 |
 |
| Plant Pathol J > Volume 33(1); 2017 > Article |
Abstract
Incidence of leaf blast on nursery plants and pitting disease on maturing banana bunches has been recorded in banana plantations during rainy season in Eastern India during 2014 to 2015. Taxonomical identification as well as DNA sequence analysis of the internal transcribed spacer region of fungus isolated from affected tissue culture derived plantlets and fruits confirmed the pathogen to be Pyricularia angulata Hashioka âin both the casesâ. Kochâs postulates were proved on young plantlets as well as on maturing fruits of cv. Grand Naine under simulated conditions. Evolutionary history was inferred and presented for our P. angulata strain PG9001 with GenBank accession no. KU984740. The analysis indicated that the P. angulata is phylogenitically distinct from other related species related to both Pyricularia and Magnaporthe. Detailed symptoms of blast lesions on young leaves, transition leaves, mid rib, petioles, peduncle, maturing bunches, bunch stalks and cushions were documented. Notably, the distinct small pitting spots on maturing bunches reduced the visual appeal of mature fruits. Appearance of pitting symptoms on fruits in relation with age of fruits and their distribution pattern on bunch and fingers was also documented in detail. Further, the roles of transitory leaves, weed hosts, seasonality on disease occurrence have also been documented.
Different varieties of banana are being cultivated in diverse climates ranging from humid tropics to dry mild subtropical regions of India (Chadha, 2001). India occupies the first position in banana production globally and during 2014 to 2015, banana was cultivated in an area of 0.88 million ha with the production of 30 million tonnes in India (Horticultural Statistics at a Glance 2015; http://agricoop.nic.in/sites/default/files/hortstat_glance.pdf).
Review of literature revealed that occurrence of similar pitting disease was known since 1931 in Australia, Africa, Asia, Central and South America (Meredith, 1963; Snowdon, 1990; Stover, 1972). The disease, referred as black pitting disease caused by Pyricularia grisea was reported to occur in severe form on Cavendish banana group in Central American countries during 1960s. The symptoms on fruits were referred as pitting disease as the pathogen produced pits on fruit surface (Kim et al., 1987; Meredith, 1963). Since it was described by Johnston (1932) for the first time, this disease was also referred as âJohnston fruit spotâ. The pathogen was initially described as P. grisea; however, later workers discriminated the species status of Pyricularia associated with banana from grisea to angulata (Hashioka, 1971; Kim et al., 1987). Occurrence of banana blast caused by P. angulata on leaves of tissue culture derived banana plantlets cv. Dwarf Cavendish was reported recently in Australia (Male et al., 2011). However, the authors opined that disease symptoms did not develop further in the field due to less favorable conditions. Association of Pyricularia sp. with banana was reported as early as in 1931 on banana shipped from Brazil to England (Tomkins, 1931). Subsequently it was reported by Wardlaw and McGuire (1932), Wardlaw (1934), Hoette (1936), Meredith (1963), Hashioka (1971), Kim et al. (1987), and Male et al. (2011) from various parts of the world. In India, association of Pyricularia sp. with tissue culture derived banana plantlets was reported by Sonah et al. (2009) from North India though the pathogen was identified as P. grisea. There had been no report on occurrence of fruit pitting symptoms and other associated symptoms on different parts of banana plants under field conditions in India. Presently, the disease occurrence is sporadic but it may cause significant economic losses under favorable weather conditions during epidemic years, as true with blast diseases caused by Pyricularia spp. in other field crops. Host specific pathotypes of Pyricularia spp. cause huge losses on many gramineous plant species, viz., rice (Ou, 1985), finger millet (Mgonja et al., 2007), wheat (Takan et al., 2004, 2012), barley (Yaegashi and Nishihara, 1976) and maize (Notteghem, 1990).
Keeping all these facts in view, the present study was undertaken with the objectives of establishing the identity, aetiology and source of inoculum of the associated pathogen. Disease occurrence in relation to prevailing weather conditions in Coastal Odisha has also been documented and presented.
The associated pathogen could not be isolated easily from infected fruits and leaf tissues, partly due to the presence of other associated saprophytic and pathogenic microorganisms and partly due to the non-competitive and slow growth habit of pathogen. Hence, diseased tissues were pre-incubated for 2-3 days, before pathogen isolation, to induce sporulation and to ensure successful isolation of monoconidial cultures and pathogen was isolated by the methodology suggested by Sangeetha et al. (2017). Monoconidial cultures for each isolate were maintained on potato dextrose agar (PDA) slants at 4°C for further use. The culture was sent to National Centre for Fungal Taxonomy, New Delhi for identification and mycological description.
Agar blocks (8-mm diameter) were cut from actively growing margins of fungal colony and inoculated on Petri plates on PDA media. Colony characters like colour, growth rate and growth patterns of the fungi were recorded on actively growing cultures incubated at ambient temperature (25 ¹ 2°C). The spore size and number of septations were recorded using image analyzer and microscopy of 50 spores for each isolate and micro-photographed. Appressoria formation by the spores were examined by incubating suspensions of conidia on leaves of 12-week-old tissue cultured plants, in a humid chamber for 14 h at 24°C.
Pathogenicity tests with representative isolates were conducted on symptomless 12-week-old tissue culture plantlets in shade net as well as in field on matured bunches of cv. Grand Naine. The pathogen was multiplied on the sterilized pseudostem bits of banana. Sterilized pseudostem bits (1-2 cm) were inoculated with the pathogen aseptically in flasks. These flasks were incubated for 15 days alternatively in dark for two days and in light for two days. The stem bits were completely covered with whitish fungal growth. To obtain conidial suspension, sterile distilled water was added in these flasks and kept on shaker at 200 rpm for 10 min to dislodge the conidia. The spore suspension was filtered and diluted to get 4 Ă 104 conidia/ml aseptically and added with 2-3 drops of Tween 20 as wetting agent. Healthy 12- to 14-week-old tissue culture plantlets of cv. Grand Naine were selected for the study. Spore suspension was sprayed on both abaxial and adaxial leaf surfaces of selected plantlets. To replicate growing conditions often found in nursery houses and to minimise the risk of natural infection, the inoculated plantlets were covered with polythene sheets with necessary support. Control plantlets were sprayed with sterile water only. Periodic observations were made on symptom development. After 10 days, the pathogen from diseased leaves was isolated, sub cultured onto PDA plates and incubated at 25°C in darkness. The resultant cultures were checked for colony and spore morphology to confirm Kochâs postulates.
Similarly for fruit inoculation, 10 healthy matured bunches of cv. Grand Naine (70 days old) were washed and surface sterilised with 70% ethanol and subsequently washed with sterile distilled water. Spore suspension of the pathogen, prepared as described above, was sprayed on bunches, while a separate set of healthy bunches sprayed with sterile distilled water served as control. The treated bunches were covered with perforated polythene bags to provide favourable condition for spore germination. Inoculated bunches were examined for lesion/spot development 5 days onwards after inoculation at periodic intervals. After 10 days the pathogen from diseased fruits was isolated, sub cultured onto PDA plates and incubated at 25°C in darkness. The resultant cultures were checked for colony and spore morphology to confirm Kochâs postulates. For cross infectivity test, Pyricularia species isolated from banana and paddy were inoculated on the blast susceptible rice (Oryza sativa) variety and on banana plants cv. Grand Naine, and vice versa, by artificial wound and non wound inoculation methods. All the above experiments were repeated twice.
The protocol used by Male et al. (2011) was adopted for molecular identification of the pathogen. The primers pairs developed by White et al. (1990), ITS1 (5â˛-TCCGTAGGTGAACCTGCGG-3â˛) and ITS4 (5â˛-TCCTCCGCTTATTGATATGC-3â˛) were used for specific amplification of ITS region of the fungi. Purified PCR products were sequenced and the resulting sequence was compared to the nucleotide sequence database (GenBank) using the nucleotide query BLASTn accessed via the National Centre for Biotechnology Information (NCBI) website. Sequences were assembled and multiple alignment was done with Clustal W 1.82 (http://www.genome.jp/tools/clustalw/). Fifty previously published sequences were obtained from GenBank for comparative analyses with our P. angulata strain PG9001 with GenBank accession no. KU984740. Evolutionary analysis were conducted in MEGA7 (Kumar et al., 2016). The neighbour-joining (NJ) method (Saitou and Nei, 1987) based on a Kimura two-parameter distance measurement (Kimura, 1980) was used to infer a phylogenetic tree. All molecular characters were unordered and given equal weight during analysis. Relative branch support was estimated with 1,000 bootstrap replications (Felsenstein, 1985) for NJ analyses. Two accessions of Pyricularia zingiberi were used to root for phylogenetic tree.
Since the pathogen was new to banana crop in India and detailed symptomotolgy in response to infection of P. angulata on banana currently not available. Hence, symptoms developing on commercial tissue cultured plants and banana fruits in the standing crops in detail with respect to plant parts infected viz., young tissue culture derived plantlets, matured leaves, midrib, petiole, peduncle, fruits, fruit stalk and cushion and so on were studied. Blast lesions appearing on leaves of tissue culture derived plantlets and maturing plants as well as pitting symptoms appearing on fruits were measured with standard scale and presented hereunder. Distribution pattern of pits on bunch as well as fingers were also studied periodically. Bunches were observed for the appearance of pitting symptoms on fruits in relation with age of fruits. For that bunches with the age of 30, 60, 90 days and harvesting stage were observed (age of bunch was calculated from the day of emergence of flower) for disease symptoms during congenial rainy season.
Role of transitory leaves and air borne inoculum in spreading the disease inoculum to developing bunches was studied under natural epiphytotic conditions in a set of four treatments consists of 5 replication with 2 trees per replication. Data was taken on banana cv. Grand Naine in the experimental farm of Directorate of Horticulture, Odisha, India. In treatment one, bunches were covered with perforated transparent poly covers along with transitory leaves and in treatment two, transitory leaves were removed at the stage of finger opening and covered with perforated transparent poly covers. In treatment three, bunches were not covered with bags but transitory leaves were removed after finger opening stage. In treatment four, transitory leaves were left to rest naturally on bunches with no bunch covering. Observations were taken once in week for pitting symptom on fruits and their distribution pattern on developing bunches to derive the role of airborne spores and transitory leaves in causing disease on developing banana bunches. Survey was also conducted in and around the infected banana field to find out the alternate weed hosts of the study pathogen. Further, seasonality of disease occurrence was also worked out by periodical field visits throughout the study period of 2014 and 2015.
Pyricularia sp. is known to cause âblastâ disease on many monocotyledonous plant species and the most economically important species is P. grisea (syn. P. oryzae), the causal agent of blast disease in rice (Hashioka, 1972). Several other species of Pyricularia are pathogenic to almost 50 plant species (Ou, 1985). Banana blast/pitting disease caused by P. angulata is known to be distributed in major banana growing regions of the world but was not known to occur in India earlier. Results of present studies on the pathogen identity and aetiological details of this emerging banana disease in India is elaborated hereunder.
Isolation of pathogen from symptomatic leaves and fruits initially yielded Fusarium spp. on most occasions but none proved pathogenecity. Modifications incorporated in the isolation method yielded Pyricularia sp. It was further established that blast pathogen is hard to recover from fruit tissue if the tissue was too wet. However, pathogen could be easily isolated from fresh blast lesions of pseudostem or peduncle. Lesions with free water standing on their surface yielded saprophytic contaminants. Infected leaf or fruit tissue incubated under high humidity induced sporulation of typical pyriform spores which aided in initial identification of the pathogen.
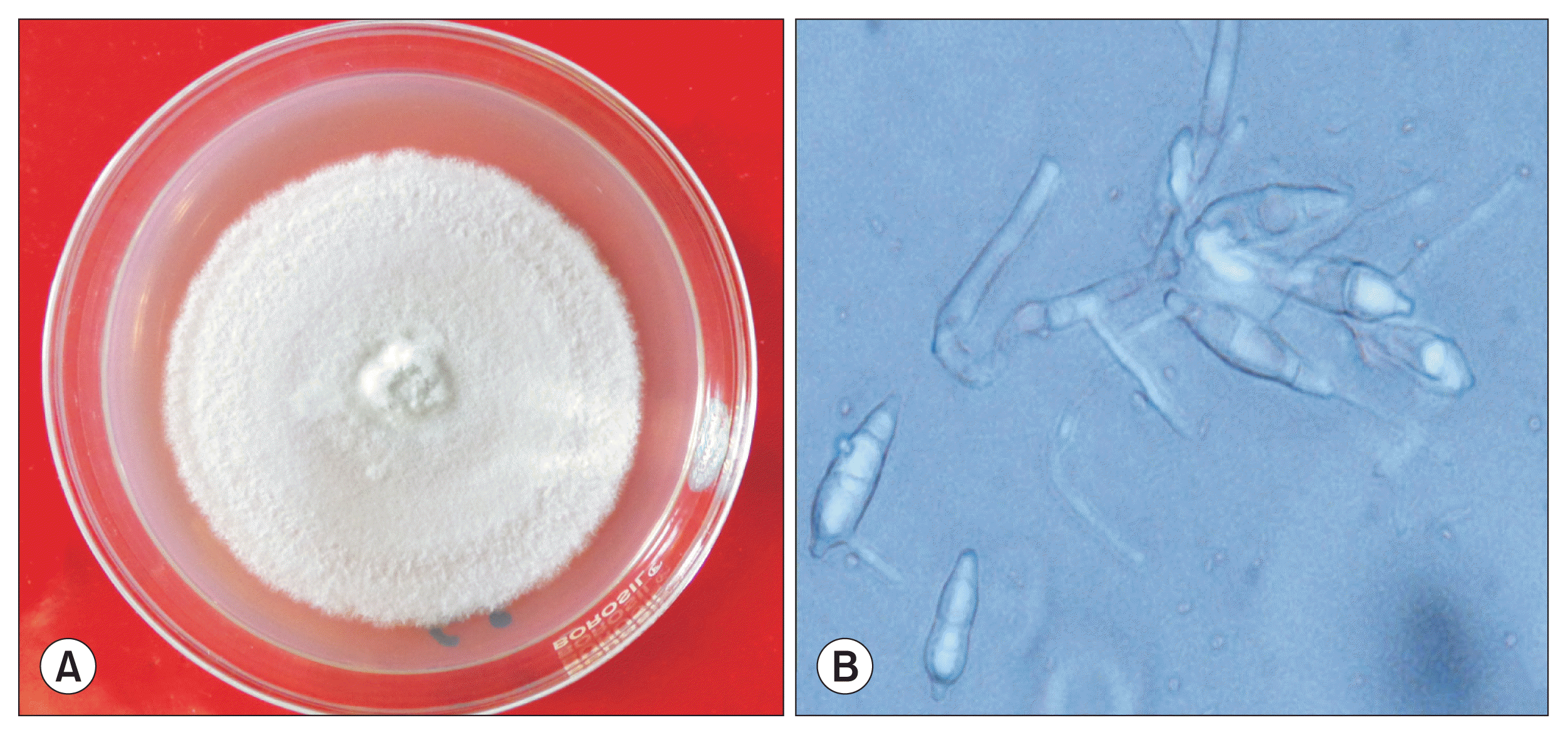
On PDA medium the pathogen produced pale delicate floccose loosely interwoven colonies which were initially creamish white in colour, becoming light brown after two weeks of incubation. Few colonies, however, were tightly floccose with dense spongy types of mycelium (Fig. 1A). Growth was slow as 8 mm disc attained 40-mm diameter colony after 7 days of inoculation. Aerial mycelium was less and sporulation was scanty. Conidia were hyaline to pale brownish in colour, two septate, ovate to obpyriform in shape, thin walled, with small protuberent hilum, measuring 20-22 Ă 6-9 Îźm in size, attached solitary at ends of denticles of conidiophores (Fig. 1B). There were minor difference in size of the conidia between the present study and Kim et al. (1987) (6.0-34.0 Ă 7.0-12.0 Îźm; average, 22.5 Ă 9.0 Îźm) and Hashioka (1971) (18.2-28.0 Ă 4.9-9.1 Îźm; average, 22.6 Ă 7.4 Îźm); however, it was in accordance with Male et al. (2011) (19-22 Ă 6.5-8.0 Îźm) who has reported banana blast incidence from Australia. Conidiophores were 2 to 3 septate (mostly two septate), branched occasionally, pale brown but thicker and darker than hyphae especially at the base and denticulate at tips. The species was identified and described as P. angulata by National Centre for Fungal Taxonomy (New Delhi, India). The appresoria were irregularly angular/stellate, which is also a differentiating character of P. angulata from P. grisea which mostly has globular appressoria. Hashioka (1971) used epithet âangulataâ to note this distinguishing feature of appresoria of P. angulata causing blast and pitting disease of banana.
The genomic DNA was extracted from the fungus, and the region between ITS1 and ITS4 was amplified with the universal primer pair ITS 1 and ITS 4. ITS sequences of the pathogen (GenBank accession no. KU984740) isolated from infected tissue culture plants and pitted fruits had maximum identity of 99% with P. angulata (GU066873, AY265322, JF719830) reported to occur on banana from other countries. To derive the evolutionary relationship of newly generated sequence of P. angulata in this study, the representative ITS sequences of Pyricularia spp. and Magnaporthe spp. were taken from Hirata et al. (2007) and Zhang et al. (2011) and used for phylogenetic analysis. The evolutionary history was inferred using the NJ method through the optimal tree with the sum of branch length of 0.26647478 (Fig. 2). The evolutionary distances were computed using the Kimura 2-parameter method in the units of the number of base substitutions per site. Supports for grouping in NJ trees were evaluated with 1,000 bootstrap replications, which produced a similar tree topology, giving high boot strap values for relevant clades. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is depicted in Fig. 2. There were a total of 359 positions in the final dataset. The analysis indicated that the P. angulata is phylogenitically distinct from other related species related to both Pyricularia and Magnaporthe, which were classified in different evolutionary groups. Male et al. (2011) from Australia confirmed the identity of P. angulata associated with banana blast by ITS-rDNA sequence analysis along with the spore characters and pathogenicity tests. Molecular evidences also clearly established the difference between P. angulata, P. grisea and 39 other Pyricularia species and allied genera (Bussaban et al., 2005).
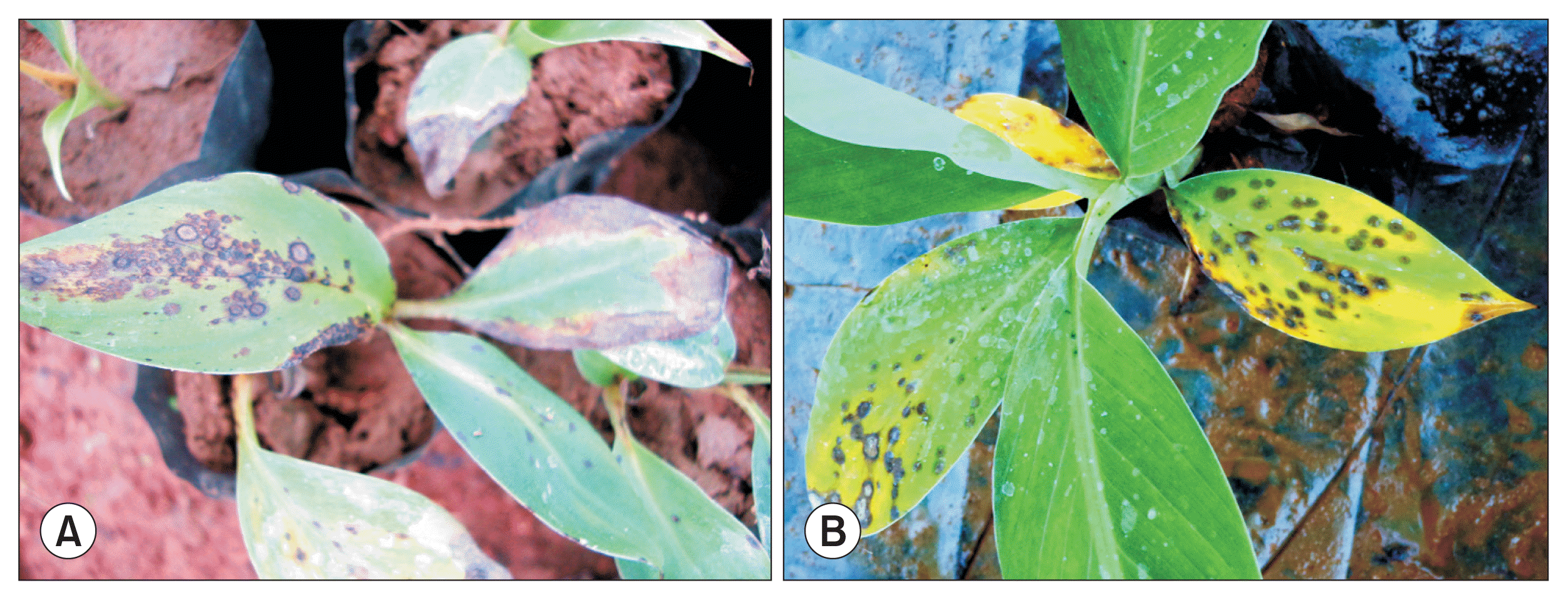
Artificial inoculation of young tissue culture derived plantlets developed typical spots within 5 days of inoculation. The blast spots initially started appearing as minute oval shaped brownish lesions with or without yellow halo (Fig. 3A). The spots coalesced with adjacent lesions and subsequently all leaves died leading to death of whole plantlet. Control plants maintained without inoculation did not show any symptoms and remained healthy throughout the study period. Similarly, small pits of 6-7 mm appeared on fruits within five days upon artificial inoculation during congenial weather conditions (Fig. 3B). P. angulata was consistently isolated from infected leaves, tender stems of plantlets and inoculated symptomatic fruits and Kochâs postulates were proved for isolates from all tissues.
Hence in the present study, based on spore characters, molecular characterization and pathogenicty tests, association of P. angulata with blast lesions of tissue culture derived plantlets and pitting symptom on fruits was confirmed and proved. The primary spread of this disease to new locations is suspected due to use of blast infected tissue culture derived plantlets which later lead to pitting symptom on fruits. However, secondary spread of the disease from infected field to nearby healthy banana field is also possible due to air borne spores of P. angulata. Wardlaw (1961) suggested that Pyricularia species causing pitting disease banana may be widely and abundantly disseminated by wind; however, these propositions needs to be experimentally established.
Up to the year 1970, it was thought that pitting disease was caused by P. grisea; however, Hashioka (1971) clearly discriminated the species identity of Pyricularia associated with Musaceae as angulata (an epithet derived from irregularly angular appresorium).
Further our study revealed that the P. angulata was pathogenic only on banana and not pathogenic to rice. Similarly P. grisea from rice also failed to infect banana plants and fruits. This results are in agreement with the work of Kim et al. (1987) who concluded that P. angulata was pathogenic only to banana and exhibited no pathogenicity on different hosts tested viz., Digitaria sanguinalis (L.) Scopo, O. sativa L., Setaria italica (L.) Beauv, and Setaria viridis (L.).
Leaf symptoms were observed on young tissue culture derived plantlets on expanded leaves and on young tender stems of commercial important Grand Naine and Banthal cultivars at nursery sale outlets. In severe case death of young plants was also observed.
Initially, blast lesions were produced distinctly in two different forms on young plantlets. Hundreds of minute tan brownish spindle shaped spots of less than one mm in diameter surrounded by yellow halo were produced all over the leaf lamina which later developed in to an elliptical or eye shaped dusty brown coloured spots of 3-4 mm diameter (Fig. 4A). Secondly, in a different way, a couple of lesions developed on the leaf lamina which enlarged to maximum of 6-8 mm in diameter with dark and light brown zonation having darker margins with or without yellow halo (Fig. 4B). Slowly adjacent spots coalesced and produced big necrotic tissue covering whole leaf leading to the blighting of leaves and finally death of whole plant in severe cases. Isolated blast lesions were also observed on midrib, petiole and pseudostem and adjoining region of petiole with pseudostem. The sequential availability of banana plantlets in nursery with favourable damp conditions throughout the year encourages spread of this fungal disease as they provide continuous susceptible host environment in nursery. Hence once established in a nursery, the disease is likely to proliferate throughout the year in the nursery. Occurrence of banana blast symptoms caused by P. angulata on leaves of tissue cultured banana plantlets cv. Grand Naine under shade net condition was also reported in Australia (Male et al., 2011). However the authors opined that disease symptoms did not develop further when affected plantlets were planted in field presumably due to prevailing unfavourable weather conditions and also presumed by authors that regular fungicide application schedule used for the control of sigatoka leaf spot might have restricted the development of pitting disease on fruits.
Initially, blast spots were observed on emerging leaves including the leaves of side suckers (Fig. 5A). On matured leaves small oval or eye shaped light brownish spots with or without yellow margin appeared (Fig. 5B). On mid rib, petiole also severe blast spots were observed during favourable weather conditions. However, blast on matured leaves observed only in some places, it was not a common phenomenon. Initially spots appeared as brown points/dots, which later enlarged up to 4 mm if condition favoured. All spots coalesced and caused extensive blighting of leaves. The potential for individual lesions to sporulate depended on the relative area of the light tan region inside the darker brown margins. Sporulating lesions appeared whitish gray as per the color of the spores. The lesions comprised of light tan centres and dark brown margins. In case of rice, susceptibility of leaves declined rapidly to blast infection with increasing leaf age (Rouman, 1992) but initial level of susceptibility of new leaves also differed greatly among the cultivars (Puri et al., 2009).
The transition leaf or flag leaf expressed the first diagnostic symptom of blast lesions in field (Fig. 5C). Typical pits, very similar to fruit pits, appeared on inner leaf sheath with maximum of 2-3 mm depth having 4-7 mm diameter (Fig. 5D) and on outer sheath purplish abundant spindle shaped blast lesions were produced. These lesions were observed at the time of its emergence itself and it was observed that, if booting stage coincided with rain for a week or two, the flag leaf/transition leaf covering the peduncle carried the infection which might be from air borne pathogenic inoculum present in the field. Shortly after booting, the transition leaf decayed and withered away within a month. The infected decaying transition leaf and bracts resting on bunches were found to be the abundant source of inoculum of the pathogen. Diamond shaped spots were also scattered over other parts of banana plant such as peduncle (Fig. 5E), fruit stalk, bunch stalk (Fig. 5F), and so on. Blast spots were also observed on terminal bud of bunch and on the hanging flowers/fingers.
Pitting symptoms were distributed on the bunch as mild, moderate or severe form depending upon age of fruit, presence of pathogenic inoculum and prevailing weather condition (Fig. 6A, B). On the surface of maturing fruits, pinprick like infection appeared initially surrounded by a reddish brown ring/circle, but inside the ring, the peel colour was green when new spots were formed (Fig. 6A, B); with an average diameter of 4-7 mm. However the pits sometimes measured up to 12 mm diameter too. Sometimes, the spots had water soaked areas instead of ring. At times, lesions also produced as direct pits without any kind of rings or water soaked halos around the pitting symptoms. Colour of peel inside the ring turned light brown within a day after the appearance of symptom and within couple of days, the centre of the spot developed into a shallow pit characterized by reddish brown/black, nearly oval, depressed spots. Symptoms on fruits are known as pitting disease as suggested by previous workers (Kim et al., 1987; Meredith, 1963; Wardlaw and McGuire, 1932) since the pathogen produces pits on fruit surface. However, pitting symptom on fruits did not extend to pulp and confined to rind only (Fig. 6C).
Even during storage, the pits did not develop further and caused no damage to pulp. In general, the size of pits remained same even when fruits matured. The different developmental stage of pitting symptoms on fruit surface of banana is clearly described in Fig. 7A. Presence of water soaked halo was observed around the pits in most of the time, but not always. Later, the centre of the pit cracked or split open diagonally (Fig. 7B) or star shaped. This symptom has been initially confused with diamond spot of banana caused by Fusarium spp. where cracking occurred in the centre of the diamond shaped spots. The diameter of the pit varied from 4-7 mm and depth was approximately 1 mm. The present findings are in conformity with Kim et al. (1987) in Jeju province of Korea who observed the blast symptoms on fruits (referred as pitting), petioles, leaf sheaths, bunch stalks, small fruit stalks and crowns of infected banana plants with the incidence of up to 100%.
In a bunch, fingers facing away from the pseudostem (proximal hand) were severely pitted than the fingers adjacent to pseudostem (Table 1). Here transition leaf acted as main source of inoculum for developing bunch and hence the proximal hand exhibited maximum level of pitting symptom. Similarly, the proximal large hand often exhibited more pitting symptoms than the distal small hands and the fingers not in contact with the transition leaf were almost free from pitting. It is due to the fact that hands and fingers in close proximity to the inoculum source gets more load of inoculum. Meredith (1963) conducted investigations in similar line in Costa Rica and reported almost the similar kind of observations; however, Kim et al. (1987) who has reported this same disease from South Korea, could not offer much information about the distribution pattern of this disease in an infected bunch. The fingers in between the proximal and distal ends were moderately pitted. On individual fingers, pits were abundant towards the finger tip, while the centre portion was less pitted. Spots were more on inner side of the bunch, near the finger stalk, on the finger stalk and crown portion. This can be presumed that the raindrops carrying pathogenic inoculum drained away and settled on lower side of inner whorl than on other portion naturally. However, the symptoms and size of pits on finger stalk/crown was smaller (2-3 mm diameter) and different compared to spots on fruits (4-7 mm), i.e., no distinct regions or cracking of spots. Here the fact is that, when there was rain, the rain droplets along with pathogenic spores rolled down on the bunch and paved the way for infection on its path. Severely infected bunches become unfit for sale due to bad appearance and breakage of fruit stalk as the infection causes the fruit to fall very easily from the bunch or from crown/cushion.
The appearance of the spots was closely correlated with the age of the fruit. Early symptoms were of few, small, reddish pits even on 30-day-old fruit bunches when weather conditions favoured disease development. However the infected bunches were scored with the disease grade of 1. Moderate level of pitting was observed in 30- to 60-day-old bunches and the bunches were scored with the disease grade of 3 and 5. However, pits appeared in severe form with profuse pitting on bunches approaching maturity, i.e., on more than 60-day-old fruits (Table 2) and bunches were scored for the disease grade of 5 and 7. In more than 90-day-old bunches, due to severe pitting on fruits and fruit stalks, the fruits were detached from the bunch and fell on the ground and this stage is categorised as 9 in the scale. Bunches aged between 60 days old to harvesting stage were the most susceptible stage of fruits. These observations were in line with the observations made by Meredith (1963). However, present study contradicts the report of Kim et al. (1987) who stated that 30-day-old fruits of banana were more susceptible to infection by P. angulata than 60-day-old fruits. In our observations, only mild symptoms of pitting were observed on 30-day-old bunch and it was clear that P. angulata had limited ability to spread through the tissue of very young fruits might be due the presence of some phenolic compounds.
In some earlier cases, as per the report, pitting symptoms were not observed on green fruits at the time of harvesting, but in the ripening rooms, new pitting symptoms were appeared on the fruits (Feakin, 1971).
Among the four treatments laid for this study, we found bunches covered with perforated poly bags along with transitory leaves exhibited pitting symptom mainly on the fingers where the transitory leaves were rested. In bunches where transitory leaves were removed and covered with perforated poly bags very few pitting spots were observed on the whole bunch. Bunches wherein transitory leaves were removed and left to open field condition without any covering exhibited almost low to moderate level of pitting. Further, in these bunches pits were almost uniformly distributed on the entire bunch and symptoms were not concentrated on proximal hand and distal end of fruits which indicated the role of air borne spores or splashing rain droplets in spreading the disease. However uncovered bunches with transitory leaves received very heavy infection on the fingers where transitory leaves were rested compared to other parts of bunches. Hence, it was confirmed that transitory leaves were one of the potential source of inoculum for disease occurrence since it emerged with abundant lesions at the time of its emergence from central whorl. It is presumed that during emergence of transition leaf out of the central whorl, spores from air drained by rainwater, collected and padded to the emerging boot caused the early infection. Since farmers are not in the practice of removing the transitory leaves even though it dried within a month of bunch emergence, these leaves rest on the developing bunches and act as a potential source of inoculum. Efforts on finding the alternate weed host of P. angulata in and around the infected field has not led to any information. Common weeds with leaf spots in and around banana plantations were examined for P. angulata infections but similar kind of lesions were not found. Halmos (1970) reported that dead leaves hanging from the plants produced potentially high levels of inoculum compared to decayed trashes on the ground as well as the host weed Commelenia erecta. He further reiterated that role of C. erecta was very less compared to other sources of inoculum in spreading the disease. However under Odisha condition, C. erecta is not a common weed. A related species Commelenia bengalenesis, though, was a common weed in and around the banana plantations; however, C. bengalensis was not found to be infected by the blast pathogen.
Odisha state experiences the average rainfall of 1,500 mm, as the result of southwest monsoon during July to September and July is the wettest month. October to November months also received little rainfall from the receding monsoon. Blast disease incidence on nursery plantlets occurred throughout the year since continuous overhead irrigation creates favorable conditions in the nursery and however outside environmental conditions playing role in increasing the severity of disease in nursery could not be ruled out. Survey of two major tissue culture banana sale outlets in and around Odisha, revealed 25% to 90% blast incidence on young plantlets depending upon the season of survey. Even in nursery, blast incidence and severity was higher during cool and rainy months but a few lesions were observed with less incidence and severity during hot and dry climate.
The survey conducted in different parts of Odisha on standing crop, during the rainy months revealed the prevalence of 39, 31% and 17% disease incidence in Boudh, Khandamal and Sundergarh districts respectively on bunches of var. Grand Naine approaching near to maturity with moderate level of disease severity. Disease incidence occurred during and weeks after moderate to heavy rainfall when maturing banana bunches coincided with rainy season. During the study period in 2014 and 2015, the highest rainfall was recorded during the months July, August, and September than other months. During initial monsoon period and towards end of July, one or two blast spots started appearing on the transition leaves, pseudostem and on leaves of side suckers and on fruit bunches of around 50% maturity. The disease severity further increased during August and September months and the trend was same in both the year. But November onwards, appearance of new lesions stopped as the monsoon was in receding phase and those infections already occurred on fruits were only present on fruits till harvest. After December up to June, it was hard to find even a single new pitting symptom on fruits and other plant parts since dry period commenced. Similar observations on seasonal nature of pitting disease were previously reported by Meredith (1963) in Central America; however, thereafter no study was conducted regarding the seasonality of pitting disease elsewhere in the world. He made it clear that on each occasion of heavy rain there was an incidence of pitting disease and further reported that rainfall with high humidity favoured the development of pitting disease. Our present study also threw some light on the influence of rainfall, high humidity, susceptible fruit stage and availability of free water for initial infection however detailed studies are underway. Effect of epidemiological factors (temperature, relative humidity, and rainfall) have been well correlated with the incidence and severity of paddy blast (P. oryzae) (Shafaullah et al., 2011) and wheat (Kohli et al., 2011). The pathogenic inoculum may be available in the plantation throughout the year in an infected field; however, only during rainy season the pathogen could cause infection. Studies on quantitative relationship between weather parameters such as temperature, humidity and rainfall and on both year on incidence and severity of pitting in banana are underway.
Emergence of pitting disease in certain plantations of Odisha state of Eastern India occurred presumably due to usage of infected tissue culture derived banana plantlets and by secondary spread of spores by air and raindrops. Even though, this disease has not yet caused serious crop loss, but its rapid progression due to favorable climatic conditions is likely to become one among the bottlenecks for quality banana production in near future. The present study has conclusively established the aetiology of this disease. Under the field condition, if maturity period of banana coincides with rainfall along with abundant inoculum, severe spotting on maturing bunches occurs. Hence, this disease has potential to cause heavy economic losses to the farmers and traders in regions with warm and moist condition. Avoidance and removal of inoculum at nursery stage coupled with suitable management strategies involving field sanitation, altering the time of planting to avoid susceptible fruit maturity period coinciding with rainy season is essential to reduce disease occurrence. Further, timely removal of transitory leaves and timely application of suitable fungicides will helpful in managing the disease. Implementation of strict certification for disease free tissue culture derived plantlets and increasing the awareness among the stakeholders regarding the implications of this new disease needs to be taken up on urgent basis to dispel the menace to banana industry in future.
Fig. 2
Evolutionary relationships of Pyricularia and Magnaporthe spp. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. All positions containing gaps and missing data were eliminated. There were a total of 425 positions in the final dataset. Two accessions of P. zingiberi were used to root for phylogenetic tree.
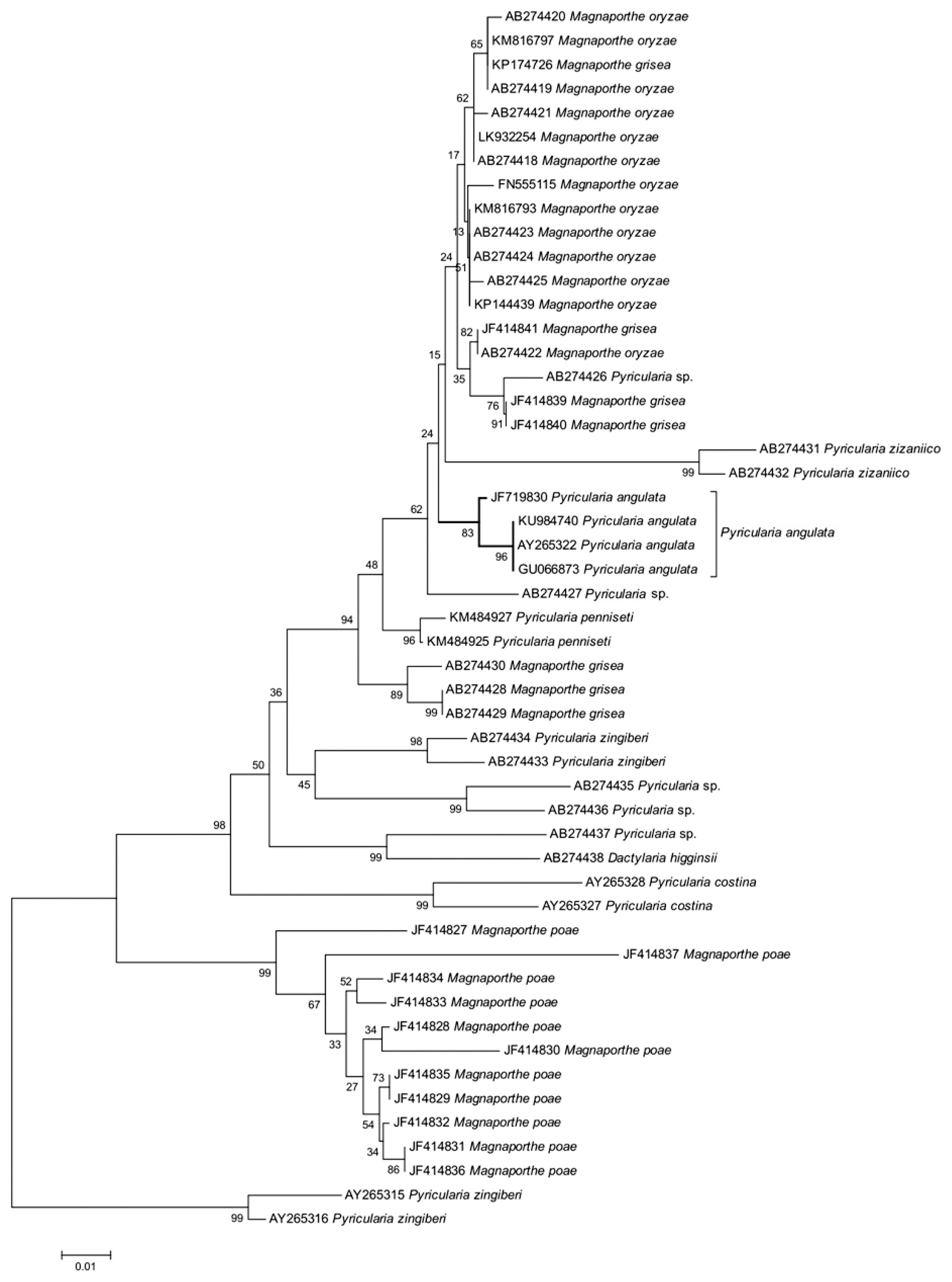
Fig. 3
Characteristic spots developed on tissue culture banana (A) and on maturing banana fruits (B) upon artifcial inoculation of Pyricularia angulata.

Fig. 5
Symptoms of blast on various plant parts. On leaves of new suckers (A); maturing leaves (B); transition leaf (C); inner side of transition leaf (D); Peduncle (E); end of bunch stalk (F).

Fig. 6
Pitting symptoms on maturing fruits; moderate infection (A), severe infection (B), and pitting symptom confined to rind of fruit (C).

Table 1
Distribution pattern of pits on banana bunch cv. Grand Naine
Table 2
Severity of pitting disease in relation with the age of fruit under field condition
| âAge of fruits (day)â | âUnder natural epiphytotic conditionsâ |
|---|---|
|
|
|
| Pitting disease in of scale 0-9* | |
| < 30 | 0 and 1 |
| 30-60 | 3 and 5 |
| 60-90 | 5 and 7 |
| > 90 | 7 and 9 |
* 0 grade, no pitting symptoms; 1 grade, presence of few pits on entire bunch; 3 grade, presence of around 10-25 pits randomly on entire bunch; 5 grade, moderate level of pits distributed over entire bunch which affects eye appeal but still marketable; 7 grade, severe level of pits distributed over entire bunch but difficult to market the fruits; 9 grade, completely pitted bunch unfit for sale and some fingers detached from bunch.
References
Bussaban, B, Lumyong, S, Lumyong, P, Seelanan, T, Park, DC, McKenzie, EH and Hyde, KD 2005. Molecular and morphological characterization of Pyricularia and allied genera. Mycologia. 97:1002-1011.


Chadha, KL 2001. Handbook of horticulture. Indian Council of Agricultural Research, New Delhi, India.
Feakin, SD 1971. Pest control in bananas. PANS manual (Volume no. 1). Centre for Overseas Pest Research, Foreign and Commonwealth Office, Overseas Development Administration, London, UK.
Felsenstein, J 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 39:783-791.



Halmos, S 1970. Inoculum sources of Pyricularia grisea, the cause of pitting disease of bananas. Phytopathology. 60:183-184.

Hashioka, Y 1971. Notes on Pyricularia I. Three species parasitic to Musaceae, Cannaceae and Zingiberaceae. Trans Mycol Soc Jpn. 12:126-135.
Hashioka, Y 1972. Fine structure of the rice blast. IX. Scanning electronmicroscopical observations on appressoria of the rice blast fungus and other species of Pyricularia. Res Bull Fac Agric Gifu Univ. 33:65-73.
Hirata, K, Kusaba, M, Chuma, I, Osue, J, Nakayashiki, H, Mayama, S and Tosa, Y 2007. Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycol Res. 111:799-808.


Hoette, S 1936. Pitting disease of bananas in Australia. Proc Royal Soc Vic. 48:90-95.
Horticultural Statistics at a Glance 2015. URL http://agricoop.nic.in/sites/default/files/hortstat_glance.pdf.
Johnston, JR 1932. The fruit spot of the banana. Bull. Res. Dep. United Fruit Co., Boston, MA, USA.
Kim, WG, Kim, CK and Lee, EJ 1987. Banana blast caused by Pyricularia angulata Hashioka. Korean J Plant Pathol. 3:114-119.
Kimura, M 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111-120.



Kohli, MM, Mehta, YR, Guzman, E, De Viedma, L and Cubilla, LE 2011. Pyricularia blast: a threat to wheat cultivation. Czech J Genet Plant Breed. 47:S130-S134.

Kumar, S, Stecher, G and Tamura, K 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870-1874.




Male, MF, Tan, YP, Vawdrey, LL and Shivas, RG 2011. Elucidation of the taxonomy and pathological status of Pyricularia associated with banana blast in Australia. Australas Plant Dis Notes. 6:22-25.


Meredith, DS 1963. Pyricularia grisea (Cooke) Sacc. causing pitting disease of bananas in Central America. Ann Appl Biol. 52:453-463.

Mgonja, MA, LennĂŠ, JM, Manyasa, E and Sreenivasaprasad, S 2007. Finger millet blast management in East Africa. Creating opportunities for improving production and utilization of finger millet. In: Proceedings of the First International Finger Millet Stakeholder Workshop, Projects R8030 and R8445 UK Department for International Development: Crop Protection Programme; September 13-14, 2005; Nairobi. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India.
Notteghem, JL 1990. Results and orientation of EC projects on rice resistance to blast. Summa Phytopathol. 16:57-72.
Ou, SH 1985. Rice diseases. 2nd ed. Commonwealth Mycological Institute, Kew, Surrey, UK.
Puri, KD, Shrestha, SM, Chhetri, GKB and Joshi, KD 2009. Leaf and neck blast resistance in tropical rice lines under green house condition. Euphytica. 165:523-532.
Rouman, EC 1992. Effect of leaf age on components of partial resistance in rice to leaf blast. Euphytica. 63:271-279.


Saitou, N and Nei, M 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406-425.

Sangeetha, G, Debasish, B, Srinivas, P and Singh, HS 2017. Improved and efficient method of isolation of Pyricularia angulata Hashioka causing blast and pitting disease of banana. J Mycopathol Res. 54:563-564.
Shafaullah Khan, MA, Khan, NA and Mahmood, Y 2011. Effect of epidemiological factors on the incidence of paddy blast (Pyricularia oryzae) disease. Pak J Phytopathol. 23:108-111.
Snowdon, AL 1990. A colour atlas of post-harvest diseases and disorders of fruits and vegetables. 1:General introduction and fruits. CRC Press, Boca Raton, FL, USA.
Sonah, H, Deshmukh, RK, Parida, SK, Chand, S and Kotasthane, A 2009. Morphological and genetic variation among different isolates of Magnaporthe grisea collected from Chhattisgarh. Indian Phytopathol. 62:469-477.
Stover, RH 1972. Banana, plantain and abaca diseases. Commonwealth Mycological Institute, Kew, Surrey, UK. 316.
Takan, JP, Akello, B, Esele, P, Manyasa, EO, Obilana, AB, Audi, PO, Kibuka, J, Odendo, M, Oduori, CA, Ajanga, S, Bandyopadhyay, R, Muthumeenakshi, S, Coll, R, Brown, AE, Talbot, NJ and Sreenivasaprasad, S 2004. Finger millet blast pathogen diversity and management in East Africa: a summary of project activities and outputs. Int Sorghum Millets Newsl. 45:66-69.
Takan, JP, Chipili, J, Muthumeenakshi, S, Talbot, NJ, Manyasa, EO, Bandyopadhyay, R, Sere, Y, Nutsugah, SK, Talhinhas, P, Hossain, M, Brown, AE and Sreenivasaprasad, S 2012. Magnaporthe oryzae populations adapted to finger millet and rice exhibit distinctive patterns of genetic diversity, sexuality and host interaction. Mol Biotechnol. 50:145-158.



Tomkins, RG 1931. Wastage in banana transport. Trop Agric (Trinidad). 8:255-264.
Wardlaw, CW 1934. Banana diseases. VI. The nature and occurrence of pitting disease and fruit spots. Trop Agric. 11:8-13.
Wardlaw, CW 1961. Banana diseases, including plantains abaca. Longmans Green and Company, London, UK.
Wardlaw, CW and McGuire, LP 1932. Pitting disease of bananas. Its nature and control. Trop Agric. 9:193-195.
White, TJ, Bruns, T, Lee, S and Taylor, JW 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications, eds. by MA Innis, DH Gelfand, JJ Sninsky and TJ White, 315-322. Academic Press, New York, NY, USA.

- TOOLS




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




