Effects of Soil Textures on Infectivity of Root-Knot Nematodes on Carrot
Article information
Abstract
This study was conducted to examine infectivity (penetration and gall and egg-mass formations) of the root-knot nematodes, Meloidogyne incognita and M. hapla, on carrots grown in soil conditions of 5 different soil textures consisting of bed-soil (b) and sand (s) mixtures (b-s mixtures) at the ratios of 10:0, 7:3, 5:5, 3:7, and 0:10. For M. incognita, the nematode penetration rates in b-s of 0:10 (100% sand) were significantly higher than in the other b-s mixtures, more greatly at 2 and 5 days after inoculation than at 10 DAI, while no significant differences in the penetration rates were mostly shown for M. hapla at the above DAI. However, for both nematodes, gall and egg-mass formations were remarkably increased in the b-s mixture of 0:10, compared to the other b-s mixtures, which is coincided with the general aspects of severe nematode infestations in sandy soils. This suggests the increased gall and egg-mass formations of M. incognita should be derived from the increased penetration rates in the sandy soil conditions, which provide a sufficient aeration due to coarse soil nature for the nematodes, leading to their mobility increased for the enhanced root penetration. For M. hapla, it is suggested that the sandy soil conditions affect positively on the healthy plant growth with little accumulation of the inhibitory materials and sufficient aeration, enhancing the nematode growth and feeding activities. All of these aspects provide information reliable for the development screening techniques efficient for the evaluation of the nematode resistance in the breeding programs.
Introduction
Agricultural economic losses caused by the plant-parasitic nematodes are estimated around 100 billion dollars (Chitwood, 2003; Oka et al., 2000; Sasser and Carter, 1985), among which the root-knot nematodes are the most important pathogens that attack a wide variety of host plants, causing serious economic losses, especially vegetable crops (Mai, 1985; Mitkowski and Abawi, 2003). The root-knot nematodes (Meloidogyne spp.) also cause one of the most serious damages to the carrot (Dacus carota var. sativus), an important root vegetable next to radish and potato in Korea, which comprises the total cultivation area of 2,849 ha and the total annual production amount of 93,694 tons in 2011 (Davis, 2004; Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea, 2012).
Four root-knot nematode species are recorded in the carrot worldwide and in Korea as well, including Meloidogyne hapla, M. incognita, M. arenaria, and M. javanica (Bridge and Starr, 2007; Korean Society of Plant Pathology, 2009). Especially, M. hapla and M. incognita are assumed to be most prevalent in Korea in open carrot cultivation fields and greenhouses, respectively, in which the environmental conditions are cool and warm that are favorable for the nematode growth and reproduction, respectively (Anwar and McKenry, 2010; Bridge and Starr, 2007; Kim, 2001; Kim et al., 2001; Sardanelli et al., 1983).
Several management tactics are applied for controlling the root-knot nematodes, among which the use of resistant plants is a powerful tool for the nematode control in sustainable agriculture that is highly effective and economically reliable with no or little additional cost for the nematode control (Kinloch and Hinson, 1972; Mitkowski and Abawi, 2003; Rhoades, 1976).
Plant diseases develop under the conditions of virulent pathogen, susceptible host and favorable environment, all of which influence the occurrences and severities of diseases, minimizing disease escape that occurs when three factors in a disease triangle do not interact at the proper time and for sufficient duration (Agrios, 2004). Host-plant resistance to root-knot nematodes (Meloidogyne spp.) is determined by incompatible responses of the plants that vary qualitatively and quantitatively even among the cultivars of the same crops and is influenced by various factors such as temperature, planting time, plant age at the inoculation time, and origin of plants, all of which affect the survival and pathogenicity of the nematode pathogens (Mendosa and Jatala, 1985; Roberts, 1987). Especially, the plant-parasitic nematodes are soil-borne pathogens whose growths and pathogenicity are influenced by the soil conditions including soil texture, moisture, aeration and osmotic potential in field soils (Van Gundy, 1985). Among these soil conditions, soil texture, estimated by the relative amounts of sand, silt and clay particles in a soil, is an important component that determines soil compactness and thus availability of aeration and moisture, which is explored as a basis for management zones of plant-parasitic nematodes within a field (Moore and Lawrence, 2013). This implies the development and infestation of the plant-parasitic nematodes vary critically depending on the soil texture, which should be applied for the selection of plants with durable resistance in the breeding program of nematode-resistant crops. Thus, in this study, effects of soil textures on the infectivity of two root-knot nematodes, M. incognita and M. hapla, on the carrot to select soil conditions suitable for the nematode disease development in the screening of the carrot plants for resistance to the plant-parasitic nematodes.
Materials and Methods
Plants, nematodes, soils and nematode inoculation
A commercial carrot cultivar Shinheukjeon-5-chon (hereafter SHC) and the maternal parent of a hybrid carrot line (13–77♀) currently developed in a Carrot Breeding Institute, Korea, were used for screening assays (nematode inoculation) in our experiments as susceptible host plants. The root-knot nematodes M. incognita Race 1 and M. hapla used in our previous studies (Park et al., 2014; Seo et al., 2014, 2015) were also used in this study. Soils used in our study were bed soil (b) (composed of 64.9% coco-peat, 15% peat-moss, 7% zeolite, 10% perlite, 2.6% dolomite, 0.03% wetting agent, and 0.47% N-P-K common fertilizer) and sand (s) at the ratios of 10:0, 7:3, 5:5, 3:7, and 0:10 (hereafter 10:0, 7:3, 5:5, 3:7, and 0:10 b-s mixtures, respectively) were used in this study. Seeds of SHC and 13–77♀ were planted in bed soil (sterilized at 15 psi, 121°C for 15 min) in a 50 cell-plug tray and grown around at 25°C for three weeks to become three-true leaf carrot seedlings, which were transplanted in 9 cm (diameter) × 8 cm (depth) plastic pots filled with b-s mixtures sterilized at 15 psi, 121°C for 15 min. The carrot seedlings were inoculated with the root-knot nematodes at three days after transplanting. Egg-masses of M. incognita and M. hapla were isolated by hand-picking with a forceps from the pure nematode cultures maintained on chili pepper cv. Bugang and tomato cv. Rutgers at 20 ± 5°C in a greenhouse, respectively. The egg-masses isolated were incubated on Baermann funnels for 3–5 days for egg hatching to second-stage juveniles (J2) of the nematodes (Son et al., 2008; Southey, 1986), which were diluted to make nematode suspensions with the concentration of about 100 J2/ml in sterile distilled water. The nematodes were inoculated on the carrot seedlings by pouring 10 ml nematode suspensions (containing about 1,000 J2) around the plant rhizosphere with thirteen replications for each nematode on each carrot cultivar of line. The carrot seedlings inoculated with the nematodes were arranged in a split-plot design of the factorial experiment in greenhouse benches and grown at 20 ± 5°C in a greenhouse, watering to the field capacity three times per week throughout the experimental period.
Examination of the nematode penetration
Nematode penetration was examined microscopically. At 2, 5, and 10 days after inoculation (DAI), the carrot seedlings were carefully uprooted from the pots, and the root systems were washed free of adhering soil with tap water. All root systems were cut into 1–2 cm root segments with a razor blade and stained with red food coloring stain following the method described by Thies et al. (2002). The root segments stained were observed under a stereomicroscope to measure the number of nematodes in root tissues with three replications for each b-s mixture.
Formation of root-knot galls and egg-masses
Six weeks after nematode inoculation, plants were carefully uprooted from pots, and the root systems were gently washed with tap water to remove adhering soil. The roots were examined visually for root-knot gall formation on each root system and the severity of root galling was graded using the gall index (GI) based on the root-knot scoring chart developed by Bridge and Page (1980); from no knots (GI = 0), few small knots difficult to find (GI = 1) ~50% of roots affected with some main roots knotting (GI = 5) through all roots severely knotted with concomitant plant death (GI = 10) with four replications for each b-s mixture. Also the number of egg-masses formed on each root system was examined by close looking into the root systems with four replications for each b-s mixture with naked eyes.
Statistical analysis
Data obtained from the experiments were subjected to analyses of variance (ANOVA) in split-plot design of 2 (nematode species) × 2 (plant cultivar/line) × 5 (b-s mixtures) factorial experiments using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Fisher’s least significant difference was employed to test for significant difference among the factors examined using critical values from the t-distribution table at P ≤ 0.05 and P ≤ 0.01.
Results
Penetration of root-knot nematodes in different soil conditions
In microscopic examination, thread-like nematodes were mostly found in the proximity of apical meristem tissues at 2 DAI, in which neither definite root-swelling nor giant cell formation was noted, regardless of the b-s mixtures, nematode species and carrot cultivar/line (Fig. 1). At 5 DAI, the root tissues of the nematode infection sites were somewhat swollen and giant cell-looking hypertrophied tissues were intermittently found in the stelar parenchyma adjacent to vascular tissues around the nematode infection sites (Fig. 2). However, the nematode morphology was mostly thread-like at this stage of infection, indicating the nematode growth should be minimal, regardless of the root-knot nematode species. At 10 DAI, the root tissues of the infection sites were more swollen than those at 5 DAI, some of which were definitely hypertrophied to form galls, in which the infecting nematodes were mostly located in the stelar parenchyma around the vascular tissues with mostly thickened shapes like sausages (Fig. 3).
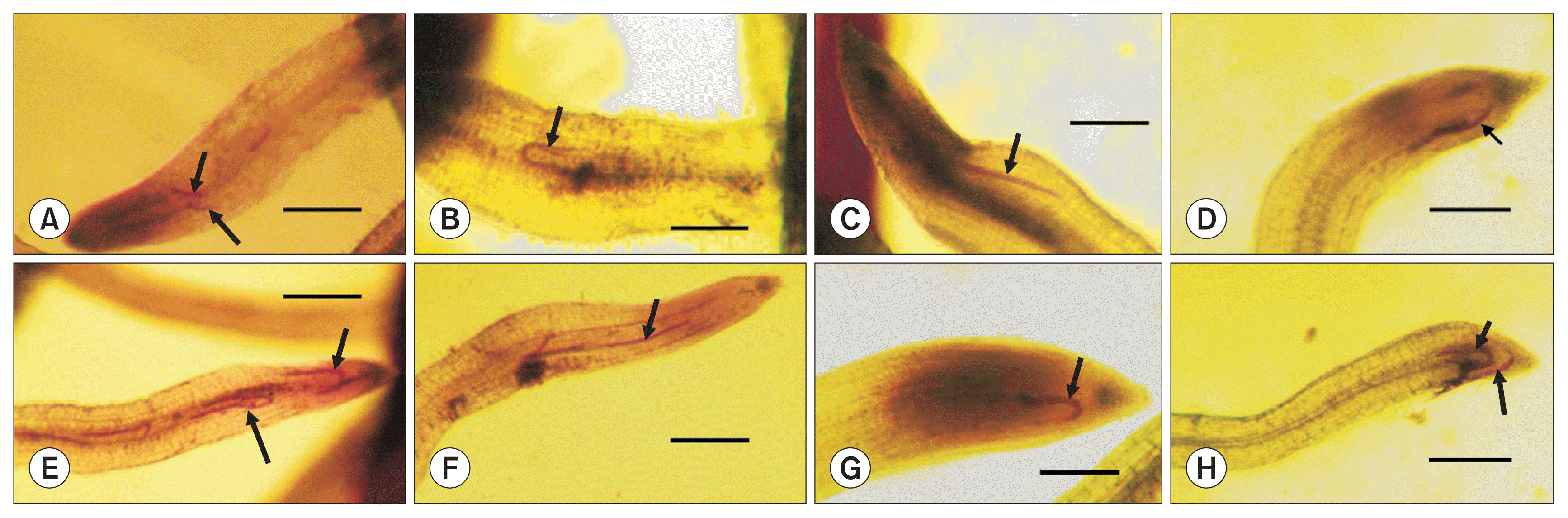
Penetration of Meloidogyne incognita (A–D) and M. hapla (E–H) in carrot cultivar Shinheukjeon-5-chon (A, B, E, F) and a crossing parent line 13–77♀ (C, D, G, H) at 2 days after inoculation, showing the nematode juveniles with no swelling (arrows) located in the proximity of the apical meristem. Scale bars = 1.0 mm.
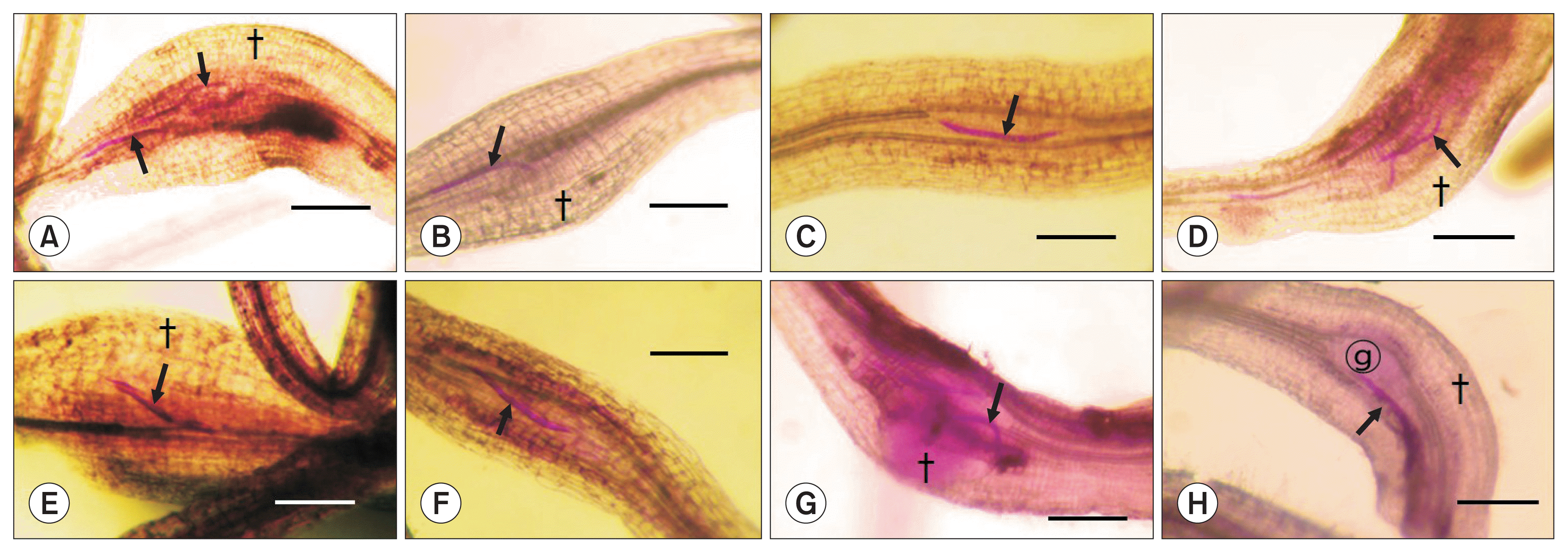
Penetration of Meloidogyne incognita (A–D) and M. hapla (E–H) in carrot cultivar Shinheukjeon-5-chon (A, B, E, F) and a crossing parent line 13–77♀ (C, D, G, H) at 5 days after inoculation, showing the nematode juveniles with little swelling (arrows) located inside the stele of upper differentiated root tissues. Note the formation of giant cells (
 ) and somewhat swelled cortical tissues (†), indicating the initial stage of gall formation. Scale bars = 1.0 mm.
) and somewhat swelled cortical tissues (†), indicating the initial stage of gall formation. Scale bars = 1.0 mm.

Penetration of Meloidogyne incognita (A–D) and M. hapla (E–H) in carrot cultivar Shinheukjeon-5-chon (A, B, E, F) and a crossing parent line 13–77♀ (C, D, G, H) at 10 days after inoculation, showing the swollen nematode juveniles (arrows) located inside the galled root tissues (asterisks) containing the giant cells (
 ). Scale bars = 1.0 mm.
). Scale bars = 1.0 mm.
The nematode penetration rates at 2, 5, and 10 DAI
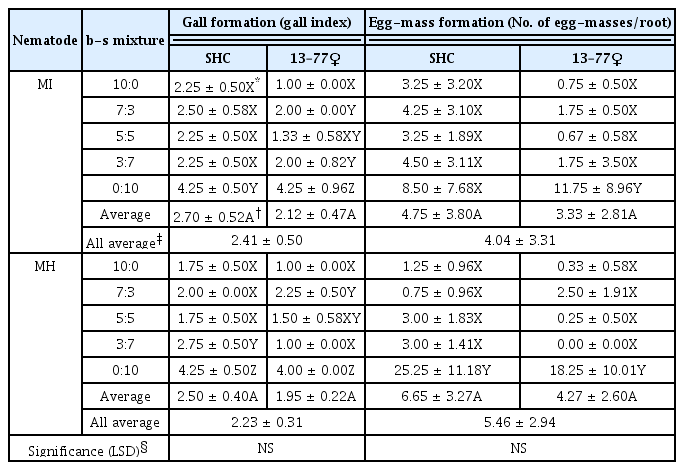
For M. incognita, the penetration rates were significantly higher in 0:10 b-s mixture (100% sand) than in the other b-s mixtures with high degrees at 2 and 5 DAI, but with a lowered degree at 10 DAI due to the increased penetration rates in the b-s mixtures other than 0:10 b-s mixture (Table 1). There were no significant differences in the penetration rates of M. incognita between the carrot cultivar SHC and the hybrid line 13–77♀. On the other hand, the penetration rates of M. hapla were not significantly different among all b-s mixtures except for SHC at 2 DAI (Table 1). Statistical comparisons of the penetration rates between the two nematode species showed significantly (P ≤ 0.05) higher penetration rates for M. hapla than M. incognita at 2 and 5 DAI, but not at 10 DAI (Table 1).
Formation of root-knot galls and egg-masses in different b-s mixtures
At 6 weeks after inoculation, root-knot nematode disease severities assessed by the gall formation were significantly higher in 0:10 b-s mixture (100% sand) than the others regardless of the nematode species and carrot cultivar/line, which revealed no significant differences between the carrot cultivar and line as well as between the nematode species (Table 2, Fig. 4). Also egg-mass formation was all higher in 0:10 b-s mixture (100% sand) than the other b-s mixtures examined, more greatly in M. hapla compared to M. incognita that even showed no significant difference among b-s mixtures in SHC (Table 2).
Formation of root-knot galls and egg-masses of MI and MH on carrot cultivar SHC and a crossing parental line (13–77♀) in pots containing b-s mixtures at the ratios of 10:0, 7:3, 5:5, 3:7, and 0:10 at 6 weeks after inoculation

Gall formation of Meloidogyne incognita (A–D) and M. hapla (E–H) in carrot cultivar Shinheukjeon-5-chon (SHC) (A, B, E, F) and a crossing parent line 13–77♀ (C, D, G, H) in 0:10 bed soil-sand (b-s) mixture (100% sand) (A, C, E, G) and the other b-s mixtures (B, D, F, H) at 6 weeks after inoculation. Note the different sizes of galls (yellow circles) formed by M. incognita (large) in SHC (A) and 13–77♀ (C) and M. hapla (small) in SHC (E) and 13–77♀ (G).
Discussion
A variety of abiotic and biotic factors affect the establishment of nematode populations in soil. Soil nematodes including plant-parasitic nematodes are mostly aerobic and originally aquatic animals, requiring proper moisture contents and aeration from their immediate surrounding soil water films, preferring most to moisture levels 40–60% of filed capacity (Dropkin, 1980; Kim, 2015; Van Gundy, 1985). Among soil conditions, soil texture, a mixture of solids (sand, silt and clay particles and organic matters) determines soil compactness and porosity (thereby availability of moisture and aeration for the nematodes) is one of the most important soil characteristics related to nematode infestations in crop fields (Moore and Lawrence, 2013; Stolzy and Van Gundy, 1968). Generally light sandy soils are more favorable to large populations of nematodes than heavy clay soils owing to more adequate aeration provided in soils consisting of coarse particles, but cause more nematode damages on plants that suffer from water stress due to easy drainage of water in coarse particulate sandy soils (Dropkin, 1980). M. incognita and Hoplolaimus columbus prefer to soils with high sand content (Koenning et al., 1996; Lewis and Smith, 1976). Soil water regimes, related with soil textures and water contents affect the penetration of the soybean cyst nematode (SCN) and the tolerance of susceptible soybean cultivars to SCN (Johnson et al., 1993b, 1994). However, little study has been made on the reasons of heavy infestation and sever damages of Meloidogyne spp. in the carrot fields in sandy soils until now.
In this study, gall and egg-mass formations of the root-knot nematodes were remarkably increased in 100% sandy soils. These results agree with the general aspects that the agricultural importance of the root-knot nematodes is associated with sandy soils and that crop damages associated with root-knot nematode infections are highly reflective of sandy soils and sandy patches within fields (Van Gundy, 1985). Infestations of M. incognita and M. hapla occur more frequently in sandy loam soils than in clay soils (Sasser, 1954).
For M. incognita in our study, the increased gall and egg-mass formations were due to the significantly increased penetration rates of the nematode J2 in the sandy soil compared to other soil textures (Table 1). This is consistent with another study that the mobility and root penetration of the nematode juveniles decreases as the clay and silt fractions in the soil increase (Prot and Van Gundy, 1981).
On the other hand, the root penetration rates of M. hapla juveniles were not much increased in 100% sandy soils as compared to M. incognita in our study, although its gall and especially egg-mass formations were significantly increased in the sandy soil over the other soil textures (Table 1, 2). This suggests that the increased nematode damage (the root-knot gall formation) and reproduction (egg-mass formation) should not be related with the nematode penetration, but with nematode growth and development after infection in the carrot root tissues, which are dependent on the giant cell formation, leading to the enhanced reproduction and root-knot galling (Seo et al., 2015). This may be supported in our study by the results that showed similar penetration rates among b-s mixtures but differentially increased gall and egg-mass formations in 100% sandy soil compared to the other b-s mixtures (Table 1, 2).
With a proper moisture content, aeration of the soil enhances stem growth and root elongation, the rate of transpiration and the intensity of the respiratory activity of the shoot, leading to healthy plant growth with a full function of nutritional absorption by root hairs and reduced accumulation of potentially inhibitory soil products (Drew and Sisworo, 1979; Hunter and Rich, 1925). Endoparasitic sedentary nematodes such as Meloidogyne and Heterodera species induce the formation of specialized nursing cells, giant cells and syncytia, respectively, from which they acquire nutrients for their growth and development (Jones, 1981; Kim et al., 1999). Soil environments such as soil water regimes and inhibitory materials influence on the location of nematode-induced syncytia by altering nematode feeding behavior and the development of giant cells and syncytia, which affects the nematode growth and development (Johnson et al., 1993a; Kim et al., 1986, 1999; Moon et al., 2010; Orion et al., 1980; Stender et al., 1986). All of these aspects suggest the increased reproduction of M. hapla in the sandy soil may be derived from the healthy plant growth with little accumulation of the inhibitory materials in the soil conditions with sufficient aeration available for the nematodes.
Minimizing the crop losses of the root-knot nematodes through the use of nematicides is expensive and provides environmental and human health problems, which makes the breeding for the nematode-resistance an attractive alternative (Fassuliotis, 1985; Oka et al., 2000; Osman and Viglierchio, 1981). Screening techniques efficient for the evaluation of the nematode resistance should be developed, for which the screening should be done under conditions that are conductive for good plant growth and that no plants escape contact with infective nematode juveniles (Fassuliotis, 1985). In our study, in b-s mixtures other than 100% sandy soil, the plant roots were not in full contact with the infective juveniles of M. incognita, resulting in low penetration rates, and not in such healthy growth status as sufficiently supporting the growth and reproduction of both root-knot nematodes, resulting in the decreased root-knot gall and egg-mass formations. This is not the true plant resistance to the nematodes, but disease escape. Therefore, we suggest the screening of the carrot for resistance to the root-knot nematodes should be done under the soil conditions of 100% sandy soil texture with proper moisture contents and fertilizers to support full contact of the infective juveniles with the plant roots and healthy plant growth to provide the soil conditions favorable for the growth and reproduction of the infecting nematodes to minimize disease escape.
Acknowledgments
This study was conducted with a financial support from the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
