Efficacy of Tissue Culture in Virus Elimination from Caprifig and Female Fig Varieties (Ficus carica L.)
Article information
Abstract
Fig mosaic disease (FMD) is a viral disease that spreads in all Tunisian fig (Ficus carica L.) orchards. RT-PCR technique was applied to leaf samples of 29 fig accessions of 15 fig varieties from the fig germplasm collection of High Agronomic Institute (I.S.A) of Chatt-Mariem, to detect viruses associated to FMD. Analysis results show that 65.5% of the accessions (19/29) and 80.0% (12/15) of the fig varieties are infected by FMD-associated viruses. From all fig accessions, 41.4% of them are with single infection (one virus) and 24.1% are with multi-infections (2 virus and more). Viruses infecting fig leaf samples are Fig mosaic virus (FMV) (20.7%), Fig milde-mottle-associated virus (FMMaV) (17.25%), Fig fleck associated virus (FFkaV) (3.45%), and Fig cryptic virus (FCV) (55.17%). A reliable protocol for FCV and FMMaV elimination from 4 local fig varieties Zidi (ZDI), Soltani (SNI), Bither Abiadh (BA), and Assafri (ASF) via in vitro culture of 3 meristem sizes was established and optimized. With this protocol, global sanitation rates of 79.46%, 65.55%, 68.75%, and 70.83% respectively for ZDI, SNI, BA, and ASF are achieved. For all sanitized varieties, the effectiveness of meristem culture for the elimination of FCV and FMMaV viruses was related to meristem size. Meristem size 0.5 mm provides the highest sanitation rates ranging from 70% to 90%.
Introduction
Fig tree (Ficus carica L.) is a valuable Mediterranean fruit crop for farmers and consumers. In Tunisia, fig tree has been cultivated traditionally since many decades and covers all areas throughout the country (Mars et al., 2008). Fig plantations cover a total area of 18,000 ha, with an annual production of about 25,000 tons (FAOSTAT, 2015). In spite of its importance, fig sector is facing serious constraints due essentially to biotic and abiotic stresses, the lack of selected caprifigs, the intensive urbanization and phytosanitary problems (Mars, 2003). Furthermore, fig tree is threatened by attacks of numerous insects and diseases on leaves and fruits causing loss of production. Among diseases, fig mosaic disease (FMD) is the viral infection the most spread all over the world (Ishikawa et al., 2012; Tzanetakis et al., 2010) and considered as serious economically problem (Yakoubi et al., 2007). The first studies of the FMD were realized by Condit and Horn (1933), who indicated the viral nature of the disease. The pathogens causing FMD can be transmitted by vegetative propagation of infected cuttings and by an eriophyid mite, Aceria ficus Cotte (Flock and Wallace, 1955). It was reported that enveloped round bodies known as double membrane bodies are associated with mosaic symptoms (Appiano et al., 1995). These round bodies were identified as particles of Fig mosaic virus (FMV) (Elbeaino et al., 2009a; Walia et al., 2009).
Actually, ten viruses, of different genera, associated to FMD have been identified in fig trees: Emaravirus, FMV; Ampelovirus, Fig leaf mottle-associated virus-2 (FLMaV-2); Trichovirus, Fig latent virus-1 (FLV-1); Closterovirus, Fig leaf mottle-associated virus-1 (FLMaV-1), Arkansas fig closterovirus-1 (AFCV-1), Arkansas fig closterovirus-2 (AFCV-2), and Fig milde-mottle-associated virus (FMMaV); Alphacryptovirus, Fig cryptic virus (FCV); Maculavirus, Fig fleck associated virus (FFkaV); and Badnavirus, Fig badnavirus-1 (FBV-1) (Elbeaino et al., 2007, 2009a, 2011a, 2011b; Gattoni et al., 2009; Laney et al., 2012; Tzanetakis et al., 2010).
In Tunisia, viruses reported to be associated to FMD are: FLMaV-1 (Nahdi et al., 2006), FLMaV-2 (Elbeaino et al., 2009b), FCV and FFkaV (Elbeaino et al., 2011a, 2011b), FMV (Elair et al., 2012), and FMMaV and FLV-1 (Elair et al., 2013). The FCV and the FMMaV were mentioned to be spread in many Tunisian fig orchards with relatively high infection rates (17.5% and 10.7%, respectively) (Elair et al., 2015; Elbeaino et al., 2011a), which inhibits the development of healthy orchards and limits the production. Meristem culture, as an effective tool for virus eradication from horticultural plants (Comlekcioglu et al., 2007; Miloŝević et al., 2012) is also applied for the production of virus-free fig plants and particularly FCV and FMMaV-free fig trees. Various molecular techniques are nowadays very efficient in the detection of viruses or viral particles in plants (Elbeaino et al., 2006; Elçi et al., 2013; Nam et al., 2015; Tzanetakis and Martin, 2008). RT-PCR was, therefore, an accuracy technique applied by many authors to detect FMD viruses (Çaglayan et al., 2009; Elair et al., 2012, 2013; Walia et al., 2014). Recently, this molecular technique was successfully applied for the detection of FCV and FMMaV on fig leaves of many countries (Aldhebiani et al., 2015; Elbeaino et al., 2011a). The application of this technique to leaf samples of some sanitized Tunisian fig varieties gives information about the reliability of the in vitro protocol used in virus elimination from in vitro plantlets. Therefore, this study aims to determine, by RT-PCR, the major viruses in some Tunisian local fig varieties and to prove, by this technique, the efficacy of meristem culture in the elimination of FCV and FMMaV from four major fig varieties.
Materials and Methods
Leaf sample collection and RT-PCR analysis
Viruses of 29 fig trees of 15 fig varieties in a germplasm collection of the High Agronomic Institute (I.S.A) of Chott-Mariem were detected. From each fig tree, a leaf sample of 5 to 10 leaves was randomly collected and analysed by RT-PCR for the presence of seven fig viruses: FLMaV-1, FMMaV, FLV-1, FLMaV-2, FCV, FMV, and FFkaV (Table 1). Total nucleic acids were extracted from 0.5 g of the main leaf veins using the silica capture protocol (Foissac et al., 2001), the tissues were homogenized in 1 ml grinding buffer (4.0 M guanidine thiocyanate, 0.2 M sodium acetate, 1 M potassium acetate, pH 5.2, 25 mM EDTA, 2.5% PVP-40 [w/v]). The extract was added to 6 M sodium iodide and 0.15 M sodium sulphite, 150 μl ethanol and 35 μl of silica particles suspension. After stripping by heat treatment in sterile water (70°C for 3 min), suspension were centrifuged and total nucleic acids were recovered and stored at −20°C until use.
A two-step protocol was used for the reverse transcription (RT) and amplification (PCR) of target RNA. Reverse transcription was performed using 1 μl of Moloney murine leukaemiavirus reverse transcriptase (M-MLV 200 units/μl), 4 μl of 5× Fs M-MLV buffer, 2 μl of DTT (0.1 M) and 0.5 μl of dNTPs (10 mM). The mixture was incubated at 39°C for 1 h and at 70°C for 10 min.
Virus detection was done by adding to the synthesized cDNA 2.5 μl of 10× Taq polymerase buffer, 1.5 mM as final concentration of MgCl2, 0.5 μl of 10 mM dNTPs, 0.5 μl of 10 μM sense and antisense of each specific primer (Table 1) (Elbeaino et al., 2006, 2007, 2009a, 2010, 2011a, 2011b), and 0.2 μl of Taq polymerase (5 units/μl) in a final volume of 25 μl. The reaction products were resolved by electrophoresis in 5% TBE polyacrylamide gel electrophoresis (PAGE) and stained with ethidium bromide.
In vitro plant regeneration
After virus molecular analysis of all fig germplasm collection of the High Agronomic Institute of Chott-Mariem, 6 accessions (02 Soltani [SNI], 01 Zidi [ZDI], 02 Bither Abiadh [BA], and 01 Assafri [ASF]) were selected to a sanitation program by meristem culture using the in vitro protocol developed by Bayoudh et al. (2015). With this reliable protocol, all steps of fig meristem initiation, shoot multiplication, vitroplant rooting and acclimatization of varieties Zidi (ZDI), Soltani (SNI), Bither Abiadh (BA), and the pollinator Assafri (ASF) were studied and successfully optimized (Bayoudh et al., 2015).
Results
FMD symptoms on leaves of surveyed fig varieties
Surveys of the fig varietal collection in High Agronomic Institute (I.S.A) of Chatt-Mariem show that fig trees are with mosaic symptoms on young and old leaves. Diseased leaves present large chlorotic spots, discoloration, mottle and necrotic bands surrounding the veins (Fig. 1). The severe attacks by FMD cause the deformation and the distortion of the leaves.
Viruses infecting the germplasm fig varieties
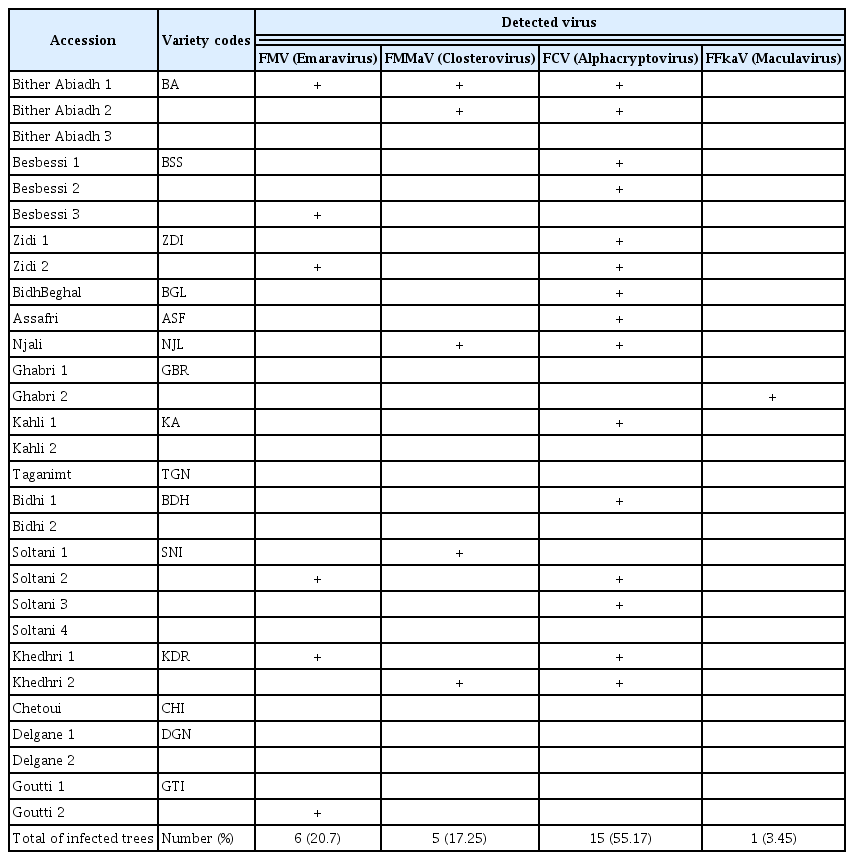
Molecular analyses, by RT-PCR, of leaf samples from 29 accessions of 15 fig varieties in germplasm collection of ISA Chatt-Mariem to detect FMV, FLMaV-1, FLMaV-2, FMMaV, FFkaV, FLV-1, and FCV viruses show that 65.5% of the analyzed accessions (19/29) are infected with FMD viruses (Fig. 2). Analytical results indicate that viruses associated to FMD are: FMV (20.7%), FMMaV (17.25%), FFkaV (3.45%), and FCV (55.17%). The FLMaV-1 and FLMaV-2 viruses are absent in all leaf samples. FCV is the most frequent and widespread virus in all analysed trees and varieties (Table 2). Results show also that 41.4% of accessions are with single infection (one virus) and 24.1% are with multi-infections (2 virus and more). The accession C1 of Bither Abiadh (BA) variety is the unique fig tree infected by three viruses: FMV, FMMaV, and FCV (Table 2).
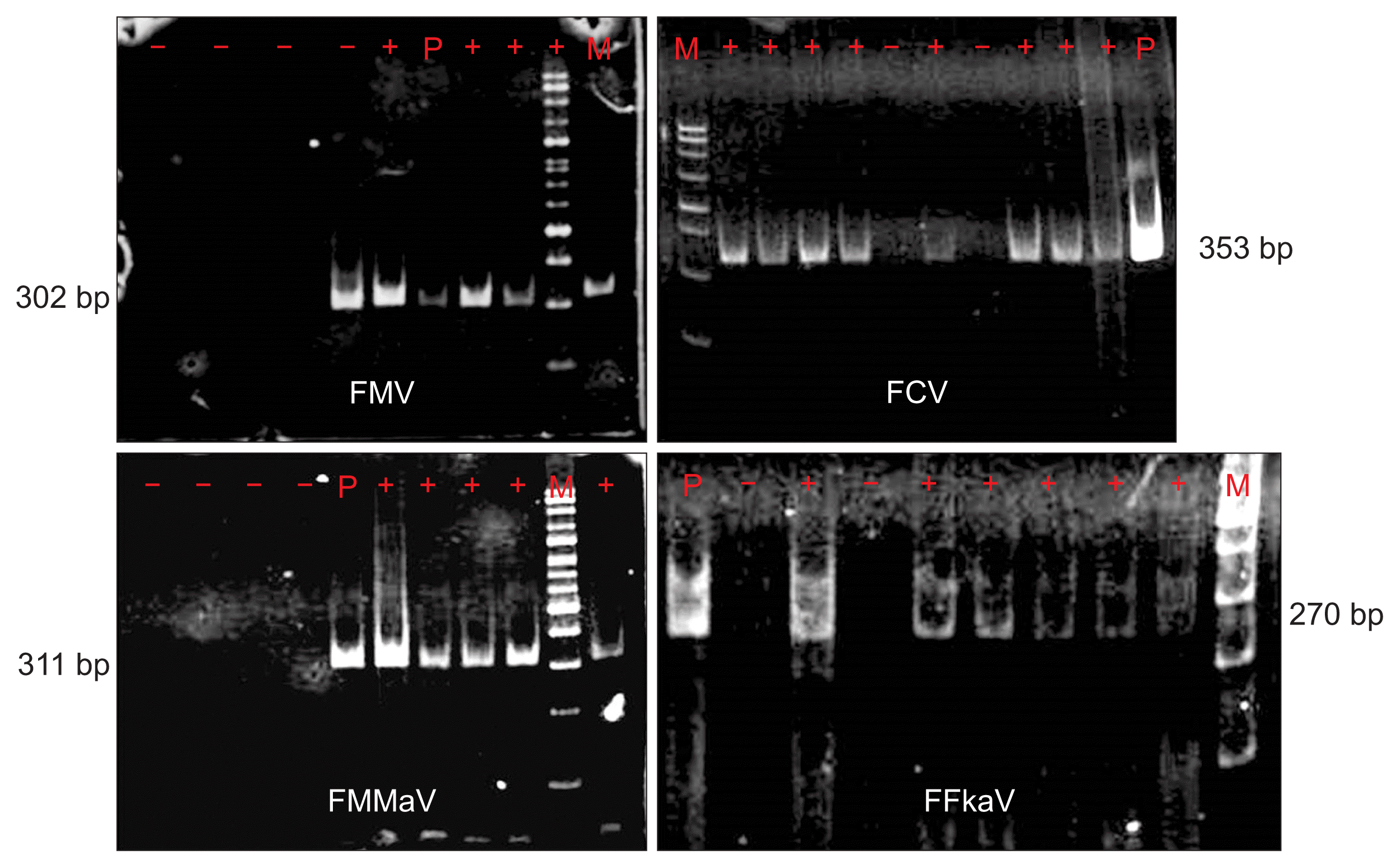
Five percent polyacrylamide gel electrophoretic profiles of RT-PCR products by specific primer amplification of Fig mosaic virus (FMV), Fig cryptic virus (FCV), Fig milde-mottle-associated virus (FMMaV), and Fig fleck associated virus (FF-kaV). +, positive sample; −, negative sample, P, positive control, M, marker 100 bp.
Concerning varieties infection, 80.0% of analysed varieties (12/15) are infected with at least one virus and 66.7% of them are infected by FCV (Table 2). Also, the varieties Soltani (SNI), Khedhri (KDR), and Zidi (ZDI) are infected by 2 viruses: FMV and FCV. The varieties Bither Abiadh (BA), Khedhri (KDR), and Njali (NJL) are infected by the 2 virus FMMaV and FCV. On the other hand, molecular analyzes indicate that varieties Taganimt (TGN), Chetoui (CHI), and Delgane (DGN) does not show any infection by FMD viruses and could be healthy.
FCV and FMMaV elimination by meristem culture
Sanitation global rate
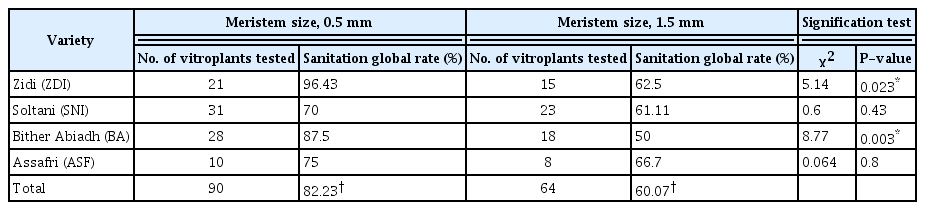
In a previous work related to in vitro fig propagation (Bayoudh et al., 2015), it was possible to establish and to optimize a reliable protocol for rapid micropropagation of 3 local fig varieties Soltani (SNI), Zidi (ZDI), Bither Abiadh (BA), and the caprifig (pollinator) Assafri (ASF) from meristem culture. With this protocol, we proceed to eliminate FCV and FMMaV viruses by regeneration of plantlets from 0.5 mm and 1.5 mm meristem sizes of 2 Soltani accessions, 2 Bither Abiadh accessions, one Zidi accession and one Assafri accession (Table 3). In fact, molecular analyzes by RT-PCR from a total of 154 vitroplants, regenerated from the 6 accessions initially infected by FCV, show that 77.27% of plantlets (119/154) were totally free of this virus and that only 22.73% (35/154) are infected. Also, in vitro culture of meristem size 0.5 mm was effective in removing FMMaV from 84.21% of regenerated plants of varieties Bither Abiadh and Soltani (32/38). The efficiency of meristem culture in viral sanitation seems widely related to meristem size and to variety to clean.
Variation of sanitation rates with varieties
The global elimination rate of the 2 virus FCV and FMMaV extends from 62.50% (40/64 plantlets) for variety Soltani to 88.89% for variety Zidi (32/36 plantlets). That of Bither Abiadh is 82.60% (38/46 plantlets) and Assafri is 72.22% (13/18 plantlets). The elimination of each virus separately also seems closely related to the sanitized variety. For Bither Abiadh and Soltani, initially infected by FMMaV and sanitized by 0.5 mm meristem size, the removal rate of each virus ranges from 70% for Soltani to 89.28% for Bither Abiadh. Which allow considering variety Bither Abiadh with higher sanitation abilities than Soltani.
Variation of sanitation rates with meristem size
Sanitation of fig plantlets from the most frequent viruses, FCV and FMMaV by meristem culture is totally dependent on the meristem size from which are issued the vitroplants. In fact, the global sanitation rate of vitroplants issued from 0.5 mm meristems (86.67%) (78/90 plantlets) is significantly higher than that of vitroplants regenerated from 1.5 mm meristem size (60.94%) (39/64 plantlets) (Table 3). For the 4 analyzed varieties, the highest sanitation rates result from 0.5 mm meristem size and the lowest rates are obtained from 1.5 mm meristems. For variety Bither Abiadh (BA) and Zidi (ZDI), we reach high elimination rates of FCV and FMMaV from the meristem size 0.5 mm of 96.43% and 87.5%, respectively. The lowest sanitation rate (50%) is obtained from meristem size 1.5 mm of BA variety. While sanitation rates of SNI, ZDI, and ASF vitroplants from 1.5 mm meristem size are very close and are 61.11%, 62.5%, and 66.7%, respectively.
Discussion
This work shows the accuracy of RT-PCR to detect viruses associated with FMD and the efficacy of in vitro tissue culture to eliminate frequent and severe viruses FCV and FMMaV from four major fig varieties BA, ZDI, SNI and ASF. Surveys carried out in fig germplasm collection of High Agronomic Institute (I.S.A) of Chatt-Mariem show the spread of FMD in almost all the varieties and the majority of the trees. Tunisian fig varieties (Saddoud et al., 2006) and most surveyed trees are virus infected and contain fig mosaic symptoms (Elair et al., 2014). The same findings of existence and dissemination of the disease in Spain, England, Albania, Cyprus, Greece, Turkey, Yemen, Algeria, Morocco, Mexico, Syria, Lebanon, Australia, South Africa, Italy, Japan, America, Saudi Arabia, Iran, Egypt, China and Palestine have been reported (Alhudaib, 2012; Alkowni et al., 2015; Elbeaino et al., 2007, 2009a, 2012). It was confirmed that global FMD attack rates and severities are not stable during the years and vary from the seasons. These variations depend mainly on the atmospheric conditions of the prospection year, the surveyed varieties and the vigorous state of the trees (Bayoudh et al., 2014). The high FMD severities of fig trees in surveyed fields prompted the need to gather information on the viruses infecting symptomatic trees in the main fig-growing areas. For this aim, we seek for the presence of seven fig infecting viruses (Table 1), by RT-PCR, in a fig germplasm collection containing varieties from main Tunisian production regions. All analyzed samples are free from FLV-1, FLMaV-1, and FLMaV-2. Similar findings are reported by Nahdi and Aljane (2014) for the non detection of FLV-1 and FLMaV-2 in fig orchards in the south region of Tunisia. In other analyze reports; FLV-1 (Elair et al., 2015), FLMaV-1 (Nahdi et al., 2006), and FLMaV-2 (Elbeaino et al., 2009b) were detected in respectively 32.4%, 28.8%, and 5.7% of Tunisian fig samples. These 3 viruses are also infecting fig orchards of many other countries (Danesh-Amuz et al., 2013; Elbeaino et al., 2009b, 2012).
The RT-PCR technique was very efficient to detect 4 viruses infecting fig samples of the germplasm collection (Fig. 2): FFkaV (3.45%), FMMaV (17.25%), FMV (20.7%), and FCV (55.17%). In this context, same viruses were detected, by RT-PCR, in some other fig Tunisian fields and other Mediterranean countries by many authors. Elbeaino et al. (2011a, 2011b) reported the presence of FFkaV (22.5%) and FCV (17.5%) in samples of Tunisian fig orchards, where it does not seem to induce a visible disease. FMV and FMMaV were recently detected by Elair et al. (2013, 2015) in many Tunisian regions.
The multiple infections of some fig varieties could confirm their susceptibility to FMD viruses (Elair et al., 2014). On the other side, it seems clear that the most cultivated and more appreciated varieties in Tunisia are in a critical virological status. In fact, the majority of accessions, Zidi, Soltani, Kahli, Goutti, and Bither Abiadh are highly infected, which urges to establish a sanitation and certification program of local fig varieties, used for large-scale installation of healthy and quality orchards.
Meristem culture is considered to be an important tool for virus disease eradication from many species (Faccioli and Marani, 1998; Miloŝević et al., 2012). The efficacy of FCV and FMMaV elimination by meristem culture is highly related to meristem size. The highest elimination virus rates, from all varieties, are obtained with smallest meristem sizes. Similar findings for fig virus elimination via meristem sizes are also reported by Chiumenti et al. (2013) and Chalak et al. (2015). This feature may be due to the fact that meristem tips in an elongating shoot are generally not connected to the vascular system of the plant and therefore are not contaminated by viruses that travel through the vascular system (Youssef et al., 2009). In addition, fig vitroplants obtained in our conditions are vigorous, with high quality and strong root system. Which was able to register high survival rates in the acclimatization step. The acclimated plants transferred to greenhouse, show no signs of somaclonal variation.
The efficiency of meristem culture in eliminating fig virus can be optimized by combining thermotherapy to this technique. Further researches on applying thermotherapy with in vitro tissues culture is to be considered to investigate the potential of these two processes in improving the elimination rates of FCV and FMMaV from fig vitroplants.