 |
 |
| Plant Pathol J > Volume 33(3); 2017 > Article |
Abstract
To develop a commercial product using the mycoparasitic fungus Simplicillium lamellicola BCP, the scaleup of conidia production from a 5-l jar to a 5,000-l pilot bioreactor, optimization of the freeze-drying of the fermentation broth, and preparation of a wettable powder-type formulation were performed. Then, its disease control efficacy was evaluated against gray mold diseases of tomato and ginseng plants in field conditions. The final conidial yields of S. lamellicola BCP were 3.3 ├Ś 109 conidia/ml for a 5-l jar, 3.5 ├Ś 109 conidia/ml for a 500-l pilot vessel, and 3.1 ├Ś 109 conidia/ml for a 5,000-l pilot bioreactor. The conidial yield in the 5,000-l pilot bioreactor was comparable to that in the 5-l jar and 500-l pilot vessel. On the other hand, the highest conidial viability of 86% was obtained by the freeze-drying method using an additive combination of lactose, trehalose, soybean meal, and glycerin. Using the freeze-dried sample, a wettable powder-type formulation (active ingredient 10%; BCP-WP10) was prepared. A conidial viability of more than 50% was maintained in BCP-WP10 until 22 weeks for storage at 40┬░C. BCP-WP10 effectively suppressed the development of gray mold disease on tomato with control efficacies of 64.7% and 82.6% at 500- and 250-fold dilutions, respectively. It also reduced the incidence of gray mold on ginseng by 65.6% and 81.3% at 500- and 250-fold dilutions, respectively. The results indicated that the new microbial fungicide BCP-WP10 can be used widely to control gray mold diseases of various crops including tomato and ginseng.
Botrytis cinerea is a causative pathogen of gray mold disease, which causes serious damage to vegetables, ornamentals, fruits, and crops throughout the world. This pathogen is well known as a plant pathogenic fungus and has been ranked second in the list of the most important plant pathogenic fungi based on their scientific and economic importance (Choi et al., 2008; Dean et al., 2012; Williamson et al., 2007). Gray mold disease is the most common disease in crops grown in greenhouse conditions. The greenhouse environment, which is optimized for plant growth, also benefits B. cinerea, with the warmth and humidity created by the lack of water vapor and air exchange with the external environment providing ideal infection conditions (Choi et al., 2008; Williamson et al., 2007). Commercial synthetic agrochemicals have been used frequently as a traditional method of controlling gray mold disease, but their frequent use has led to the emergence of resistant B. cinerea and the control of gray mold disease has become more difficult. As an alternative, biofungicidal approaches using antagonistic microorganisms or microorganism-derived natural products have been actively studied, and numerous bio-disinfectant candidates have been reported for the prevention of gray mold diseases (Choi et al., 2009; Hedin, 1982; Le Dang et al., 2014; Moyano et al., 2004). However, there has been no effective bio-disinfectant until now.
The fungus Simplicillium lamellicola BCP, which is a mycoparasitic fungus of B. cinerea, was isolated from B. cinerea mycelia by Choi et al. (2008) and re-identified via nucleotide sequence analyses of the 28S rRNA and internal transcribed spacer regions by Le Dang et al. (2014). The BCP strain can produce the antifungal compound verlamelin, which has a strong and broad-spectrum antifungal activity (Kim et al., 2002b). The spore suspension and culture filtrate of S. lamellicola BCP can significantly reduce the incidence of gray mold disease in several crops such as cucumber, tomato, and strawberry when they are cultivated in growth chambers, greenhouses, and fields (Choi et al., 2008). In addition, BCP cells can rapidly produce a large quantity of spores in a broth medium. All these features suggest the possibility that S. lamellicola BCP could be developed as a commercial biofungicide. However, to develop and commercialize S. lamellicola BCP as a biofungicide, a series of processes such as culture optimization, scale-up for fermentation, optimum formulation, and the evaluation of disease efficacy in field conditions are necessary.
In this study, the culture conditions of S. lamellicola BCP, which has an inhibitory effect on gray mold disease, were optimized in a 5-l jar and scaled-up to a 5,000-l pilot bioreactor for the production of its conidia. In addition, harvest and formulation techniques for improving the storage stability of the conidia were developed and the disease control efficacy of the formulation against gray mold of tomato and ginseng in field conditions was evaluated.
Conidia of S. lamellicola BCP were maintained in 1.2-ml Cryovial┬« tubes (Simport, Montreal, QC, Canada) at ŌłÆ80┬░C to avoid any genetic changes due to successive culturing. For the production of S. lamellicola BCP conidia, a fermentation medium composed of 3% fructose (Duksan Pure Chemicals, Ansan, Korea), 3% soybean meal (Gaemi Food, Seongnam, Korea), 0.5% MgSO4┬Ę7H2O (Duksan Pure Chemicals), 0.5% KCl (Duksan Pure Chemicals), 0.1% K2HPO4 (Duksan Pure Chemicals), and 0.001% FeSO4┬Ę7H2O (Duksan Pure Chemicals) was used. Czapek-Dox broth (CDB; Difco Laboratories, Livonia, MI, USA) was used as a positive control.
For seed cultures, a 1.2-ml Cryovial® tube (Simport) was thawed at 25°C and cultured in a 500-ml Erlenmeyer flask containing 100 ml of fermentation medium. The flasks were incubated on a rotary shaker (IS-971RF; Jeio Tech, Daejeon, Korea) at 25°C and 150 rpm for 48 h. For laboratory-scale fermentation, 3% (v/v) of seed culture was inoculated into a 5-l jar containing 3 l of fermentation medium and equipped with a dissolved-oxygen meter and a pH meter (KoBioTech, Incheon, Korea). Cultivations were performed for 96 h at 25°C with agitation at 300 rpm and an aeration rate of 1 vvm. For the scaled-up 500-l pilot fermentation (KoBioTech), 3% (v/v) of seed culture was transferred into a vessel containing 300 l of fermentation medium. Cultivations were performed for 96 h at 25°C and 150 rpm with an aeration rate of 0.5 vvm. For the 5,000-l scale fermentation (KoBioTech), seed cultures were grown for 48 h in a 500-l pilot vessel as described above. Then, the culture broth was added to a 5,000-l vessel containing 3,000 l of fermentation medium at a ratio of 3% (v/v). The pilot fermentation was performed for 96 h at 25°C and 80 rpm with an aeration rate of 0.5 vvm. To determine the number of conidia in the fermentation broth samples, they were serially diluted with 0.85% NaCl solution. The diluted solutions were spread on potato dextrose agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and the number of conidia was counted after 3 days by the plate viability count method (Oliveira et al., 2015).
To effectively harvest viable S. lamellicola BCP conidia, optimization of the freeze-drying method was performed. To select the optimum additive, various additives and combinations were mixed with the culture broth of S. lamellicola BCP as shown in Table 1. For each of 11 samples, 3 l of the sample solution was placed in a 5-l rectangular aluminum tray, frozen at ŌłÆ20┬░C for 24 h, and then sequentially freeze-dried using a 500-kg freeze dryer (PYTFD-500R; ilShin Lab, Seoul, Korea). The temperature was programmed at ŌłÆ20┬░C for 600 min, ŌłÆ10┬░C for 600 min, 0┬░C for 600 min, ŌłÆ15┬░C for 900 min, and ŌłÆ30┬░C for 1,200 min. The number of conidia in each of the 11 freeze-dried samples was determined as described above. The experiment was repeated twice with three replicates per sample.
The combination of 10% lactose, 7.5% trehalose, 2% soybean meal, and 0.5% glycerin was selected as an optimum additive mixture for the freeze-drying of the fermentation broth of S. lamellicola BCP. Using the freeze-dried sample, a wettable powder-type formulation (BCP-WP10) was prepared; 10 g of the freeze-dried sample was mixed with 89.5 g of flux-calcined diatomaceous earth (MW25, 68855-54-9; Dow AgroSciences, Indianapolis, IN, USA), 0.25 g of polyethylene glycol monomethyl ether acetate (28-00379-01; Wako Pure Chemical Industries, Osaka, Japan), and 0.25 g of 2-(acetoxy[polyethyleneoxy]propyl) heptamethyltrisiloxane (125997-17-3; Jiangxi Hito Chemical Co., Jiangxi, China).
To investigate the stability of conidia in BCP-WP10, it was packed into aluminum packs containing 100 g each and stored at 40┬░C in an oven (FO600M; Jeio Tech) for 22 weeks. To count the number of viable conidia in the formulation, samples were collected at 2-week intervals and serially diluted with 0.85% NaCl solution (Papavizas et al., 1984). The diluted solutions were spread on potato dextrose agar and the number of conidia was counted after 3 days by the plate viability count method (Oliveira et al., 2015). The experiment was repeated twice with three replicates per sample.
To investigate the control effect of BCP-WP10 against tomato gray mold, an experiment was performed in a farmerŌĆÖs greenhouse in Hwaseong-si, Gyeonggi Province, South Korea. Three-week-old tomato plants of the ŌĆśSeokwangŌĆÖ cultivar at the 4- to 5-true-leaf stage were planted on February 16th at a planting distance of 40 cm. BCP-WP10 was applied three times at 500- and 250-fold dilutions at 7-day intervals (calendar dates 3/25/2009, 4/1/2009, and 4/8/2009) by foliar spray. Fludioxonil (suspension concentrate; active ingredient 20%) was applied at a 1,000-fold dilution as a positive control and plants treated with distilled water served as controls. The tomato plants were infected naturally with B. cinerea. Plots of 50 m2 were arranged as a randomized complete block with three replicates per treatment. Disease incidence was determined by counting the number of diseased plants among 30 plants per treatment at 7 days after the third treatment and then the control efficacy was calculated using the following formula:
To evaluate the potential use of BCP-WP10 as a biocontrol agent against ginseng gray mold, a trial was conducted in a farmerŌĆÖs field in Yangpyeong-gun, Gyeonggi Province, South Korea. BCP-WP10 at 500- and 250-fold dilutions was applied three times to 4-year-old ginseng plants (cv. ŌĆśCheng-pungŌĆÖ) at 7-day intervals (calendar dates 4/16/2009, 4/23/2009, and 4/30/2009). Fenhexamid (wettable powder; active ingredient 50%), at a 1,000-fold dilution and water were applied as positive and negative controls, respectively. The plots, consisting of 50 plants per plot, were arranged as a randomized complete block with three replicates per treatment. Disease incidence was determined by counting the number of diseased plants among of 50 plants per treatment at 7 days after the third treatment and then the control efficacy was calculated as described above.
Optimum culture conditions for maximizing the conidial yield of S. lamellicola BCP were investigated in 5-l jar fermenters. Inoculum size, culture temperature and pH, and agitation speed were treated as the key factors for optimization. The highest conidial yield was obtained under culture conditions of 25┬░C and pH 4.5, an impeller speed of 300 rpm, and an inoculum size of 3% (v/v) in a 5-l jar. After 4 days of cultivation, the highest conidial yield of 3.3 ├Ś 109 conidia/ml was obtained. The conidial yield obtained using the optimized fermentation medium was about 33 times higher than that of CDB medium (Table 2). The yield coefficient (Yp/s) in a 5-l jar was 11.0 for cultures using the optimized fermentation medium, whereas a Yp/s of only 0.3 was obtained with CDB medium.
Conidial production was scaled up from a 5-l jar to a 340 5,000-l vessel. An agitation speed of 300 rpm in a 5-l jar corresponded to 150 rpm in a 500-l pilot and 80 rpm in a 5,000-l vessel, respectively. Comparable conidial yields were obtained in the 5-l, 500-l, and 5,000-l bioreactors, with final yields of 3.3 ├Ś 109 conidia/ml for a 5-l jar, 3.5 ├Ś 109 conidia/ml for a 500-l pilot vessel, and 3.1 ├Ś 109 conidia/ml for a 5,000-l vessel, respectively (Fig. 1). The volumetric productivity in a 5,000-l pilot bioreactor was comparable to that in a 5-l jar and a 500-l pilot vessel. These findings indicated that the scale-up of fermentation culture from 5-l to 5,000-l was successfully achieved.
The yield and viability of conidia in the freeze-dried samples varied according to the different additives used. Among the 11 tested additives, the combination of 10% lactose, 7.5% trehalose, 2% soybean meal, and 0.5% glycerin produced the highest conidial yield in a freeze-dried sample (7.1 ├Ś 109 conidia/g) (Table 1). Compared to the number of conidia counted under a light microscope, the conidial viability of that sample was 85.7% and it was used for the preparation of BCP-WP10.
The storage stability of BCP-WP10 was tested at 40┬░C for 22 weeks. The results showed that conidial viabilities of about 50-100% (more than 30.0 ├Ś 107 conidia/g) were maintained for the first 8 months. After that time, the viability dramatically decreased to approximately 17% (5.0 ├Ś 107 conidia/g) after 20 weeks and 8.7% (2.6 ├Ś 107 conidia/g) after 22 weeks (Fig. 2).
The disease control efficacy of BCP-WP10 was investigated against gray mold diseases of tomato and ginseng in farmersŌĆÖ fields. As shown in Fig. 3, BCP-WP10 suppressed the development of tomato gray mold in a dose-dependent manner (Fig. 3). The disease incidence of the untreated control was 26.8%. At 500- and 250-fold dilutions, the control efficacies of the formulation were 64.7% and 82.6%, respectively. The control efficacy of BCP-WP10 at 250-fold dilution was similar to that of fludioxonil.
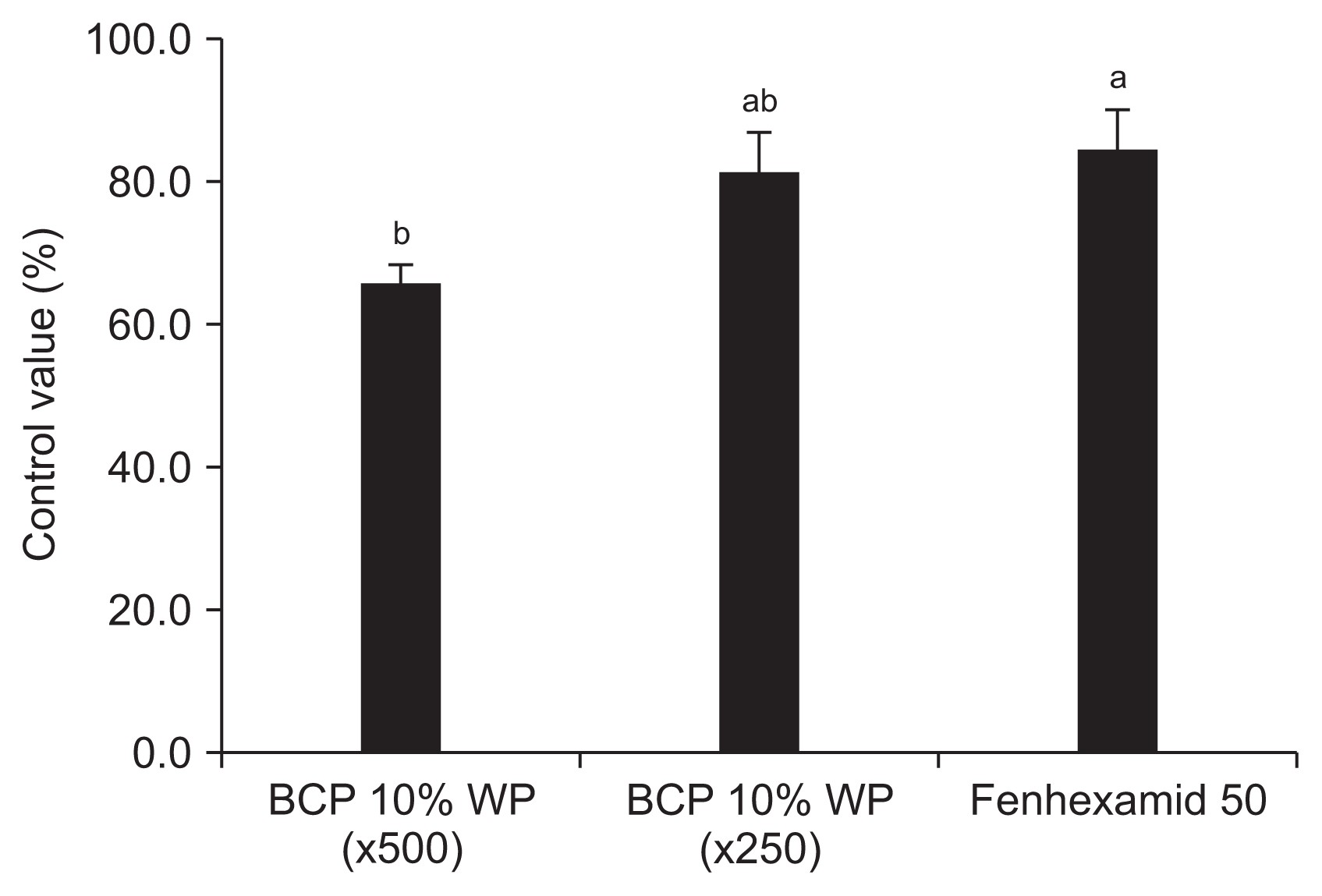
In addition, BCP-WP10 effectively reduced the development of ginseng gray mold. The disease incidence of the untreated control was 21.3%. The control efficacies of BCP-WP10 were 65.6% for the 500-fold dilution treatment and 81.3% for the 250-fold dilution treatment (Fig. 4).
Gray mold is one of the major causes of economic damage to various crops in greenhouse conditions, and it is also damaging to a lesser extent under field conditions. Various chemical pesticides have been used for the control of gray mold in the agricultural ecosystem (Choi et al., 2008; Williamson et al., 2007). However, chemical pesticides have been known to cause environmental pollution, lethal toxicity, and the emergence of resistant pathogens (Chung et al., 2006; Li et al., 2008), leading many scientists to study the development of alternative measures to control gray mold using antagonistic microorganisms (Ahn et al., 2009; Choi et al., 2009; Kim et al., 2007, 2013; Lee et al., 2006; Qu et al., 2016; Wang et al., 2010; Zhao et al., 2003). However, few microbial fungicides have been widely used because the currently available industrialized microbial pesticides may have poor control efficacies for gray mold diseases under field conditions (Hong et al., 2012; Kim et al., 2012). Therefore, to develop a microbial fungicide having a uniform and high control efficacy, it is crucial to develop an optimized pilot fermentation protocol, conidial harvesting process, and formulation (Berger et al., 1996; Hong et al., 2012; Paulitz and B├®langer, 2001).
To the best of our knowledge, it is the first report on industrial development of biocontrol agent using biocontrol fungus S. lamellicola. To meet the line of cost effectiveness, successful scale-up in submerged culture is very essential because volumetric productivity in submerged culture is generally higher than that in solid culture. During scale-up process, however, the reduction of productivity have been reported (Kim et al., 2002a, 2005). As the fermentation scale increased from a 5-l jar to a 300 l vessel, the pigment yield of Monascus J101 was reduced up to 16% (Kim et al., 2002a). In Saccharomyces cerevisiae culture, product yield of enterokinase in a 300-l vessel was lower than that in a 5-l jar (Kim et al., 2005). In this study, the conidia yield of 3.1 ├Ś 109 conidia/ml in a 5,000-l vessel was comparable to that of a 5-l jar fermenter. The difference in conidia yields was only 6%. Considering reduction of product yield in larger scale fermentation, this result is fairly acceptable in the process development, indicating that scale-up process was developed in our study.
The conidial viability of our solid culture (unpublished data) was reduced to 10% of the original by grinding. Eventually, liquid culture using a jar fermenter appeared to have some merits for the cultivation and post-cultivation processes of conidia as compared to solid culture. Additionally, for scale-up to industrial processes, the use of liquid culture in a jar would be more favorable if the problem of a low conidial viability can be solved. In this study, an optimized freeze-drying method for harvesting S. lamellicola conidia from a liquid culture was developed. Several drying methods such as freeze-drying, spray-drying, and convection oven-drying are often used in industries. However, becuase the conidia of S. lamellicola produced in a liquid culture are sensitive to heat, a freeze-drying method was used in this study. When lactose, trehalose, soybean meal, and glycerin were used as additives for the freeze-drying method, the conidial viability was maintained at 85.7%. The BCP-WP10 formulation was prepared and 17% (5.0 ├Ś 107 conidia/g) of conidia survived storage at 40┬░C for 20 weeks. Each 8 weeks of the experimental period is converted to 1 year based on the pesticide registration and storage stability test standard. Considering the effective conidial density, this result indicates that BCP-WP10 should be stable for at least 2 years at room temperature. Thus, these processes could possibly be applied to commercial production.
BCP-WP10 effectively controlled the development of gray mold diseases of tomato and ginseng plants in field conditions. At a 500-fold dilution, BCP-WP10 showed control efficacies of 65% for tomato gray mold and 78% for ginseng gray mold. Kim et al. (2012) reported that one Bacillus biofungicide reduced tomato gray mold incidence by 55.4%. Although the experimental conditions such as disease incidence, treatment frequency, and environment differed from those of our experiment, the disease control efficacy of BCP-WP10 was higher than that of the Bacillus biofungicide. Additionally, the control efficacy of a 250-fold dilution treatment of BCP-WP10 was similar to that of the chemical fungicide fludioxonil. These results suggest that BCP-WP10 can be used to control gray mold disease of tomato plants in an environmentally friendly manner.
Ginseng is a health food and a high-value crop, but it needs a long cultivation period and is susceptible to plant diseases such as gray mold. Many farms depend on the use of chemical fungicides for the control of diseases in ginseng plants. However, since gray mold causes damage not only during plant growth, but also during storage and transportation after harvest (Agrios, 2005), it is essential to develop a biological control agent that can replace chemical fungicides. Many experiments have investigated plant-derived antifungal metabolites (Lee et al., 2001) and antagonistic microorganisms (Yang et al., 2014), but to date there has been no biofungicide that can effectively reduce the development of ginseng gray mold. In this study, the control efficacy of BCP-WP10 for ginseng gray mold was 65.6% at a 500-fold dilution. The control efficacy of BCP-WP10 at a 250-fold dilution (81.3%) was not significantly different from that of the chemical fungicide fenhexamid (84.4%). This indicates that BCP-WP10 can be used as an alternative measure for the control of ginseng gray mold.
In conclusion, processes for the scale-up of fermentation culture from 5-l to 5,000-l, freeze-drying, and wettable powder-type formulation of S. lamellicola BCP were developed in this study. The developed formulation, BCP-WP10, effectively suppressed the incidence of gray mold diseases on tomato and ginseng under field conditions. BCP-WP10 can be used to control gray mold diseases in farmlands where chemical fungicide resistance or residual toxicity exists and the use of chemical pesticides is prohibited. Furthermore, the use of BCP-WP10 as a new biofungicide will reduce the use of chemical fungicides and the costs associated with the development of new chemical fungicides.
Acknowledgments
This research was supported by a grant (315007-03) from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Advanced Production Technology Development Program funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.
Fig.┬Ā1
Cultivation patterns of Simplicillium lamellicola BCP in 5-l (A), 500-l (B), and 5,000-l (C) bioreactors based on a constant impeller tip speed. DOT, dissolved oxygen tension.

Fig.┬Ā2
Storage stability of the wettable powder-type formulation of Simplicillium lamellicola BCP at 40┬░C. Each value represents the mean ┬▒ standard deviation of two runs with three replicates per run.
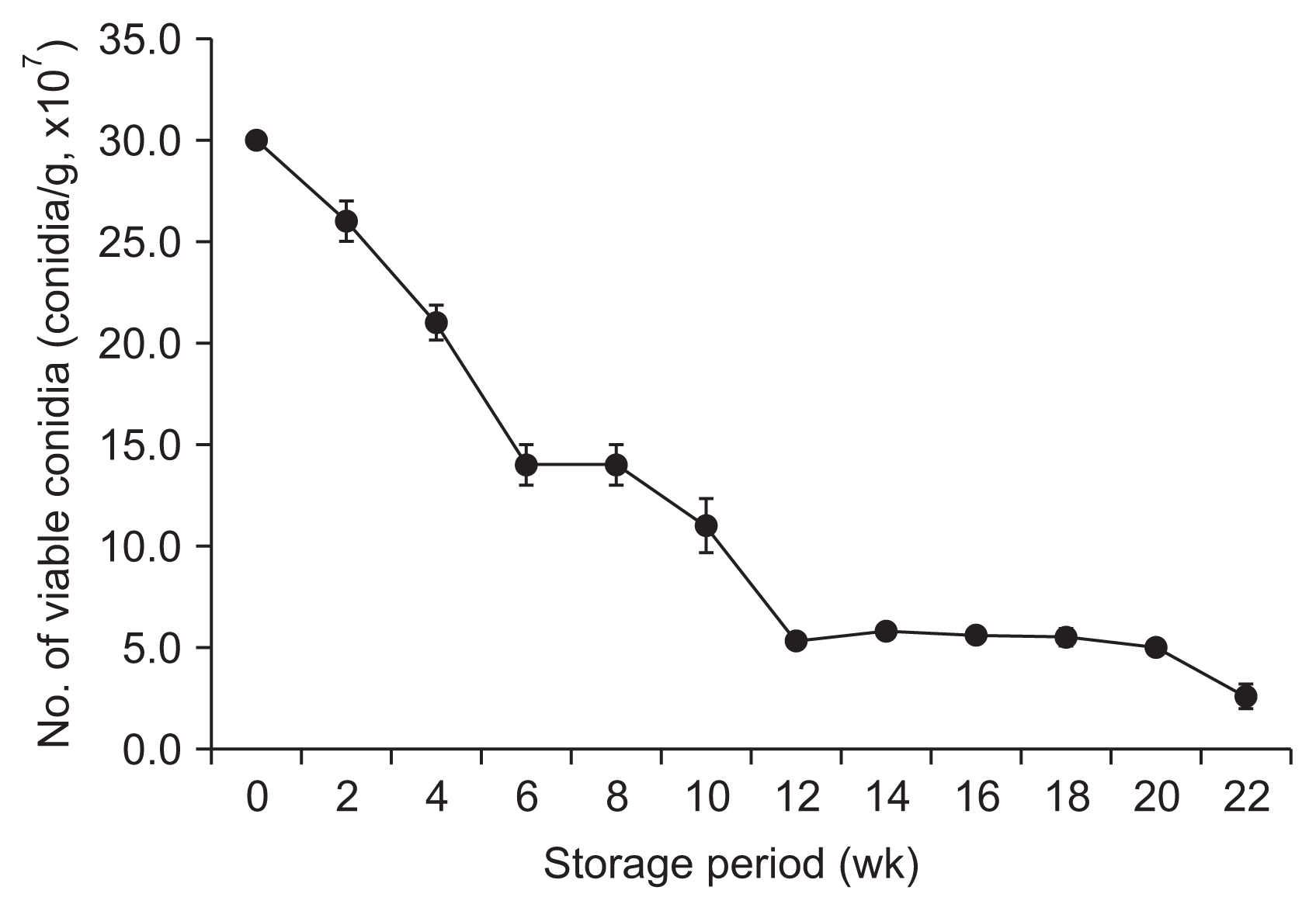
Fig.┬Ā3
Disease control efficacy of the wettable powder-type formulation of Simplicillium lamellicola BCP (BCP-WP10) against tomato gray mold in a field. Disease incidence (DI) was assessed 7 days after the third application of BCP-WP10, and DI was calculated as the percentage of tomato plants that were infected with Botrytis cinerea. Control efficacy was calculated using the following formula: control efficacy (%) = 100 ├Ś (DI of control ŌłÆ DI of treatment)/DI of control. Data shown are the means of three replicates. Means followed by the same letter are not significantly different (P = 0.05) according to TukeyŌĆÖs honest significant difference test.

Fig.┬Ā4
Disease control efficacy of the wettable powder-type formulation of Simplicillium lamellicola BCP (BCP-WP10) against ginseng gray mold in a field. Disease incidence (DI) was assessed 7 days after the third application of BCP-WP10, and DI was calculated as the percentage of ginseng plants that were infected with Botrytis cinerea. Control efficacy was calculated using the following formula: control efficacy (%) = 100 ├Ś (DI of control ŌłÆ DI of treatment)/DI of control. Data shown are the means of three replicates. Means followed by the same letter are not significantly different (P = 0.05) according to TukeyŌĆÖs honest significant difference test.
Table┬Ā1
Composition of 11 additives for freeze-drying the fermentation broth of Simplicillium lamellicola BCP and the numbers of viable conidia in the freeze-dried samples
| No. | Additives (%) | Viable conidia* (conidia/g, ├Ś109) |
|---|---|---|
| Control | Culture broth before freeze dry | 3.3 ┬▒ 0.2 |
| 1 | Lactose 20% | 2.8 ┬▒ 0.2 |
| 2 | Lactose 10% + glucose 10% | 2.2 ┬▒ 0.3 |
| 3 | Lactose 10% + skim milk 10% | 2.4 ┬▒ 0.8 |
| 4 | Skim milk 20% | 3.5 ┬▒ 1.4 |
| 5 | Skim milk 10% + glucose 10% | 2.8 ┬▒ 0.7 |
| 6 | Trehalose 20% | 4.0 ┬▒ 0.3 |
| 7 | Trehalose 10% + lactose 10% | 2.2 ┬▒ 0.3 |
| 8 | White carbon 10% + lactose 10% | 3.5 ┬▒ 0.4 |
| 9 | White carbon 10% + trehalose 10% | 3.8 ┬▒ 0.3 |
| 10 | White carbon 10% + lactose 5% + trehalose 5% | 4.9 ┬▒ 0.4 |
| 11 | Lactose 10% + trehalose 7.5% + soybean meal 2% + glycerin 0.5% | 7.1 ┬▒ 0.3 |
References
Agrios, GN 2005. Plant pathology. 5th ed. Elsevier Academic Press, New York, NY, USA. 510-514.
Ahn, JY, Park, MS, Kim, SK, Choi, GJ, Jang, KS, Choi, YH, Choi, JE, Kim, IS and Kim, JC 2009. Suppression effect of gray mold and late blight on tomato plants by rhamnolipid B. Res Plant Dis. 15:222-229.

Berger, F, Li, H, White, D, Frazer, R and Leifert, C 1996. Effect of pathogen inoculum, antagonist density, and plant species on biological control of Phytophthora and Pythium damping-off by Bacillus subtilis Cot1 in high-humidity fogging glasshouses. Phytopathology. 86:428-433.

Choi, GJ, Kim, JC, Jang, KS, Cho, KY and Kim, HT 2008. Mycoparasitism of Acremonium strictum BCP on Botrytis cinerea, the gray mold pathogen. J Microbiol Biotechnol. 18:167-170.

Choi, GJ, Kim, JC, Jang, KS, Nam, MH, Lee, SW and Kim, HT 2009. Biocontrol activity of Acremonium strictum BCP against botrytis diseases. Plant Pathol J. 25:165-171.

Chung, KC, Kim, CB, Kim, DK and Kim, BJ 2006. Isolation of antagonistic bacteria against major diseases in Panax ginseng C. A. Meyer. Korean J Med Crop Sci. 14:202-205.
Dean, R, Van Kan, JA, Pretorius, ZA, Hammond-Kosack, KE, Di Pietro, A, Spanu, PD, Rubb, JJ, Dickman, M, Kahmann, R, Ellis, J and Foster, GD 2012. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 13:414-430.



Hong, SJ, Park, JH, Kim, YK, Jee, HJ, Han, EJ, Shim, CK, Kim, MJ, Kim, JH and Kim, SH 2012. Study on the control of leaf mold, powdery mildew and gray mold for organic tomato cultivation. Korean J Organic Agric. 20:655-668.

Kim, HJ, Kim, JH, Oh, HJ and Shin, CS 2002a. Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochem. 38:649-655.

Kim, HJ, Kim, YH, Roh, YH, Seong, BL and Shin, CS 2005. Optimization of enterokinase fermentation using a recombinant Saccharomyces cerevisiae. Process Biochem. 40:717-722.

Kim, JC, Choi, GJ, Kim, HJ, Kim, HT, Ahn, JW and Cho, KY 2002b. Verlamelin, an antifungal compound produced by a mycoparasite, Acremonium strictum. Plant Pathol J. 18:102-105.

Kim, JH, Lee, SH, Kim, CS, Lim, EK, Choi, KH, Kong, HG, Kim, DW, Lee, SW and Moon, BJ 2007. Biological control of strawberry gray mold caused by Botrytis cinerea using Bacillus licheniformis N1 formulation. J Microbiol Biotechnol. 17:438-444.

Kim, TS, Ko, MJ, Lee, SW, Han, JH, Park, KS and Park, JW 2012. Effect of microbial agent on control of tomato gray mold and powdery mildew. Korean J Pestic Sci. 16:364-368.

Kim, YS, Song, JG, Lee, IK, Yeo, WH and Yun, BS 2013. Bacillus sp. BS061 suppresses powdery mildew and gray mold. Microbiology. 41:108-111.

Le Dang, Q, Shin, TS, Park, MS, Choi, YH, Choi, GJ, Jang, KS, Kim, IS and Kim, JC 2014. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J Agric Food Chem. 62:3363-3370.


Lee, SG, Ahn, YJ, Park, JD, Kim, JC, Cho, KY and Lee, HS 2001. Fungicidal activity of 46 plant extracts against rice leaf blast, rice sheath blight, tomato late blight, cucumber gray mold, barley powdery mildew and wheat leaf rust. Korean J Pestic Sci. 5:18-25.
Lee, SK, Shon, HB, Kim, GG and Chung, YR 2006. Enhancement of biological control of Botrytis cinerea on cucumber by foliar sprays and bed potting mixes of Trichoderma harzianum YC459 and its application on tomato in the greenhouse. Plant Pathol J. 22:283-288.

Li, X, Han, JS, Jin, X, Yin, D and Choi, JE 2008. Control of alternaria leaf blight of ginseng by microbial agent and fungicides. Res Plant Dis. 14:102-106.

Moyano, C, G├│mez, V and Melgarejo, P 2004. Resistance to pyrimethanil and other fungicides in Botrytis cinerea populations collected on vegetable crops in spain. J Phytopathol. 152:484-490.

Oliveira, DG, Pauli, G, Mascarin, GM and Delalibera, I 2015. A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J Microbiol Methods. 119:44-52.


Papavizas, GC, Dunn, MT, Lewis, JA and Beagle-Ristaino, J 1984. Liquid fermentation technology for experimental production of biocontrol fungi. Phytopathology. 74:1171-1175.

Paulitz, TC and B├®langer, RR 2001. Biological control in greenhouse systems. Annu Rev Phytopathol. 39:103-133.


Qu, H, Zhao, L, Zhao, F, Liu, Y and Yang, Z 2016. Biocontrol of gray mold decay in pear by Bacillus amyloliquefaciens strain BA3 and its effect on postharvest quality parameters. Polish J Microbiol. 65:171-176.

Wang, YF, Yu, T, Xia, JD, Yu, DS, Wang, J and Zheng, XD 2010. Biocontrol of postharvest gray mold of cherry tomatoes with the marine yeast Rhodosporidium paludigenu. Biol Control. 53:178-182.
Williamson, B, Tudzynski, B, Tudzynski, P and Van Kan, JA 2007. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 8:564-580.

Yang, HJ, Jeong, SJ, Jeong, SY and Jeong, DY 2014. Screening of antagonistic bacteria having antifungal activity against various phytopathogens. Korean J Mycol. 42:333-340.

Zhao, J, Li, J and Kong, F 2003. Biocontrol activity against Botrytis cinerea by Bacillus subtilis 728 isolated from marine environment. Ann Microbiol. 53:29-35.
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




