear due to very limited evidence. Therefore, it is necessary to monitor the dynamics of the canker bacterial population in Korea, which will be useful to operate a systematic control strategy effectively, such as the development of canker resistant cultivars of kiwifruit and a national early warning and surveillance system for canker epidemics.
Acknowledgments
This study was carried out with the support of the Cooperative Research Program for Agricultural Science and Technology Development (PJ01019702), Rural Development Administration, Republic of Korea, and this work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (315019-02-1-SB020).
Fig. 1
Annual trend of kiwifruit bacterial canker epidemics based on location and biovar group (Psa2 or Psa3) of Psa from 2013 to 2015. Psa2, Pseudomonas syringae pv. actinidiae biovar 2; Psa3, P. syringae pv. actinidiae biovar 3.
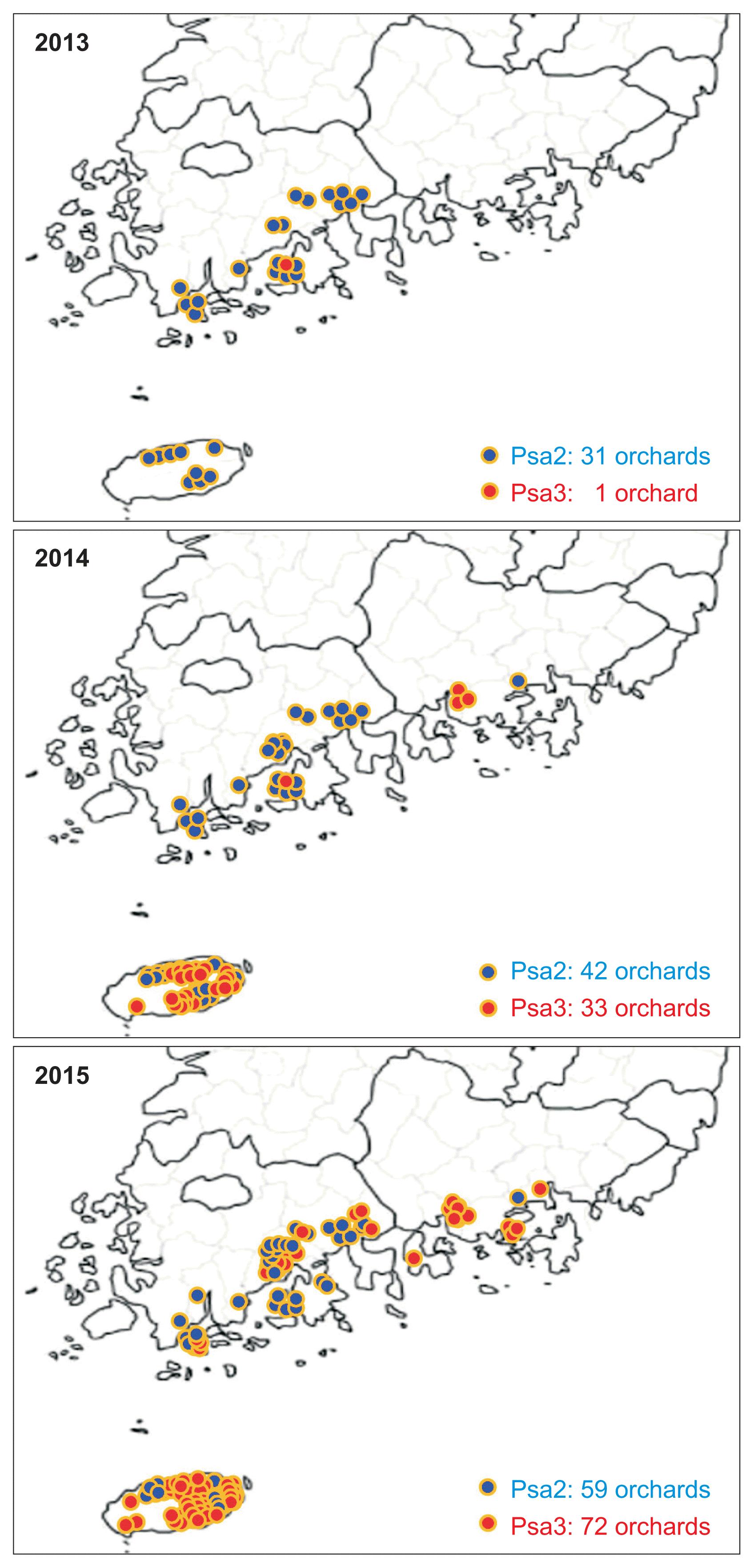
Fig. 2
Kiwifruit bacterial canker symptoms. (A) Dark-red bacterial ooze running down on the trunk of Yellow-king cultivar (May 2011, Goheung-gun, Jeonnam Province in Korea). (B) Bacterial ooze on the trunk of Zecy-gold cultivar (May 2014, Sacheon-si, Gyungnam Province in Korea). (C) Typical leaf symptom of canker of Hort16A cultivar (July 2014, Jeju-si, Jeju Island in Korea). (D) Bacterial ooze on the fruit-bearing branch of Enza-Red cultivar (July 2014, Jeju-si, Jeju Island in Korea). (E) Dieback symptom of the fruit-bearing branch of Hort16A cultivar (July 2014, Jeju-si, Jeju Island in Korea).

Table 1
Bacterial canker occurrence on different kiwifruit cultivars by Psa2 or Psa3 in 2015
References
Balestra, GM, Mazzaglia, A, Quattrucci, A, Renzi, M and Rossetti, A 2009. Current status of bacterial canker spread on kiwifruitfruit in Italy. Australas Plant Dis Notes. 4:34-36.
Butler, M, Jung, JS, Kim, GH, Lamont, I, Stockwell, P, Koh, YJ and Poulter, R 2015. Genome features of Pseudomonas syringae pv. actinidiae recently isolated in Korea. Acta Hortic. 1095:75-80.

Butler, MI, Stockwell, PA, Black, MA, Day, RC, Lamont, IL and Poulter, RT 2013. Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PLoS One. 8:e57464



Chapman, JR, Taylor, RK, Weir, BS, Romberg, MK, Vanneste, JL, Luck, J and Alexander, BJ 2012. Phylogenetic relationships among global populations of Pseudomonas syringae pv. actinidiae. Phytopathology. 102:1034-1044.


Everett, KR, Cohen, D, Pushparajah, IPS, Vergara, MJ, Curtis, CL, Larsen, NJ and Jia, Y 2012. Heat treatments to kill Pseudomonas syringae pv. actinidiae on contaminated pollen. N Z Plant Prot. 65:8-18.
Everett, KR, Taylor, RK, Romberg, MK, Rees-George, J, Fullerton, RA, Vanneste, JL and Manning, MA 2011. First report of Pseudomonas syringae pv. actinidiae causing kiwifruit bacterial canker in New Zealand. Australas Plant Dis Notes. 6:67-71.


Ferrante, P and Scortichini, M 2009. Identification of Pseudomonas syringae pv. actinidiae as causal agent of bacterial canker of yellow kiwifruit (Actinidia chinensis Planchon) in central Italy. J Phytopathol. 157:768-770.

Gallelli, A, Talocci, S, L’Aurora, A and Loreti, S 2011. Detection of Pseudomonas syringae pv. actinidiae, causal agent of bacterial canker of kiwifruit, from symptomless fruits, and twigs, and from pollen. Phytopathol Mediterr. 50:462-472.
Han, HS, Koh, YJ, Hur, JW and Jung, JS 2003. Identification and characterization of coronatine-producing Pseudomonas syringae pv. actinidiae. J Microbiol Biotechnol. 13:110-118.
Koh, HS, Kim, GH, Lee, YS, Koh, YJ and Jung, JS 2014. Molecular characteristics of Pseudomonas syringae pv. actinidiae strains isolated in Korea and a multiplex PCR assay for haplotype differentiation. Plant Pathol J. 30:96-101.



Koh, YJ, Cha, BJ, Chung, HJ and Lee, DH 1994 Outbreak and spread of bacterial canker in kiwifruit. Korean J Plant Pathol. 10:68-72 (in Korean).
Koh, YJ, Kim, GH, Jung, JS, Lee, YS and Hur, JS 2010. Outbreak of bacterial canker on Hort16A (Actinidia chinensis Planchon) caused by Pseudomonas syringae pv. actinidiae in Korea. N Z J Crop Hortic Sci. 38:275-282.

Koh, YJ, Kim, GH, Koh, HS, Lee, YS, Kim, SC and Jung, JS 2012. Occurrence of a new type of Pseudomonas syringae pv. actinidiae strain of bacterial canker on kiwifruit in Korea. Plant Pathol J. 28:423-427.

Lee, YS, Kim, GH, Koh, YJ, Zhuang, Q and Jung, JS 2016. Development of specific markers for identification of biovars 1 and 2 strains of Pseudomonas syringae pv. actinidiae. Plant Pathol J. 32:162-167.



McCann, HC, Rikkerink, EH, Bertels, F, Fiers, M, Lu, A, ReesGeorge, J, Andersen, MT, Gleave, AP, Haubold, B, Wohlers, MW, Guttman, DS, Wang, PW, Straub, C, Vanneste, JL, Rainey, PB and Templeton, MD 2013. Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathog. 9:e1003503



Sawada, H, Kanaya, S, Tsuda, M, Suzuki, F, Azegami, K and Saitou, N 2002. A phylogenomic study of the OCTase gene in Pseudomonas syringae pathovars: the horizontal transfer of the argK-tox cluster and the evolutionary history of OC-Tase gene on their genomes. J Mol Evol. 54:437-457.

Scortichini, M 1994. Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathol. 43:1035-1038.

Scortichini, M, Marcelletti, S, Ferrante, P, Petriccione, M and Firrao, G 2012. Pseudomonas syringae pv. actinidiae: a reemerging, multi-faceted, pandemic pathogen. Mol Plant Pathol. 13:631-640.



Serizawa, S, Takikawa, Y, Ichikawa, T, Tsuyumu, S and Goto, M 1989. Occurrence of bacterial canker of kiwifruit in Japan: description of symptoms, isolation of the pathogen and screening of bactericides. Ann Phytopathol Soc Jpn. 55:427-436.

Stefani, E and Giovanardi, D 2012. Dissemination of Pseudomonas syringae pv. actinidiae through pollen and its epiphytic life on leaves and fruits. Phytopathol Mediterr. 50:489-496.
Takikawa, Y, Serizawa, S, Ichikawa, T, Tsuyumu, S and Goto, M 1989. Pseudomonas syringae pv. actinidiae pv. nov.: the causal bacterium of kiwifruitfruit canker in Japan. Ann Phytopathol Soc Jpn. 55:437-444.
Vanneste, JL 2012. Pseudomonas syringae pv. actinidiae (Psa): a threat to the New Zealand and global kiwifruit industry. N Z J Crop Hortic Sci. 40:265-267.

Vanneste, JL, Giovanardi, D, Yu, J, Cornish, DA, Kay, C, Spinelli, F and Stefani, E 2011. Detection of Pseudomonas syringae pv. actinidiae in kiwifruit pollen samples. N Z Plant Prot. 64:246-251.
- TOOLS
-
METRICS

- Related articles
-
Halo Blight of Kudzu Vine Caused by Pseudomonas syringae pv. phaseolicola inKorea2006 June;22(2)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



