 |
 |
| Plant Pathol J > Volume 33(1); 2017 > Article |
Abstract
Barley yellow dwarf virus (BYDV) belongs to Luteovirus and is limited only at phloem related tissues. An open reading frame (ORF) 4 of BYDV codes for the movement protein (MP) of BYDV gating plasmodesmata (PD) to facilitate virus movement. Like other Luteoviruses, ORF 4 of BYDV is embedded in the ORF3 but expressed from the different reading frame in leaky scanning manner. Although MP is a very important protein for systemic infection of BYDV, there was a little information. In this study, MP was characterized in terms of subcellular localization and programmed cell death (PCD). Gene of MP or its mutant (ΔMP) was expressed by Agroinfiltration method. MP was clearly localized at the nucleus and the PD, but ΔMP which was deleted distal N-terminus of MP showed no localization to PD exhibited the different target with original MP. In addition to PD localization, MP appeared associated with small granules in cytoplasm whereas ΔMP did not. MP associated with PD and small granules induced PCD, but ΔMP showed no association with PD and small granules did not exhibit PCD. Based on this study, the distal N-terminal region within MP is seemingly responsible for the localization of PD and the induction small granules and PCD induction. These results suggest that subcellular localization of BYDV MP may modulate the PCD in Nicotiana benthamiana.
Barley yellow dwarf virus (BYDV) has been known to a worldwide virus infecting grass species, including barley, wheat, rice, maize, and so on (Miller et al., 2002). It belongs to Luteovirus which is a positive sense single-stranded RNA virus (Miller and Rasochová, 1997). Like other Luteovirus, BYDV is a type of phloem-limited viruses transmitted by aphids in circulative nonpropagative manner (Xu et al., 1994).
Plant cells communicate each other to keep the normal life cycle by transporting in and out necessary materials through plasmodesmata (PD) found in plant system only (Ding, 1998). Water, ions, small metabolites, growth factors, and even marcromolecules including autonomous and nonautonomous protein can be transported to adjacent cells for local and long-distance movement (Citovsky and Zambryski, 1991; Lucas, 2006; Lucas and Lee, 2004). Movement of virus from cell to cell is a type of nonautonomous movements during infection (Lucas, 2006; Lucas and Lee, 2004). Unlike an animal virus, plant virus movement to adjacent cells occurs via the specialized intercellular channels called PD (Lazarowitz and Beachy, 1999). The size of PD’s micro-channel is about 3 nm in diameter whereas the smallest virus is about 10 nm in diameter (Ding, 1998; Ding et al., 1992). However, the size of a channel is too small to allow virus’ movement if there is no any other stimulative action on PD to be enlarged.
Movement protein (MP) of a virus has been known to act as the regulator for PD gating (Deom et al., 1992). Thereby plant virus can move through this small channel (Lazarowitz and Beachy, 1999). In higher plants, this system involves in the trafficking of proteins and various forms of RNA that function non-cell-autonomously to affect developmental processes.
ORF4 of BYDV encodes 17 kDa called MP (Nass et al., 1998). Like other Luteoviruses, the ORF4 expressed in leaky scanning manner is embedded in ORF3 which encodes 22 kDa call coat protein (CP) (Miller and Rasochová, 1997). In addition to cell-to-cell movement, MP is required for systemic infection of BYDV in plants (Chay et al., 1996; Sambade et al., 2008). BYDV capsid is composed of two forms of CP, the major P3 protein (ORF3) and the minor P3/P5 protein (ORF3 + ORF5) which is the product of a translational readthrough of P3 (Wang et al., 1995). Like P3/P5 in Polerovirus, that of BYDV is responsible for insect transmission and acts to facilitate long-distance virus movement in phloem tissue (Taliansky et al., 2003). From many cases, viral CP has been known to the factor triggering resistant responses of host plants against viral infection (Baulcombe et al., 1994; Beachy, 1999; Bendahmane et al., 1995, 1997; Clark et al., 1995a, 1995b; Deom et al., 1994; Lim et al., 1997; Osbourn et al., 1990).
In plants, programmed cell death (PCD) has been known as the essential elements for the plant development and can be local, systemic, micro, or macro (van Doorn and Woltering, 2005). Some elements of the defense response in plants may potentially be toxic to the plant cell resulting in that they could induce directly cell death. The hypersensitive response (HR) is a form of PCD often associated with plant responses to biotic or abiotic stresses (van Breusegem and Dat, 2006). Various plant pathogens act as agent for biotic stress against plants (Gechev et al., 2006). When pathogens infect plants, they show several responses such as severe, mild or no disease symptom by host-pathogen interaction. These phenomena occur due to the plant evolution against the attack of plant pathogen (Dong, 1998; Govrin and Levine, 2000). PCD is one of mechanisms disease resistance as the result of the rapid development of cell death by perturbation of physiological processes of plants, called HR (Agrios, 2006; Carr et al., 2010; Goodman and Novacky, 1994). Their evolutions have been occurred in various manners to avoid efficiently pathogens’ invasion resulting in less damage on plant system or in no disease at all. The earliest changes observed following pathogen recognition are an oxidative burst, resulting in the production of reactive oxygen species (ROS) and necrosis in that region (Baker and Orlandi, 1995; Levine et al., 1994; May et al., 1996). Common symptoms of virus infection to plant are chlorosis, retarded growth or stunting, and necrosis of the initial infection sites. Necrosis can often be seen in the vascular and cortical regions and phloem related tissues display necrosis in some virus-host interaction. TGBp3 of Potato virus X (PVX) induced cell death in leaves of Nicotiana benthamiana (Ye et al., 2011). Slows barley growth due to BYDV infection might be related to the death of phloem related cells (D’Arcy and Domier, 2000; Miller and Rasochová, 1997). The precise information is not available that BYDV causes PCD in phloem related tissues, or that BYDV infection induces real HR, a type of PCD.
Therefore, this study was conducted to visualize the localization of the MP and to investigate the potential function of the MP in PCD, which might provide additional insights of MP’s role in figuring out all aspects of the BYDV life cycle.
Full-genomic BYDV was kindly provided by Dr. J. Y. Yoon from National Institute of Horticultural and Herbal Science (Jeonju, Korea). The V15 (pPZP-P35S-3′PinII-Bar with spectinomycin resistant gene) vector was obtained from Dr. S. C. Park at National Academy of Agricultural Science (Jeonju, Korea) and modified to generate the pV15-RFP shown at Fig. 1A. The CP gene and MP gene of BYDV were introduced into the pV15-RFP binary vector to construct pV15-CP:RFP and pV15-MP:RFP (Fig. 1B). Before inserting CP and MP into the pV15-RFP, TA cloning was carried out using into pGEM-T Easy vector. The coding sequences of CP and MP of BYDV were amplified by PCR, using the primer sets containing XbaI sequence (Table 1). The XbaI-treated plasmids (pGEM-CP and pGEM-MP) was gel-purified and inserted into the XbaI site of V15-RFP to construct binary plasmid for agroinfection. Final clone was called pV15-CP:RFP for CP or pV15-MP:RFP for MP (Fig. 1B). The pV15-CP:RFP or the pV15-MP:RFP was introduced into the Agrobacterium tumefaciens strain GV3101 and transformed the A. tumefaciens was used to express the target gene at N. benthamiana, an experimental host used in this study.
In order to study regarding the effect of the MP on PCD, the other binary plasmid vector, pPZP-P35TP-LxAB (Fig. 1C) provided by Dr. Y. H. Lim at National Academy of Agricultural Science (Jeonju, Korea), was used for cloning platform. In order to make the deletion mutant (ΔMP:RFP) of MP:RFP, highly conserved sequences (QWLWS, SQST, SNSVPIY, PT, SQVS, L) of the amino acid (AA) of BYDV MP were identified by aligning multiple sequences of BYDV MP acquired from National Center for Biotechnology Information database using “Clustal Omega” Program to find important domain responsible for the PD trafficking in plants (Link et al., 2011) (Fig. 2). The coding sequences of MP:RFP and of ΔMP:RFP were amplified by PCR from pV15-MP:RFP, using the primer sets containing SpeI sequence for the forward primer and SacI sequence for the reverse primer (Table 1). PCR fragment for ΔMP:RFP was created by deleting the nucleotides corresponding to AAs 1 to 55 of MP (Fig. 1D). Constructs for agroinfection, pPZP-MP:RFP and pPZP-ΔMP:RFP, were made by replacing the LxAB coding sequences between SpeI and SacI of pPZP-P35TP-LxAB with PCR products digested with restriction enzymes and gel purified MP and its deletion mutant, respectively (Fig. 1C, D).
The leaves of the N. benthamiana were infiltrated with A. tumefaciens based on the previous method (Bendahmane et al., 2000) with a little modification. For Agroinfiltration, A. tumefaciens harboring a gene encoding the interesting protein was overnight-cultured in 10 ml of LB liquid medium containing spectinomycin (100 mg/l) in the shaking incubator with 250 rpm at 28ºC. Bacterial cells were harvested by centrifugation at 2,000g for 20 min and washed two times with 25 ml of ddH2O. Then, bacterial pellet were resuspended to an OD600 of 0.4 in infiltration buffer (10 mM MgCl2, 10 mM MES, 20 mM glucose, pH 5.6). In order to promote gene expression, 200 μM of acetosyringone in final concentration was added into infiltration buffer prior to use (Goodin et al., 2002). A. tumefaciens harboring a gene encoding the interesting protein was infiltrated into leaves of 4-week-old N. benthamiana of using a 1 ml syringe. After Agroinfiltration, N. benthamiana plants were maintained at 25ºC for transient gene expression.
In order to visualize subcellular localization of each viral protein, an epifluorescence microscope (MoticamPro 205A; Motic, Xiamen, China) and confocal microscope (SP8 X; Leica, Wetzlar, Germany) were used at approximately 40 h post-infiltration with Agrobacterium containing binary plasmid to express corresponding proteins, RFP, CP:RFP, MP:RFP and ΔMP:RFP. Images were taken using the epifluorescence microscope and the confocal microscope equipped with Active Measure (Shinhan Scientific Optics, Seoul, Korea) and Leica Application Suite X (Leica), respectively. All subsequent image manipulations and figure processing were carried out using Photoshop 5.0 (Adobe Systems, San Jose, CA, USA).
To determine the optimum density of Agrobacterium cell to induce PCD, OD600 value of bacterium cell was resuspended to 0, 0.2, 0.4, 0.6, 0.8, and 1.0 with infiltration buffer. If there is no mention on Agrobacterium cell density, it means that OD600 is 0.4.
To test a gradual change of HR, each leaf was infiltrated with Agrobacterium containing each construction at different time points and maintained at 25°C. An experiment was conducted to verify the effect of light on PCD. Each plant with leaf infiltrated with Agrobacterium containing each construction was placed on the light of dark condition for 48 h.
Diaminobenzidine (DAB) staining method was employed to visualize ROS generated by dead cells. DAB staining was carried out based on previous research done by Wong’s group (Tran et al., 2014; Wong et al., 2007) with little modifications. Briefly, leaves agroinfiltrated were placed in petridish and incubated in solution containing 1 mg/ml of DAB made with PBS buffer (pH 7.2) for 3 h. Then 95% ethanol was applied to the leaves at 65°C for overnight to bleach chlorophyll out. The stained leaves were digitalized using the camera built in cellular phone (Galaxy Note 4; Samsung, Seoul, Korea).
Ion leakage from leaf discs was measured to examine the occurrence of cell death as used previously by Rizhsky et al. (2004) with a little modification. Five discs of leaves (9 mm in diameter) were placed in ddH2O for 5 min to eliminate ion leakage owing to injury. All leaf discs for each treatment were transferred into a 50 ml tube with 35 ml of ddH2O and incubated for 3 h at room temperature. Then, the conductivity of the fluid was measured with a conductivity meter considering this conductivity as value A. After the leaf discs were then returned back to the fluid in the 50 ml tube and incubated at 65°C overnight, the tubes were kept at room temperature to cool down to measure the conductivity of the fluid. This second measurement of conductivity was referred to as value B. Ion leakage for each sample was calculated and expressed by as a percentage;
Epifluorescence microscope was used to visualize the subcellular localization of RFP, CP:RFP, and MP:RFP in an epidermal cell of N. benthamiana leaf. When RFP was expressed within leaf cells of N. benthamiana by Agroinfiltration to observe the RFP localization, RFP was localized at the nucleus (white arrows) and the cytoplasm (blue arrowhead) (Fig. 3B). CP:RFP (Fig. 3C) showed the similar subcellular localization with the pattern of the RFP localization (Fig. 3B) exhibiting red fluorescence at the nucleus (white arrows) and the cytoplasm (blue arrowheads). However, fluorescent uniformity of CP:RFP (Fig. 3C) differed from that of RFP (Fig. 3B). RFP (Fig. 3B) expressed the similar brightness for the nucleus and the cytoplasm, whereas CP:RFP (Fig. 3C) exhibited stronger red fluorescence signals at the nucleus than those at the cytoplasm. Although it was not very clear, it seemed that CP:RFP was rarely localized at the PD (white arrowhead) (Fig. 3C). MP:RFP (Fig. 3D) showed the totally different pattern of the subcellular localization compared with the localization of RFP and CP:RFP. RFP and CP:RFP were mainly exhibited red fluorescence at the nucleus (white arrows) and the cytoplasm (blue arrowheads) (Fig. 3B, C) but the red fluorescent signals by the expression of MP:RFP were mainly at the nucleus (Fig. 3D, white arrows) and the PD (Fig. 3D, white arrowheads) with a little red fluorescent background. The patterns of fluorescent signals owing to expression of MP:RFP in cell showed less nucleus localization and more distinct PD localization (data not shown).
Since there was clear MP:RFP localization at PD (Fig. 3D), an experiment was carried out in order to examine the effect of the MP mutation on the PD targeting. MP:RFP and its mutant were expressed in leaf’s epidermal cell of N. benthamiana and N. tabacum by Agroinfiltration and then observed their expression patterns through laser confocal microscope (Fig. 4). In cells expressing RFP, red fluorescence was mainly observed at the cytoplasm and at the nucleus as expected from other experiment shown at Fig. 3B (Fig. 4B). Mutation of MP:RFP, ΔMP:RFP, showed different subcellular localization compared to that of MP:RFP. MP:RFP showed clear localization at the nuclear (white arrowhead) (Fig. 4C-1) and at the PD (white arrows) (Fig. 4C-2). There were also many red fluorescent small granular vesicles (blue arrowheads) within a cell expressing MP:RFP (Fig. 4C-1). However, the fluorescence from cells expressing the ΔMP:RFP was more similar with that of RFP with a little different pattern showing the targeting nucleus (white arrowhead) (Fig. 4D) and the cytoplasmic pattern/streaming (blue arrow) (Fig. 4D-1) by exhibiting red fluorescence along with endoplasmic reticulum (ER) from cells expressing ΔMP:RFP (Fig. 4D, D-1).
It has been known that viral protein induces PCD which is one of defense mechanisms. However, there was no clear information how BYDV involves in PCD. It, therefore, thought that it was worthy to find the viral protein related to PCD. In this experiment, two viral proteins, CP and MP, were tested whether they involve in PCD.
N. benthamiana and N. tabaccum were infiltrated with Agrobacterium with binary vector containing gene of RFP, CP:RFP, or MP:RFP. When N. benthamiana and N. tabaccum were observed at 48 h after Agroinfiltration to express each protein mentioned above. N. benthamiana (Fig. 5A, B) and N. tabaccum (Fig. 5D, E) showed similar responses on PCD phenomenon. For both species, PCD was observed only from the region expressing MP:RFP but no PCD were observed from area expressing RFP or CP:RFP.
DAB staining experiment was conducted using the same leaf of N. benthamiana shown at Fig. 5B for the indirect visualization of ROS which led PCD (Fig. 5C). It showed that region of leaf expressing MP:RFP was stained by DAB but there was no stain at the region expressing RFP or CP:RFP and at mock inoculated area, indicating MP:RFP induced PCD.
MP:RFP cloned into V15 vector and into pPZP vector were expressed in order to examine the effect of type of vector. Although pPZP expression system displayed a little more dark DAB stain than V15 system, it was clear that MR:RFP expressed from both vector system induced PCD at N. benthamiana and N. tabaccum, indicating there was seemingly no plasmid effect on PCD development (Fig. 5B, C, E).
Since it was clear that MP involved in PCD, the further systemic experiment was conducted to determine minimal concentration of the Agrobacterium driving PCD. Agrobacterium harboring binary plasmid vector containing gene to express MP:RFP was suspended with infiltration buffer to prepare 5 different concentrations based on by adjusting optical densities, 0.2, 0.4, 0.6, 0.8, and 1.0. These five mixtures were applied to the same leaf of two different species by infiltration (Fig. 6). Mock infiltration was carried out for negative control. Mock inoculated area on the leaf of N. benthamiana and N. tabaccum did not show any PCD sign. There was no PCD at the inoculated area with Agrobacterium expressing RFP, regardless of optical density and species (Fig. 6). PCD was observed at leaf inoculated with Agrobacterium to express MP:RFP. PCD at N. benthamiana was weakly observed when Agrobacterium mixture with 0.2 in OD600 was applied (Fig. 6A). It was not easy to ascertain PCD without DAB staining. By DAB staining, PCD sign was clearly displayed from N. benthamiana infiltrated with Agrobacterium with 0.2 in OD600 (Fig. 6B). When more Agrobacterium, such as 0.4, 0.6, 0.8, and 1.0 in OD600, was used, PCD sign was observed from N. benthamiana even without DAB staining. Similar results were acquired from an experiment conducted with N. tabaccum, showing clear PCD sing at the region inoculated with Agrobacterium with 0.4, 0.6, 0.8, and 1.0 in OD600 (Fig. 6C). Based on these combined results, there was no big difference for PCD sign at more than 0.4 in OD600. Therefore, Agrobacterium was adjusted to 0.4 in OD600 for other experiments.
It has been reported the importance of the N-terminal domain involving in self-interaction (Xia et al., 2012). Therefore, it hypothesized the N-terminal domain of MP might be related to PCD. To determine the role of the N-terminal domain of MP on PCD, the ΔMP:RFP not containing the distal end of the N-terminal region of MP, was used. Control (buffer filtrated), RFP, MP:RFP (WT), and ΔMP:RFP were expressed by Agroinfiltration into the leaf of the N. benthamiana (Fig. 7B) and into the leaf of the N. tabaccum (Fig. 7E).
Like control and RFP used as negative controls, the ΔMP exhibited no PCD from the leaf of the N. benthamiana and from the leaf of the N. tabaccum (Fig. 7B, E). However, leaves of the N. benthamiana and the N. tabaccum which were expressed MP:RFP showed distinct PCD (Fig. 7B, E). Since ion leakage is usually linked to ROS and PCD, ion leakage was measured to confirm that MP-induced PCD at leaves of N. benthamiana. Leaf disks obtained from mock-, RFP-, and ΔMP:RFP-expressing N. benthamiana did not showed significant increases in ion leakage after 48 h infiltration (Fig. 8). In contrast, sample acquired from MP:RFP expressing N. benthamiana displayed increased in ion leakage after 48 h infiltration (Fig. 8).
These combined results indicate that the distal end of N-terminal region MP might be essential for initiating PCD from two plant species used in this study. DAB stained N. benthamiana also showed the same result with clear PCD at 48 h after argoinfiltration to express MP:RFP (Fig. 7C).
Plant cells communicate each other to keep the normal life cycle by transporting in and out necessary materials through PDs, a special structure located between cells, found in plant system only (Ding, 1998). In addition to their fundamental function in transport of intrinsic metabolites, PDs function as special pathway for cell-to-cell movement in virus spreading at infected tissue (Chen et al., 2000). Virus-encoded MPs mediate cell-to-cell movement of plant virus and are a critical factor in the interactions between plant viruses and their plant hosts for the successful systemic infection (Cruz et al., 1998). MP function for the cell-to-cell and localization to PD have been reported for many viruses, Apple chlorotic leaf spot virus, Cucumber mosaic virus, Potato leaf roll virus (PLRV), PVX, Rice dwarf virus, Rice stripe virus, Tobacco mosaic virus (TMV), and so on (Chen et al., 2000; Cruz et al., 1998; Itaya et al., 1997; Li et al., 2004; Satoh et al., 2000; Schmitz et al., 1997; Vogel et al., 2007; Xiong et al., 2008). Although it has been known that MP plays a key role in virus movement and localized at PD, interestingly there was no report that BYDV MP localizes at PD.
Nass et al. (1998) showed that MP of BYDV was localized in the cytoplasm and nucleus associating with a filamentous material. Although they failed to show the PD localization of BYDV- MP from the in situ immunolocalization assays, mentioning that MP association with PD was overlooked by cell disruption from the cytological change induced by BYDV, they still believed that BYDV MP would be localized at PD in order to facilitate PD gating like MP of other plant viruses. To eliminate cell disruption induced by full-genomic BYDV infection, only RFP fused MP (MP:RFP) was expressed transiently in N. benthamiana plants. MP localization to the nucleus was supported by Nass et al. (1998). In addition, although they fail to detect MP at PD (Nass et al., 1998), we are able to detect MP localization to PD. This contradictory observation is not surprised because of different experiment system. They expressed MP from full-length BYDV whereas we expressed MP only. The other different targeting compared to the previous report was MP distribution through cytoplasm. Nass et al. (1998) reported that MP was also clearly observed at the cytoplasm but there was no clear cytoplasmic localization of MP from this study. It is possible that BYDV MP interaction with viral RNA replicated in cytoplasm might be inhibited the MP trafficking to PD when MP was expressed from full-genomic BYDV.
CP:RFP was mainly detected at the nucleus and at the cytoplasm (Fig. 3C). This result was supported by the previous report by Nass et al. (1995). Since BYDV belongs to the similar group with PLRV, BYDV CP localization to nucleolus also was expected based on the report by Haupt et al. (2005). From in this study, however, the CP of BYDV localized at the nucleus but not at nucleoli. Although there was the different result with expected one, it is not surprising because these two viruses are not the same. In addition to this difference, there was the other difference compared to localization of PLRV CP which was not detected from the PD (Haupt et al., 2005). However, in the case of BYDV, CP:RFP was detected at PD indicating CP localization to PD (Fig. 3C) even it was not many compared to MP (Fig. 3D). It is worth to note that many plant viruses require CP in addition to MP for cell-to-cell or for long-distance communication (Dolja et al., 1995; Yang et al., 2000), providing a rationale for the possibility of CP’s localizing at PD.
The ΔMP:RFP lack of distal N-terminal region of MP was made to define the important region of MP:RFP for PD targeting (Fig. 1). The deletion mutant showed different subcellular localizations shown from MP:RFP (Fig. 4C, D). The ΔMP:RFP lost PD localization and exhibit red fluorescence at ER (Fig. 4C, D). In addition, while red small granules appeared in cell where MP:RFP was expressed (Fig. 4C-1, blue arrowheads), red small granules granules were not detected from the cell expressing ΔMP:RFP (Fig. 4D). These results combined all may indicates that distal N-terminal region may be the significant key region for the PD trafficking and small granular targeting. Previous researches conducted with full-genomic BYDV by Nass et al. (1995, 1998) support the formation of small granules by MP:RFP.
When BYDV infect barley plant showed slow growth which might be from the death of phloem cells (D’Arcy and Domier, 2000). It has been known that PCD is the essential process of cell death which is a response to changes occurred internally, externally, or both during development for the normal life (Dat et al., 2000; Fink and Cookson, 2005; Greenberg, 1997; Sager and Lee, 2014). Although oxygen is required for plant’s aerobic life, it is the origin of toxic byproducts called ROS generated through general metabolism pathways such as photosynthesis, photorespiration, and respiration in subcellular organelles including chloroplasts, mitochondria, and peroxisomes (Apel and Hirt, 2004; Gill and Tuteja, 2010; Guan et al., 2000; Kwon et al., 2002; Xiong et al., 2002). Since the level of ROS increase because of many kinds of environmental stresses such as oxygen deprivation, temperature, high light intensity and others resulting in PCD (Apel and Hirt, 2004; Blokhina et al., 2003; Jiang and Zhang, 2002), ROS has been used as an indicator of PCD. Visualization of ROS production by DAB staining and the ion leakage measurement have been used for PCD indicator (Tran et al., 2014). In this study, we were able to correlate PCD with the DAB staining and the ion leakage (Fig. 7, 8).
It has been known PCD is well known common defense mechanism used by plants against infecting plant viruses to inhibit the systemic infection of viruses (Carr et al., 2010). It is common that CP functions as an elicitor of host defense mechanism initiating HR that causes necrosis and PCD at virus infection sites (Carr et al., 2010; Tran et al., 2014) in virus and host-pspecific manner for TMV (Berzal-Herranz et al., 1995; Culver and Dawson, 1991; Komatsu et al., 2010). However, interestingly, during conducting the experiment to investigate the subcellular localization of MP and CP (Fig. 5), PCD phenomena were observed from the N. benthamiana and N. tabaccum leaves infiltrated with Agrobacterium to express MP:RFP but plants expressing ΔMP:RFP or expressing CP:RFP did not show. It seemed that intact MP:RFP was responsible for PCD but CP did not involve in PCD. To clarify the observation, a separate set of experiments was conducted for PCD study (Fig. 5,-8).
The concentration of Agrobacterium has been considered as an important factor when researchers transiently express interesting proteins through Agroinfiltration. The result of the optimization experiment was seemingly agreed with previous studies (Amoah et al., 2001; Hosein et al., 2012; Kwon et al., 2002; Tran et al., 2014). Many previous studies revealed that OD600 values, an indication of the concentration of Agrobacterium, from 0.5 to 1.0 displayed the best experimental result (Amoah et al., 2001; Hosein et al., 2012; Tran et al., 2014; Wydro et al., 2006). As the OD600 values induced PCD/HR were between 0.4 and 1.0, minimum optical density inducing distinct PCD was determined to 0.4 and used for rest experiment to minimize side effect which might occur by use of high concentration of Agrobacterium.
As mentioned above, red fluorescence was associated with many PD and many small vesicles when MP:RFP was expressed in cells of N. benthamiana, whereas red fluorescence was no association with PD and with small vesicles when ΔMP:RFP was expressed in cells of N. benthamiana. Interestingly, MP:RFP localized to PD and small granules showed PCD phenomenon but no PCD for ΔMP:RFP with different targeting compared to MP:RFP, suggesting that modulation of the protein localization linked to the change of physiological functions within cells against viral protein expression. It is not surprising that mutation regulates host-pathogen interaction via altering protein trafficking. As reported by Ju et al. (2007), mutation of viral protein alters subcellular localization resulting in inhibiting virus infection. Deletion mutations of PVX TGBp2 in the central region eliminated the formation capability of small granular vesicles which was typical for PVX TGBp2 was expressed and also inhibited virus movement.
The study results combined all together may suggest that localization of BYDV MP modulates PCD or these two events are highly linked to the development of disease resistance.
Acknowledgments
This research was supported by Rural Development Administration fund PJ01004202, Republic Korea.
Fig. 1
The schematic maps of plasmid vectors used in this study for the transient expression of gene. (A) pV15-RFP is made by inserting RFP gene between XbaI and BamHI of pV15 (pPZP-P35S-3′PinII-Bar). (B) pV15-CP:RFP and -MP:RFP are modified plasmid vector at “A” by inserting coat protein (CP) and movement protein (MP) into XbaI site, respectively. (C) pPZP-P35TP-LxAB used to make plasmid at “D”. (D) Plasmid vector to express Barley yellow dwarf virus (BYDV) MP and its mutant (ΔMP). MP is intact MP of BYDV, no mutation. The amino acids of MP from 1 to 55 (dotted line) were deleted for ΔMP.
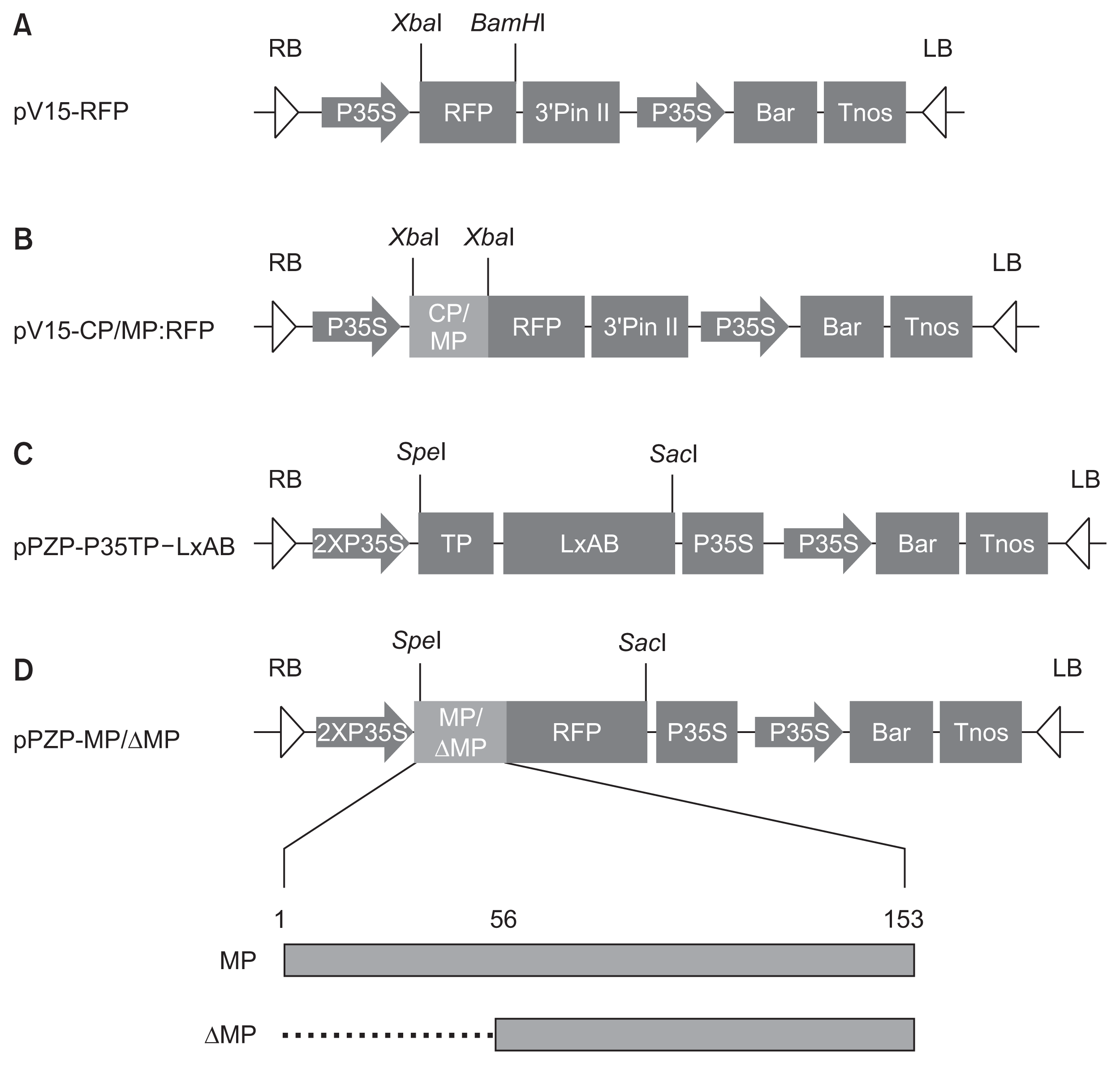
Fig. 2
Sequence alignment of movement proteins (MPs) of different Barley yellow dwarf virus (BYDV) strains. Highly conserved amino acid sequences of MP were identified by aligning multiple sequences of BYDV MP acquired from National Center for Biotechnology Information database using “Clustal Omega” Program (http://www.ebi.ac.uk/Tools/msa/clustalo/). Conserved sequence regions of MPs of BYDV strains are highlighted with different colors. Asterisks indicate BYDV strain used in this study.

Fig. 3
Observation of subcellular localization of CP:RFP and MP:RFP in the leaf cell of the Nicotiana benthamiana under an epifluorescence microscope. (A) Negative control (buffer infiltrated leaf). (B) RFP expressing in leaf cells. (C) CP:RFP expressing in leaf cells. (D) MP:RFP expressing in leaf cells. Blue arrowheads, white arrowheads, and white arrows indicate cytoplasm, plasmodesmata, and nucleus, respectively. CP, coat protein; MP, movement protein.
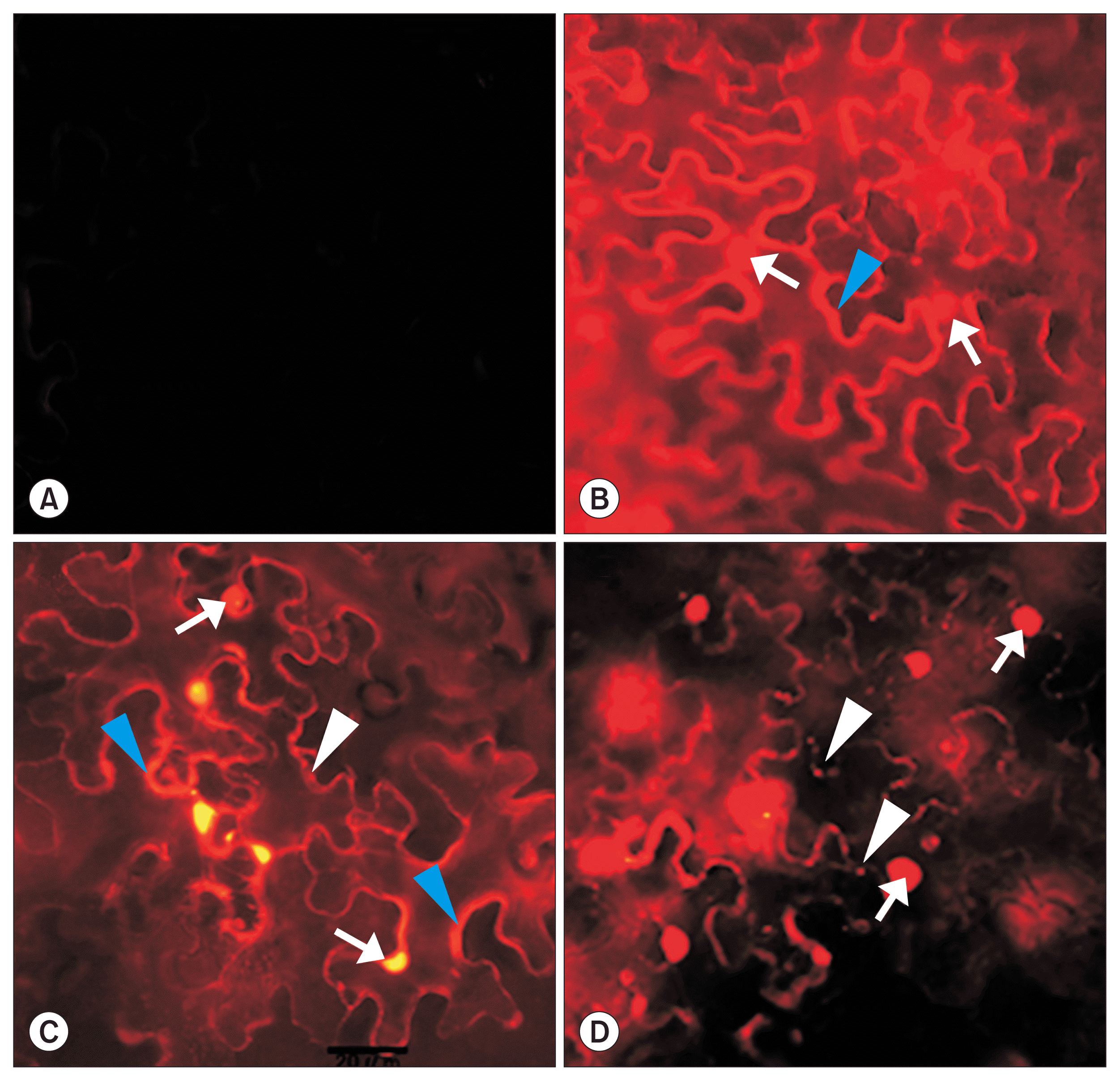
Fig. 4
Confocal microscopy of subcellular localization of movement protein (MP) and its deletion mutation (ΔMP) in leaf’s cell of Nicotiana benthamiana. (A) Negative control (buffer treated leaf). (B) RFP expressing in leaf cells. (C) MP:RFP expressing in leaf cells. (D) ΔMP:RFP expressing in leaf cells. White arrowheads, white arrows, blue arrowheads, and blue arrow indicate nucleus, plasmodesmata, unidentified vesicle, and cytoplasmic stream, respectively. Image in the white box of each panel (C-1, C-2, D-1) was magnified to highlight.
Fig. 5
Appearance of leaf showing programmed cell death by expressing the viral protein fused to RFP and diaminobenzidine (DAB) stained leaf. Viral protein is designate in yellow circles on each leaf. (A-C) Nicotiana benthamiana. (D, E) N. tabaccum. (B) Appearance of leaf “A” at 48 h after Agroinfiltration. (C) DAB stained leaf shown at “B”. Cell death area is indicated in dotted line. MP, movement protein; CP, coat protein.

Fig. 6
Physical appearance according to the different concentrations of Agrobacterium to express movement protein. (A, B) Nicotiana benthamiana. (C) N. tabaccum. (A, C) Physical appearance of leaf at 48 h after Agroinfiltration. (B) Physical appearance of leaf “A” which was applied diaminobenzidine (DAB) staining. The left side of each leaf, was infiltrated with RFP and the right side was infiltrated with MP:RFP (WT). Concentration of Agrobacterium was adjusted based on optical density at 600 nm (OD600 = 0.2, 0.4, 0.6, 0.8, 1.0 and C [buffer infiltrated as negative control]). MP, movement protein.
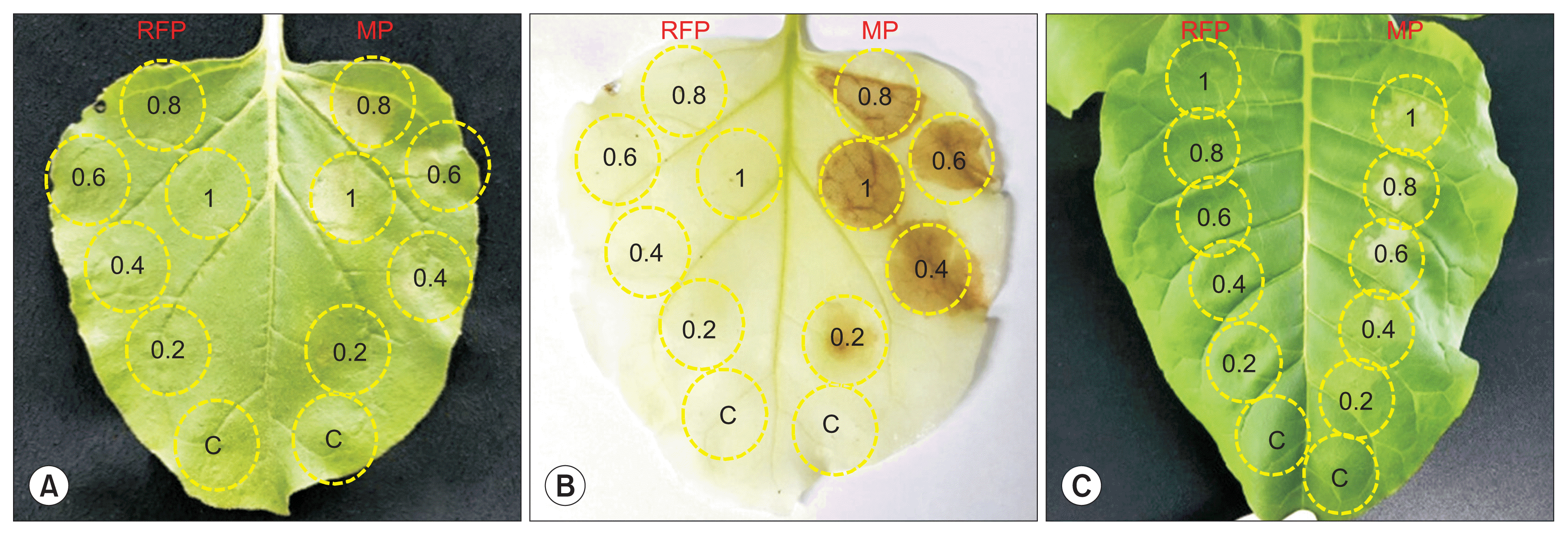
Fig. 7
Appearance of leaves expressing MP:RFP and its mutant according to different time. On each leaf, the upper left side, the lower left side, the upper right side and the lower right side are expressing no protein (as negative control [buffer infiltrated]), MP:RFP (WT), ΔMP:RFP, and RFP, respectively. (A-C) Nicotiana benthamiana leaves. (C) Physical appearance of leaf “B” which was applied diaminobenzidine (DAB) staining. (D, E) N. tabaccum leaves. Cont., control; MP, movement protein.

Fig. 8
Cell death monitoring by ion leakage. The leaf discs were obtained from Nicotiana benthamiana infiltrated with buffer (mock), RFP, ΔMP:RFP, or MP:RFP at 0 and 48 h after infiltration (hai). MP, movement protein.
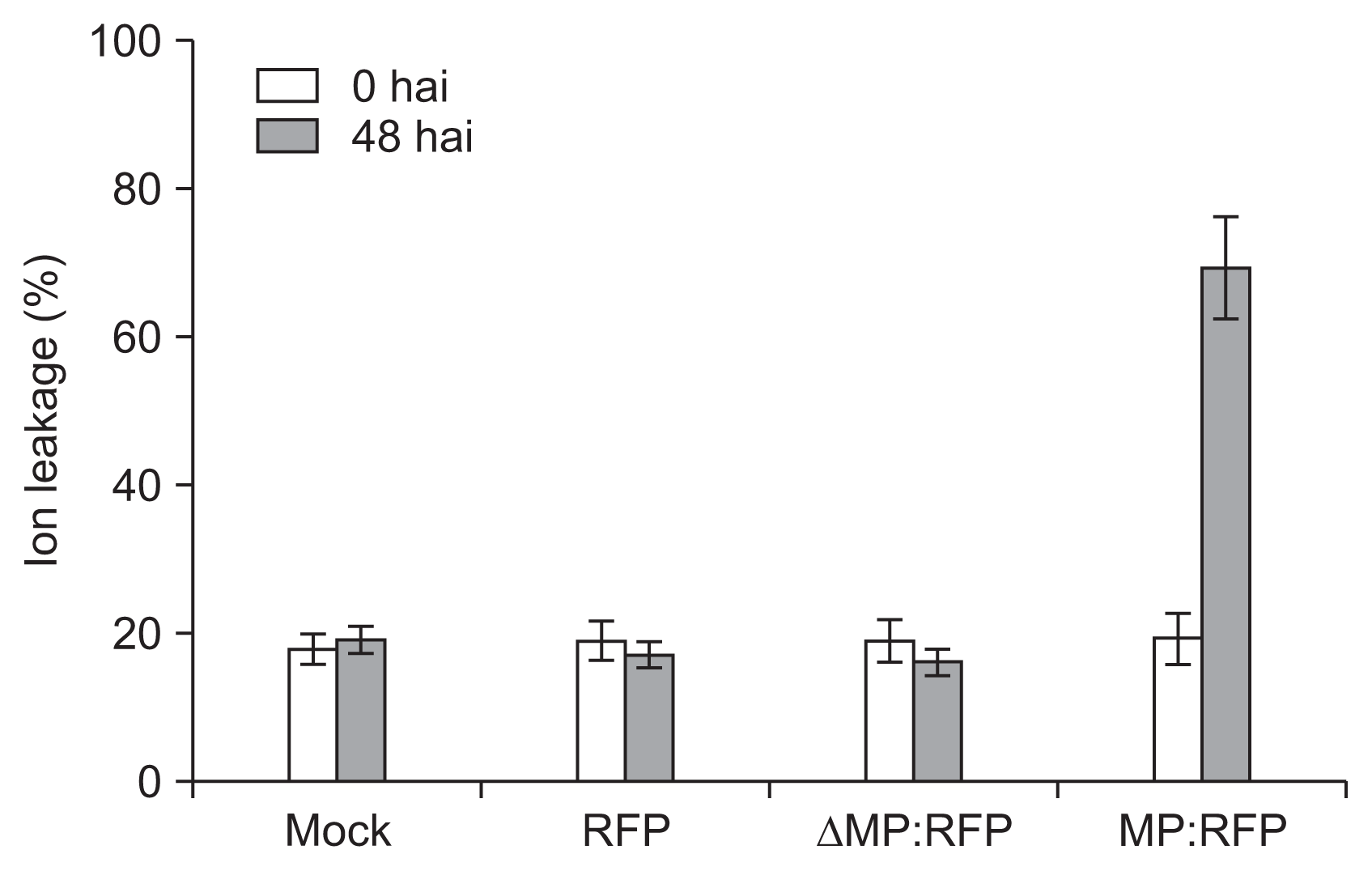
Table 1
Primer sets used for cloning of coat protein (CP), movement protein (MP), and deletion mutant of MP (ΔMP)
References
Agrios, GN 2006. Plant pathology. 5th ed. World Science, Seoul, Korea. 929.(Trans., in Korean).
Amoah, BK, Wu, H, Sparks, C and Jones, HD 2001. Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot. 52:1135-1142.


Apel, K and Hirt, H 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373-399.


Baker, CJ and Orlandi, EW 1995. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 33:299-321.


Baulcombe, DC, Gilbert, J, Goulden, M, Köhm, B and Cruz, SS 1994. Molecular biology of resistance to Potato virus X in potato. Biochem Soc Symp. 60:207-218.

Beachy, RN 1999. Coat-protein-mediated resistance to tobacco mosaic virus: discovery mechanisms and exploitation. Philos Trans R Soc Lond B Biol Sci. 354:659-664.




Bendahmane, A, Köhn, BA, Dedi, C and Baulcombe, DC 1995. The coat protein of potato virus X is a strain-specific elicitor of Rx1-mediated virus resistance in potato. Plant J. 8:933-941.


Bendahmane, A, Querci, M, Kanyuka, K and Baulcombe, DC 2000. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. Plant J. 21:73-81.



Bendahmane, M, Fitchen, JH, Zhang, G and Beachy, RN 1997. Studies of coat protein-mediated resistance to tobacco mosaic tobamovirus: correlation between assembly of mutant coat proteins and resistance. J Virol. 71:7942-7950.




Berzal-Herranz, A, de la Cruz, A, Tenllado, F, Díaz-Ruíz, JR, López, L, Sanz, AI, Vaquero, C, Serra, MT and García-Luque, I 1995. The Capsicum L3 gene-mediated resistance against the tobamoviruses is elicited by the coat protein. Virology. 209:498-505.


Blokhina, O, Virolainen, E and Fagerstedt, KV 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 91:179-194.



Carr, JP, Lewsey, MG and Palukaitis, P 2010. Signaling in induced resistance. Adv Virus Res. 76:57-121.


Chay, CA, Gunasinge, UB, Dinesh-Kumar, SP, Miller, WA and Gray, SM 1996. Aphid transmission and systemic plant infection determinants of Barley yellow dwarf luteovirus-PAV are contained in the coat protein readthrough domain and 17-kDa protein, respectively. Virology. 219:57-65.


Chen, MH, Sheng, J, Hind, G, Handa, AK and Citovsky, V 2000. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 19:913-920.



Citovsky, V and Zambryski, P 1991. How do plant virus nucleic acids move through intercellular connections? BioEssays. 13:373-379.


Clark, WG, Fitchen, JH and Beachy, RN 1995b. Studies of coat protein-mediated resistance to TMV. I. The PM2 assembly defective mutant confers resistance to TMV. Virology. 208:485-491.


Clark, WG, Fitchen, J, Nejidat, A, Deom, CM and Beachy, RN 1995a. Studies of coat protein-mediated resistance to tobacco mosaic virus (TMV). II. Challenge by a mutant with altered virion surface does not overcome resistance conferred by TMV coat protein. J Gen Virol. 76:2613-2617.


Cruz, SS, Roberts, AG, Prior, DA, Chapman, S and Oparka, KJ 1998. Cell-to-cell and phloem-mediated transport of potato virus X: the role of virions. Plant Cell. 10:495-510.



Culver, JM and Dawson, WO 1991. Tobacco mosaic virus elicitor coat protein genes produce a hypersensitive phenotype in transgenic Nicotiana sylvestris plants. Mol Plant-Microbe Interact. 4:458-463.

Dat, J, Vandenabeele, S, Vranová, E, van Montagu, M, Inzé, D and van Breusegem, F 2000. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 57:779-795.



Deom, CM, He, XZ, Beachy, RN and Weissinger, AK 1994. Influence of heterologous tobamovirus movement protein and chimeric-movement protein genes on cell-to-cell and long-distance movement. Virology. 205:198-209.


Ding, B, Turgeon, R and Parthasarathy, MV 1992. Substructure of freeze-substituted plasmodesmata. Protoplasma. 169:28-41.


Dolja, VV, Haldeman-Cahill, R, Montgomery, AE, Vandenbosch, KA and Carrington, JC 1995. Capsid protein determinants involved in cell-to-cell and long distance movement of Tobacco etch potyvirus. Virology. 206:1007-1016.


Fink, SL and Cookson, BT 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 73:1907-1916.




Gechev, TS, Van Breusegem, F, Stone, JM, Denev, I and Laloi, C 2006. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 28:1091-1101.


Gill, SS and Tuteja, N 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 48:909-930.


Goodin, MM, Dietzgen, RG, Schichnes, D, Ruzin, S and Jackson, AO 2002. pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31:375-383.


Goodman, RN and Novacky, AJ 1994. The hypersensitive reaction in plants to pathogens: a resistance phenomenon. American Phytopathological Society, St. Paul, MN, USA. 256.
Govrin, EM and Levine, A 2000. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 10:751-757.


Greenberg, JT 1997. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 48:525-545.


Guan, LM, Zhao, J and Scandalios, JG 2000. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22:87-95.


Haupt, S, Stroganova, T, Ryabov, E, Kim, SH, Fraser, G, Duncan, G, Mayo, MA, Barker, H and Taliansky, M 2005. Nucleolar localization of potato leafroll virus capsid proteins. J Gen Virol. 86:2891-2896.


Hosein, FN, Lennon, AM and Umaharan, P 2012. Optimization of an Agrobacterium-mediated transient assay for gene expression studies in Anthurium andraeanum. J Am Soc Hortic Sci. 137:263-272.

Itaya, A, Hickman, H, Bao, Y, Nelson, R and Ding, B 1997. Cell-to-cell trafficking of cucumber mosaic virus movement protein: green fluorescent protein fusion produced by biolistic gene bombardment in tobacco. Plant J. 12:1223-1230.

Jiang, M and Zhang, J 2002. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot. 53:2401-2410.


Ju, HJ, Brown, JE, Ye, CM and Verchot-Lubicz, J 2007. Mutations in the central domain of potato virus X TGBp2 eliminate granular vesicles and virus cell-to-cell trafficking. J Virol. 81:1899-1911.



Komatsu, K, Hashimoto, M, Ozeki, J, Yamaji, Y, Maejima, K, Senshu, H, Himeno, M, Okano, Y, Kagiwada, S and Namba, S 2010. Viral-induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol Plant-Microbe Interact. 23:283-293.


Kwon, SY, Jeong, YJ, Lee, HS, Kim, JS, Cho, KY, Allen, RD and Kwak, SS 2002. Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant Cell Environ. 25:873-882.


Lazarowitz, SG and Beachy, RN 1999. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell. 11:535-548.



Levine, A, Tenhaken, R, Dixon, R and Lamb, C 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 79:583-593.


Li, Y, Bao, YM, Wei, CH, Kang, ZS, Zhong, YW, Mao, P, Wu, G, Chen, ZL, Schiemann, J and Nelson, RS 2004. Rice dwarf phytoreovirus segment S6-encoded nonstructural protein has a cell-to-cell movement function. J Virol. 78:5382-5389.




Lim, PO, Ryu, JS, Lee, HJ, Lee, U, Park, YS, Kwak, JM, Choi, JK and Nam, HG 1997. Resistance to tobamoviruses in transgenic tobacco plants expressing the coat protein gene of pepper mild mottle virus (Korean isolate). Mol Cells. 7:313-319.


Link, K, Vogel, F and Sonnewald, U 2011. PD Trafficking of potato leaf roll virus movement protein in arabidopsis depends on site-specific protein phosphorylation. Front Plant Sci. 2:18



Lucas, WJ 2006. Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology. 344:169-184.


Lucas, WJ and Lee, JY 2004. Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol. 5:712-726.



May, MJ, Hammond-Kosack, KE and Jones, J 1996. Involvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf-gene-dependent defense response of tomato cotyledons induced by race-specific elicitors of Cladosporium fulvum. Plant Physiol. 110:1367-1379.



Miller, WA, Liu, S and Beckett, R 2002. Barley yellow dwarf virus: Luteoviridae or Tombusviridae? Mol Plant Pathol. 3:177-183.



Nass, PH, Domier, LL, Jakstys, BP and D’Arcy, CJ 1998. In situ localization of barley yellow dwarf virus-PAV 17-kDa protein and nucleic acids in oats. Phytopathology. 88:1031-1039.


Nass, PH, Jakstys, BP and D’Arcy, CJ 1995. In situ localization of barley yellow dwarf virus coat protein in oats. Phytopathology. 85:556-560.

Osbourn, JK, Sarkar, S and Wilson, TM 1990. Complementation of coat protein-defective TMV mutants in transgenic tobacco plants expressing TMV coat protein. Virology. 179:921-925.


Rizhsky, L, Shulaev, V and Mittler, R 2004. Measuring programmed cell death in plants. In: Apoptosis methods and protocols, eds. by HJM Brady, 197-189. Humana Press, Totowa, NJ, USA.

Sager, R and Lee, JY 2014. Plasmodesmata in integrated cell signalling: insights from development and environmental signals and stresses. J Exp Bot. 65:6337-6358.



Sambade, A, Brandner, K, Hofmann, C, Seemanpillai, M, Mutterer, J and Heinlein, M 2008. Transport of TMV movement protein particles associated with the targeting of RNA to plasmodesmata. Traffic. 9:2073-2088.


Satoh, H, Matsuda, H, Kawamura, T, Isogai, M, Yoshikawa, N and Takahashi, T 2000. Intracellular distribution, cell-to-cell trafficking and tubule-inducing activity of the 50 kDa movement protein of Apple chlorotic leaf spot virus fused to green fluorescent protein. J Gen Virol. 81:2085-2093.


Schmitz, J, Stussi-Garaud, C, Tacke, E, Prüfer, D, Rohde, W and Rohfritsch, O 1997. In situ localization of the putative movement protein (pr17) from potato leafroll luteovirus (PLRV) in infected and transgenic potato plants. Virology. 235:311-322.


Taliansky, M, Mayo, MA and Barker, H 2003. Potato leafroll virus: a classic pathogen shows some new tricks. Mol Plant Pathol. 4:81-89.



Tran, PT, Chol, H, Kim, SB, Lee, HA, Choi, D and Kim, KH 2014. A simple method for screening of plant NBS-LRR genes that confer a hypersensitive response to plant viruses and its application for screening candidate pepper genes against Pepper mottle virus. J Virol Methods. 201:57-64.


van Breusegem, F and Dat, JF 2006. Reactive oxygen species in plant cell death. Plant Physiol. 141:384-390.




van Doorn, WG and Woltering, EJ 2005. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 10:117-122.


Vogel, F, Hofius, D and Sonnewald, U 2007. Intracellular trafficking of Potato leafroll virus movement protein in transgenic Arabidopsis. Traffic. 8:1205-1214.


Wang, JY, Chay, C, Gildow, FE and Gray, SM 1995. Readthrough protein associated with virions of Barley yellow dwarf luteovirus and its potential role in regulating the efficiency of aphid transmission. Virology. 20:954-962.

Wong, HL, Pinontoan, R, Hayashi, K, Tabata, R, Yaeno, T, Hasegawa, K, Kojima, C, Yoshioka, H, Iba, K, Kawasaki, T and Shimamoto, K 2007. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 19:4022-4034.




Wydro, M, Kozubek, E and Lehmann, P 2006. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta Biochimica Polonica. 53:289-298.



Xia, Z, Cao, R, Sun, K and Zhang, H 2012. The movement protein of barley yellow dwarf virus-GAV self-interacts and forms homodimers in vitro and in vivo. Arch Virol. 157:1233-1239.



Xiong, L, Schumaker, KS and Zhu, JK 2002. Cell signaling during cold, drought, and salt stress. Plant Cell. 14:Suppl. S165-S183.



Xiong, R, Wu, J, Zhou, Y and Zhou, X 2008. Identification of a movement protein of the tenuivirus rice stripe virus. J Virol. 82:12304-12311.




Xu, SJ, Banks, PM, Dong, YS, Zhou, RH and Larkin, PJ 1994. Evaluation of Chinese Triticeae for resistance to barley yellow dwarf virus (BYDV). Genet Resour Crop Evol. 41:35-41.


- TOOLS
-
METRICS

- Related articles
-
Seed Transmission of
Tomato yellow leaf curl virus in White Soybean (Glycine max )2017 ;33(4)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



