A Novel Recombined Potato virus Y Isolate in China
Article information
Abstract
This study reports the findings of a distinct Potato virus Y (PVY) isolate found in Northeast China. One hundred and ten samples (leaves and tubers) were collected from potato plants showing mosaic symptoms around the city of Harbin in Heilongjiang province of China. The collected tubers were planted and let to grow in a greenhouse. New potato plants generated from these tubers showed similar symptoms, except for one plant. Subsequent serological analyses revealed PVY as the causing agent of the disease. A novel PVY isolate (referred to as HLJ-C-44 in this study) was isolated from this sample showing unique mild mosaic and crisped leaf margin symptoms. The complete genome of this isolate was analyzed and determined. The results showed that HLJ-C-44 is a typical PVY isolate. Phylogenetic analysis indicated that this isolate belongs to the N-Wi strain group of PVY recombinants (PVYN-Wi) and also shared the highest overall sequence identity (nucleotide and amino acid) with other members of this strain group. However, recombination analysis of isolate HLJ-C-44 revealed a recombination pattern that differed from that of other PVYN-Wi isolates. Moreover, biological assays in four different potato cultivars and in Nicotiana tabacum also revealed a different phenotypic response than that of a typical PVYN-Wi isolate. This data, combined, suggest that HLJ-C-44 is a novel PVY recombinant with distinct biological properties.
Introduction
Potato virus Y (PVY) is by far the most widely studied and distributed potato infecting virus. It is present in all potato growing areas of the world. PVY is the type member of the genus Potyvirus, family Potyviridae (Gray et al., 2010). It causes serious diseases in potato and tobacco and brings huge economic losses (Gao et al., 2013; Hu et al., 2011; Ogawa et al., 2008). Infection of potatoes by PVY can result in a variety of symptoms ranging from mild to severe mosaic and vein necrosis, depending on the potato cultivars and virus strains. In addition, viral infection can result in a decrease in yield production of up to 80% or even more in severe cases (Glais et al., 2002; Nolte et al., 2007; Whitworth et al., 2006). PVY is a single-stranded, positive-sense RNA virus of approximately 9700 nucleotides (nt) in size, which is encapsidated within a flexuous rod shape virion. The genomic RNA contains one single open reading frame (ORF) which encodes a polyprotein of 3061 amino acids (aa) (Riechmann et al., 1992). After translation, the viral polyprotein is subsequently cleaved into nine mature peptides by the action of the viral encoded proteinase (Nichol et al., 2005). An additional viral protein is produced by a +2 reading frame shift of the P3 cistron (Chung et al., 2008).
Initially, PVY was classified into three strains groups: PVYN (necrotic), PVYC (stipple streak), and PVYO (ordinary), based on reactions observed in potato cultivars carrying the Ny and Nc genes and in the induction of venial necrosis in tobacco. PVYN isolates induce veinal necrosis in tobacco whereas PVYO and PVYC induce a hypersensitive response (HR) on Ny and Nc carrying potato cultivars, respectively (Jones, 1990). Nevertheless, other strain groups, which deviate from the three main strain groups (PVYN, PVYO, and PVYC) have been described (Jones, 1990). PVY isolates that react serologically to PVYO antibodies, but induce typical PVYN necrosis in tobacco are classified as PVYN-Wi (Beczner et al., 1984; Chrzanowska, 1991; Mcdonald and Singh, 1996a, 1996b; Ohshima et al., 2002; Singh et al., 2008). The PVYN-Wi strain group is composed of isolates which are genetic recombinants of PVYN and PVYO (Gray et al., 2010). PVYN-Wi isolates can be divided into two groups based on the recombination patters. One group contains two recombination junctions (in the P1 and HC-Pro/P3 enconding regions) and the second group, which is designated as PVYN:O in North America (Gray et al., 2010), has only one (in the HC-Pro/P3 enconding region) (Fig. 1; Karasev and Gray, 2013; Piche et al., 2004). Furthermore, PVY isolates which fall into the PVYN classification due to their ability to induce necrosis in tobacco and their positive reaction to PVYN antibodies, but are associated with the occurrence of potato tuber necrosis disease (PTNRD) are classified as PVYNTN (Beczner et al., 1984; Gray et al., 2010; Romancer et al., 1994; van den Heuvel et al., 1994). Similar to PVYN-Wi, PVYNTN can also occur as recombinants of PVYN and PVYO. Some PVYNTN isolates contain three recombination junctions (in the HC-Pro/P3, VPg encoding regions and the C-terminus of the CP) (Karasev and Gray, 2013) and other isolates contain four recombination junctions (in the P1, HC-Pro/P3, VPg encoding regions and the C-terminus of the CP) (Fig. 1; Gao et al., 2014). In 2010, Ali et al. (2010a) reported a new PVYNTN recombinant associated with PTNRD which differs from the previously described since it shares the recombination characteristics of both PVYNTN and PVYNWi (Ali et al., 2010a; Karasev and Gray, 2013). Nevertheless, non-recombinant PVYNTN isolates also exist, and have been reported in North America and Japan (Nie and Singh, 2003; Ogawa et al., 2008; Ohshima et al., 2000).

Schematic representation of the recombination events within PVY isolates. Recombination events occurring at a breakpoint estimated at position 522bp of the sequence alignment for the HLJ-C-44 were identified using RDP4 analysis software.
This study reports the findings of a unique PVY isolate found during field studies in Northeast China; one of the biggest potato producing area in China. In this study, one hundred and ten samples (leaves and tubers) were collected from potato plants showing mosaic symptoms around the city of Harbin (Heilongjiang, China). The collected tubers were planted in the greenhouse and let to grow. New potato plants generated from these tubers showed similar symptoms, except for one plant. Subsequent analyses revealed PVY as the causing agent of the disease. We hence further characterized this isolate. Results show that this isolate (referred to as HLJ-C-44 in this study) is genetically and biologically distinct from previously characterized isolates, indicating that HLJ-C-44 is possibly a novel PVY recombinant.
Materials and Methods
Virus isolate, host plant assay and serology
One hundred and ten samples were collected from a field in the vicinity of Harbin city, Heilongjiang province, China. Leaves and tubers were collected from potato plants showing mosaic symptoms. Subsequently, tubers were planted in the greenhouse and let to grow. Isolate HLJ-C-44 was selected for further characterization due to its unique symptomatological characteristics (mild mosaic symptoms and crisped leaf margin) (Fig. 2A, 2B).
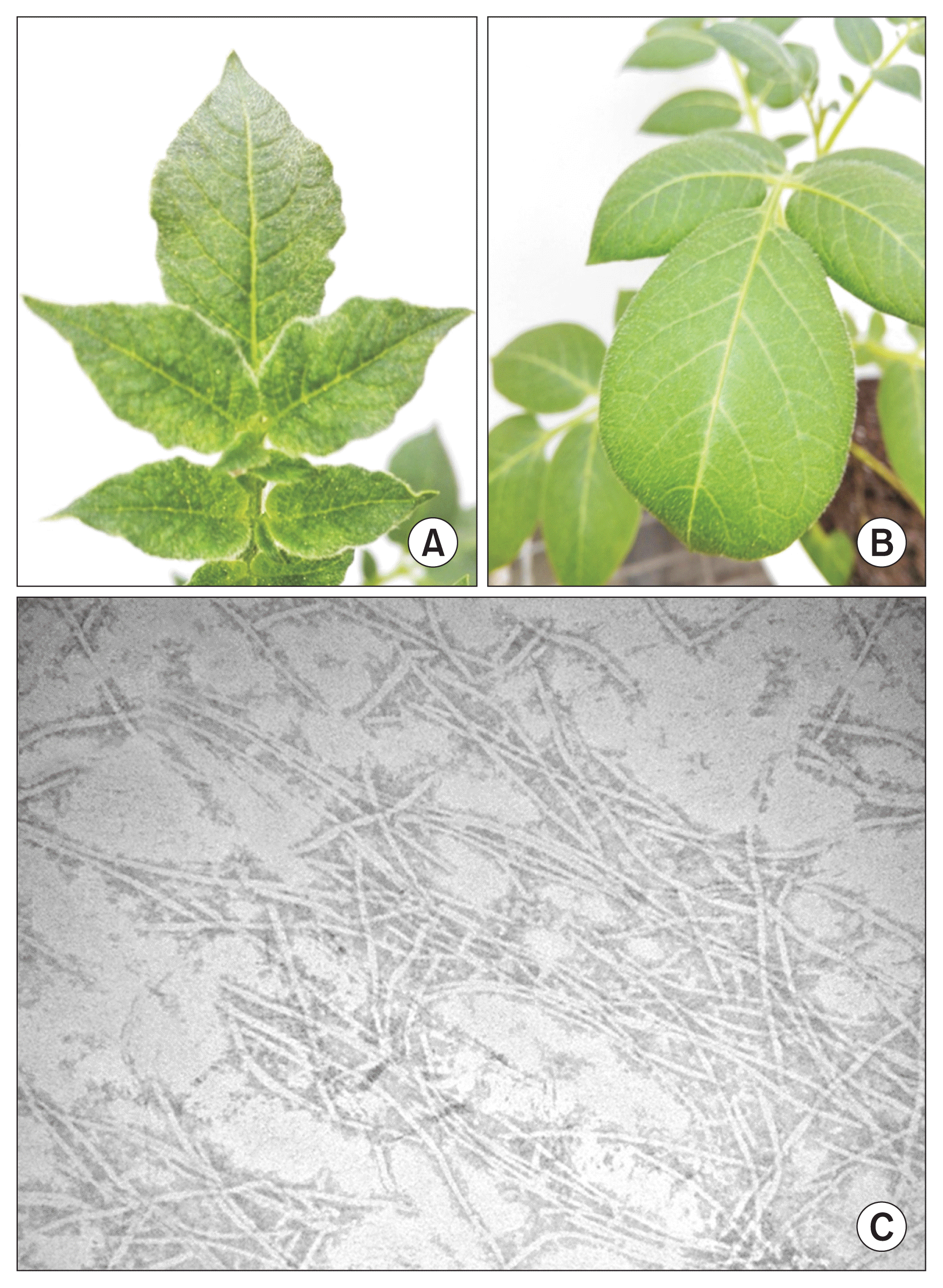
Symptoms of isolate HLJ-C-44 and viral purification. (A) Mild mosaic and crisped leaf marginsymptoms of isolate HLJ-C-44 in potato variety KeXin 13. (B) Healthy potato leaves of potato variety KeXin 13. (C) Visualization of efficient viral purification by electron microscopy.
HLJ-C-44 was screened for the presence of common potato viruses (PVY, Potato virus X, Potato virus A, Potato virus M, Potato virus S, Potato leaf roll virus, Tobacco rattle virus, Alfalfa mosaic virus, Cucumber mosaic virus, Potato virus V, Potato virus T, Potato mop top virus, Tomato spotted wilt virus, Potato aucuba mosaic virus, Tomato black ring virus and Tobacco mosaic virus) by DAS-ELISA (Adgen, Auchincruive, Ayr, UK and Agdia, Elkhart, USA). In addition, Potato spindle tuber viroid was tested by nucleic acid spot hybridization (Fig. 3) as described by Lü et al (2009). The results showed only positive infection of PVY (Table 1). Subsequently, HLJ-C-44 was mechanically inoculated to Nicotiana tabacum and propagated.

Nucleic acid spot hybridization assay for detection of PSTVd. (A, B) positive controls. (C, D) negative controls. (E, F) HLJ-C-44 samples.
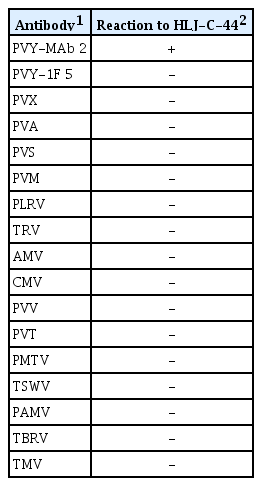
Serological reactions of HLJ-C-44 to antibodies used for detection of the most common potato-infecting viruses
The serotype of HLJ-C-44 was investigated by compound direct ELISA using two PVY monoclonal antibodies, MAb2 and 1F5 (Agdia, Elkhart, USA). The assays were conducted in duplicate following the manufacturer’s instructions.
The following potato varieties were selected to further characterize HLJ-C-44: KeXin 13, KeXin 18, HeLan 15 and XingJia 2. These varieties are the main varieties cultivated in Heilongjiang province. Virus particles were purified from tobacco plant inoculated with HLJ-C-44 using a sucrose gradient and high speed centrifugation as described by Fan (2010). The viral purification was verified by electron microscopy (Fig. 2C) and diluted to the appropriate concentration (about 0.1 mg/ml). Purified virus was used for the mechanical inoculations. Young potato and tobacco seedlings were inoculated on six to eightcarborundum dusted leaves per plant. Once a systemic infection occurred, symptomatic leaves were collected and tested by PCR.
Due to its genetic similarity with PVYN-Wi, a PVYN-Wi isolate (HLJ-BDH-2, genebank accession number KX032614) from our genebank collection was selected for comparison. This isolate was purified, inoculated to the same potato varieties (KeXin 13, KeXin 18, HeLan 15 and XingJia 2) and tested as described above.
RNA extraction and genome sequencing
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufactures instructions. After extraction, the RNA was verified by gel electrophoresis and quantified by spectrophotometry. cDNA was synthesized from total RNA using the ImProm-II Reverse Transcriptase (Promega, Madison, WI, USA) in accordance with the manual. The synthesized cDNA was stored at −20°C until use.
The 5′ and 3′terminus of the PVY genome were obtained using 5′and 3′ RACE kit (Roche, Mannheim, Germany) and the assay was conducted in duplicate following the manufacturer’s instructions. The complete PVY genome was amplified using PCR and three primer pairs (Table 2). Three overlapping fragments were amplified using PrimeSTAR Max DNA Polymerase (Takara, Dalian, China) following the manufacturer’s instructions. The annealing temperature for the three primer pairs is the following: PVY4F-PVY3980 is 50°C, PVY2F-PVY2R is 57°C and PVY3F-PVY3R is 53°C.
Amplified fragments were gel purified, cloned into pEASY-T5 (TransGen, Beijing, China) and propagated in Trans 1 cells (TransGen). For every PCR product, 5 clones were selected, purified and sequenced in the Beijing Genomics Institute Ltd for sequencing (BGI). The full length PVY sequence was assembled using DNAMAN (version 8.0; Lynnon Biosoft, San Ramon, USA) phylogenetic analysis software.
Phylogeny, sequence comparison and recombination analysis
Phylogenetic analysis, nucleotide and amino acid comparisons were carried out using the Bio Edit software and the online Blast software at NCBI (http://www.blast.ncbi.nlm.nih.gov/). The phylogenetic tree was generated using 32 isolates, from which the complete polyprotein-encoding region (9186 nt) was available in the NCBI GenBank. Isolates were to chosen to cover a representative number of isolates per strain group. The 5′NTR and 3′NTR regions were not included in the analysis. The ClustalX software was subsequently used to conduct multiple sequence alignments. The MrModeltest software was used for the nucleotide substitution analysis using the GTR+G+I in Akaike Information Criterion (AIC) standard model. This data was subsequently fed into the MEGA 6.0 software to generate a Maximum likelihood (ML) tree with a 1000 bootstrap confidence. Nucleotide and amino acid sequence comparison was carried out with the 15 PVY isolates (out of the 32 used for the phylogenetic tree) from which the complete genome (including the 5′ and 3′ NTR) were available (Table 3, 4). These isolates also represented all strain groups mentioned in this study. Pairwise comparisons were carried out using Blast online service.

Non-coding and coding regions of HLJ-C-44 nucleotide sequence identity with 15 representative PVY isolates
Recombination analysis was carried out using RDP, GENECONV, BOOTSCAN, MAXCHI and SISCAN methods implemented in RDP4 (version 4.71) pack (University of Cape Town, Cape Town, South Africa) (Martin and Rybicki, 2000). The complete sequence of non-recombinant PVY isolates was obtained from the GenBank and was used for recombination analysis (Table 5). The analyses were done using default settings and a Bonferroni- corrected P-value cutoff of 0.01. Only recombination breakpoints detected by a minimum of five methods within the program were considered as significant data.
Multiplex RT-PCR for determining HLJ-C-44
In order to further verify the strain of HLJ-C-44 isolates, the multiplex RT-PCR system established by Ali et al. (2010b) was adopted. Through the single or mixed infection of PVY samples by multi-sets of specific primers, this system amplifies RT-PCR. The major PVY strain was determined through the band size and the different band combinations produced by gel electrophoresis of the PCR products, including PVYO, PVYN and PVYN-Wi.
Results
Screening for other common potato viruses, viral purification and symptoms expression of isolate HLJ-C-44
DAS-ELISA and NASH results showed that within the viruses and viroid tested for, only PVY was present (Table 1, Fig. 3). In addition, sucrose gradient and high speed centrifugation viral purification resulted in purification of typical PVY flexuous rod shape virions (Fig. 2C). Moreover, inoculation of these purified particles to potato variety KeXin 13, resulted in mosaic and leaf crisping symptoms (Fig. 2A). These symptoms were identical to the ones observed in the potato plant which the PVY isolate was purified.
Genome analysis
The full-length sequence of HLJ-C-44 was amplified and sequenced using a primer-walking strategy and standard cloning techniques. The length of isolate HLJ-C-44 was found to be 9710 nt (excluding poly A tail). The coding region is composed of 9186 nt encoding a polyprotein of 3061 aa. The length of 5′ and 3′ NTR are 185 and 339 nt, respectively. Within the polyprotein, nine putative protein cleavages were identified. The cleavage sites (following the N-terminus to Carboxyl-terminus orientation) were predicted to be: Q/G, G/G, Q/R, Q/S, Q/A, Q/G, E/A, Q/A and Q/G. Furthermore, the recently discovered PIPO protein (Chung et al., 2008) produced by a +2 phase reading frame shift was found in the P3 cistron. The genome of isolate HLJ-C-44 has been submitted to the GenBank database and given the accession number KU569326.
Pairwise nucleotide sequence identity analysis was carried out with the coding regions of 15 PVY isolates representing all strain groups of this study from which the complete sequence (including NTRs) is available from the NCBI (Table 3). HLJ-C-44 shared the highest nucleotide similarity (over 96%) with the PVYN-Wi members SGS-AG and Mb112; and the lowest (under 86%) with isolates RRA-1 (PVYNTN), N605 (PVYN) and Mont (PVYN). At the amino acid level, HLJ-C-44 also shared the highest similarity (over 97%) with SGS-AG and Mb112 and the lowest (93%) with RRA-1, N605, Mont and Adgen (PVYC) (Table 4).
All the protein-encoding regions of HLJ-C-44 shared more than 96% (nt) and 97% (aa) sequence similarities with PVYN-Wi SGS-AG and Mb112, except for the P1 (Table 3 and Table 4). The P1 of HLJ-C-44 shared 91% (nt) and 92% (aa) sequence similarity with SGS-AG; and only 81% (nt) and 82% (aa) sequence similarity with Mb112. Computer assisted sequence alignment revealed that the difference localizes to nucleotides 310–522 in SGS-AG and was even longer in the P1 of Mb112 (nucleotides 1–522). Interestingly, from all the isolates included in this study, the P1 of HLJ-C-44 shared the highest sequence identity [97% (nt) and 98% (aa)] with a PVYNTN isolate (SYR-III-L4). The P1 similarities with other isolates ranged from 80%–91% (nt) and 76%–92% (aa) level.
Phylogenetic and recombination analysis
The phylogenetic analysis indicated that 32 different PVY isolates can be grouped into four main clusters, N/NTN, N-Wi, O and C and are supported by bootstrap values over 74%. Individual isolates incorporated into these four main clusters are supported by reliable bootstrap values (over 51%) and agree with their previous strain grouping (Wang et al., 2013; Gao et al., 2015). Isolate HLJ-C-44 was grouped within the N-Wi cluster with a bootstrap confidence value of 86% (Fig. 4).
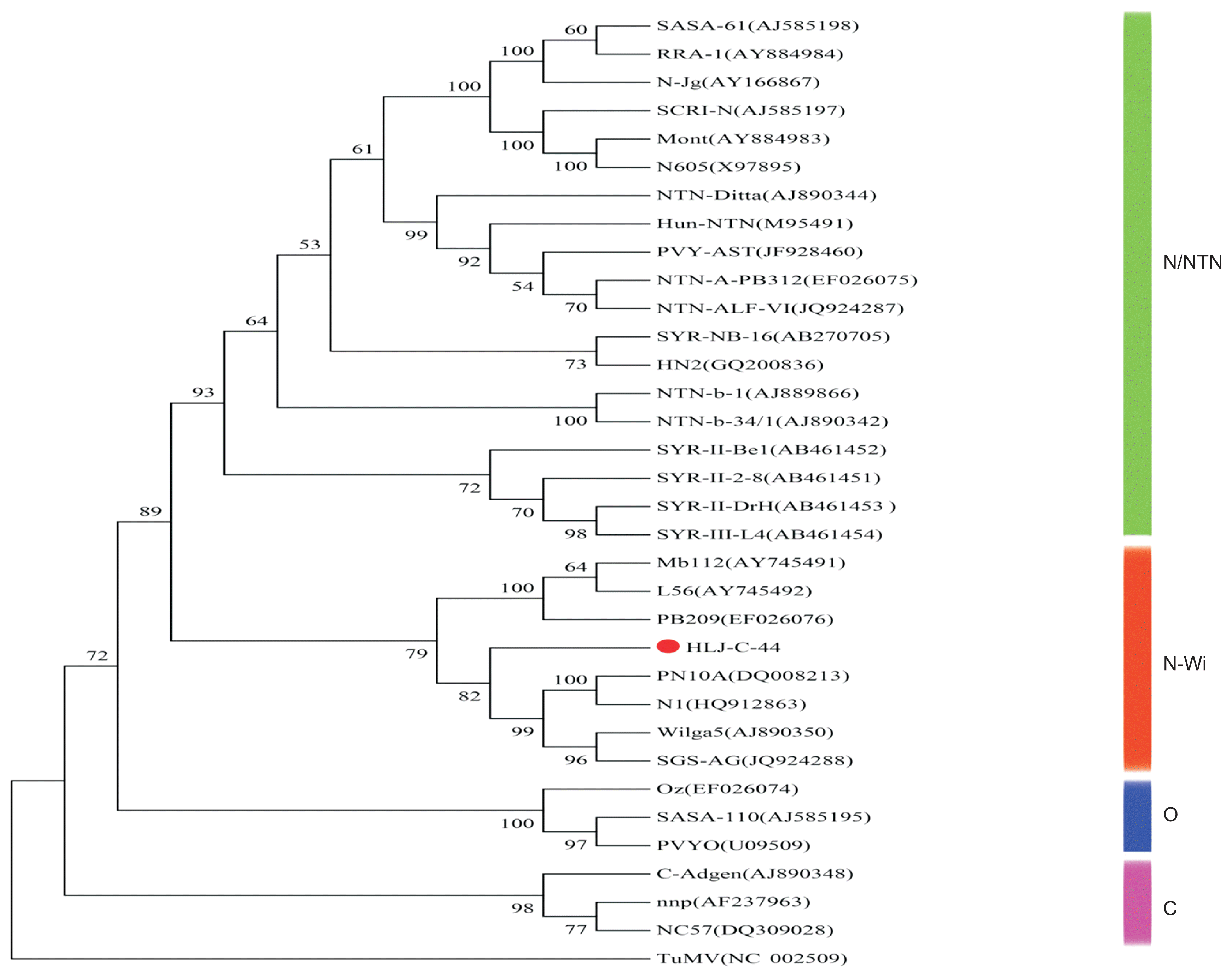
Evolutionary relationship of HLJ-C-44 with selected PVY strains. The following PVY isolates, with their respective Genbank ID number in parenthesis, were used: NTN/N: EF026075, JQ924287, JF928460, M95491, AJ890744, AJ585198, AY884984, AY166867, AJ585197, AY884983, X97895, AB270705, GQ200836, AJ889866, AJ890342, AB461452, AB461451, AB461453 and AB461454; N-Wi: AY745491, AY745492, EF026076, DQ008213, HQ912863, AJ890350 and JQ924288; O: EF026074, AJ585195 and U09509; C: AJ890348, AF237963 and DQ309028; Out group: NC_002509.
Previous studies indicate that PVYN-Wi strain group members are recombinants between PVYN and PVYO (Glais et al., 2002). RDP4 analysis revealed one recombination event consisting of spliced fragments from two non-recombinant PVY isolates, Mont (AY884983) and SASA-110 (AJ585195). The recombinant breakpoint was detected by five methods implemented in RDP4 [RDP (4.496×10−179), GENECONV (6.579×10−171), Boot Scan (7.074×10−174), Max Chi (2.031×10−41), and SiScan (6.134×10−43)] which confirmed the recombination event. The results revealed two recombination junctions within the HLJ-C-44 genome, at nucleotide 522 and 2220 (Fig. 1). Recombination analysis was also carried out with two other PVYN-Wi isolates (SGS-AG and Mb112). Isolate SGS-AG also contained two recombination junctions. It shared the same end recombination junction as HLJ-C-44 (nucleotide 2220) but differed in the first recombination junction. The first recombination junction of SGS-AG was located in nucleotide 310 rather than 522. Isolate Mb112 showed only one recombination junction at nucleotide 2220 (the same as HLJ-C-44; Fig. 1).
Physiological symptoms
Greenhouse experiments showed that HLJ-C-44 was unique since it showed mosaic symptoms and crisped leaf margins in leaves, but was lacking systemic veinal necrosis. DAS-ELISA and nucleic acid spot hybridization assays revealed only the presence of PVY.
Mechanical inoculation of the purified virus isolate HLJ-C-44 to four potato varieties (KeXin 13, KeXin 18, HeLan 15 and XingJia 2) resulted in a range of symptoms (Table 6). In addition, a PVYN-Wi isolate was also inoculated to the 4 potato varieties. PVYN-Wi was selected for the biological assay comparison since it was the most closely genetically related PVY [97% (nt), 98% (aa) similarity over the complete coding region]. HLJ-C-44 caused similar symptoms (systemic mild mosaic) in varieties KeXin 18, HeLan 15 and XingJia 2. However, inoculation of HLJ-C-44 to variety KeXin 13 resulted in a more pronounced systemic mosaic accompanied with leaf crisping. On the other hand, PVYN-Wi differed from HLJ-C-44 in that it only induced a mild mosaic in KeXin 13 but induced leaf deformation and systemic mosaic in XingJia 2. PVYN-Wi induced similar symptoms in Kexin 18 and HeLan 15 as HLJ-C-44. No symptoms were observed in tubers harvested from HLJ-C-44 and PVYN-Wi infected plants (observation of tubers after storage for 1 month) (Fig. 5). HLJ-C-44 systemically infected tobacco plants generating only mild mosaic symptoms, whereas infection of tobacco plants with PVYN-Wi resulted in veinal necrosis and mosaic.
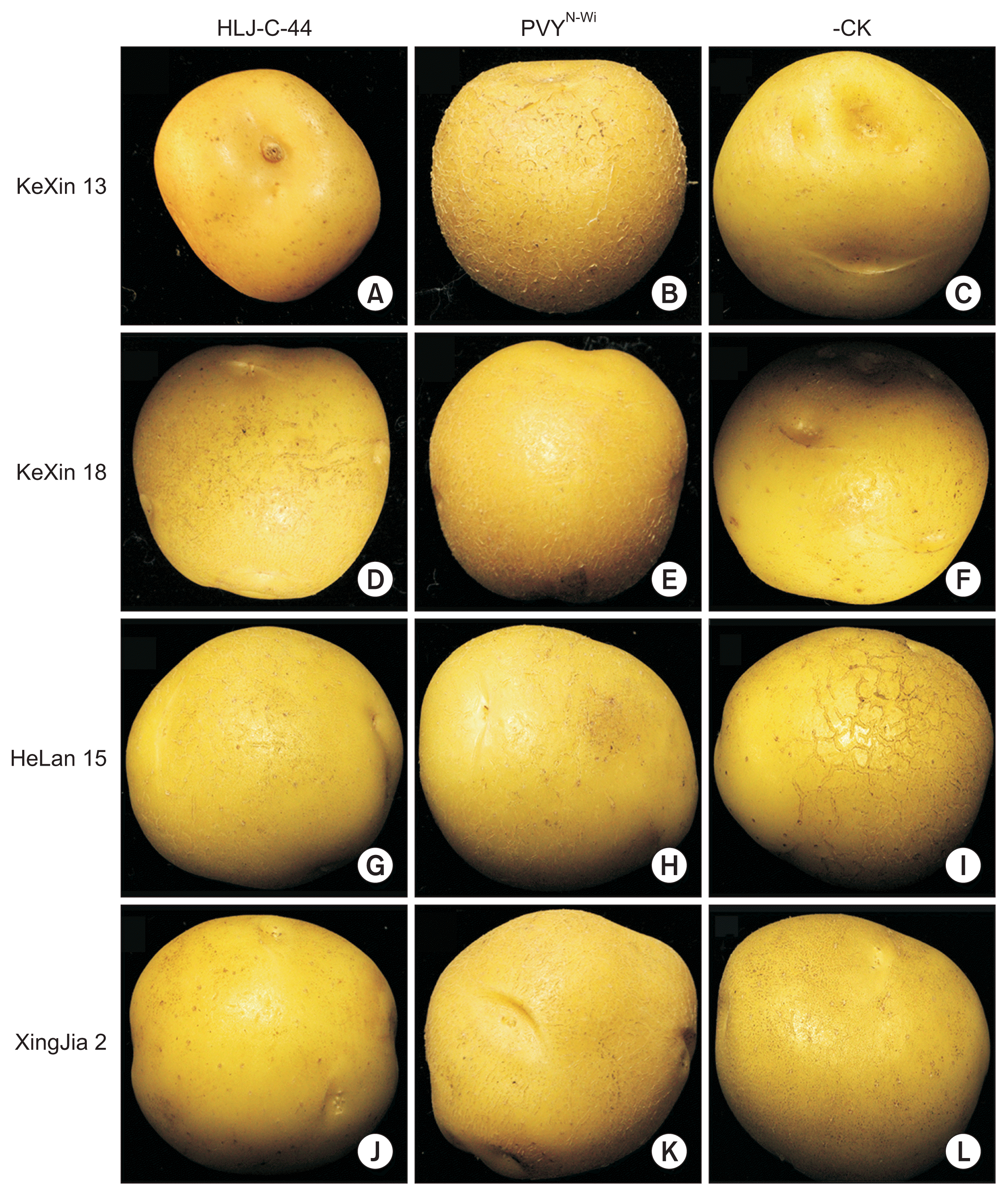
No Symptoms of potato tubers after infected with HLJ-C-44 and PVYN-Wi. Observation of tubers after storage for 1 month, no symptoms in the tubers. A, D, G, J: infected with HLJ-C-44, A: KeXin 13, D: KeXin 18, G: HeLan 15, J: XingJia 2; B, E, H, K: infected with PVYN-Wi, B: KeXin 13, E: KeXin 18, H: HeLan 15, K: XingJia 2; C, F, I, L: Negative control, C: KeXin 13, F: KeXin 18, I: HeLan 15, J: XingJia 2.
Determination of results of the HLJ-C-44 multiplex RT-PCR
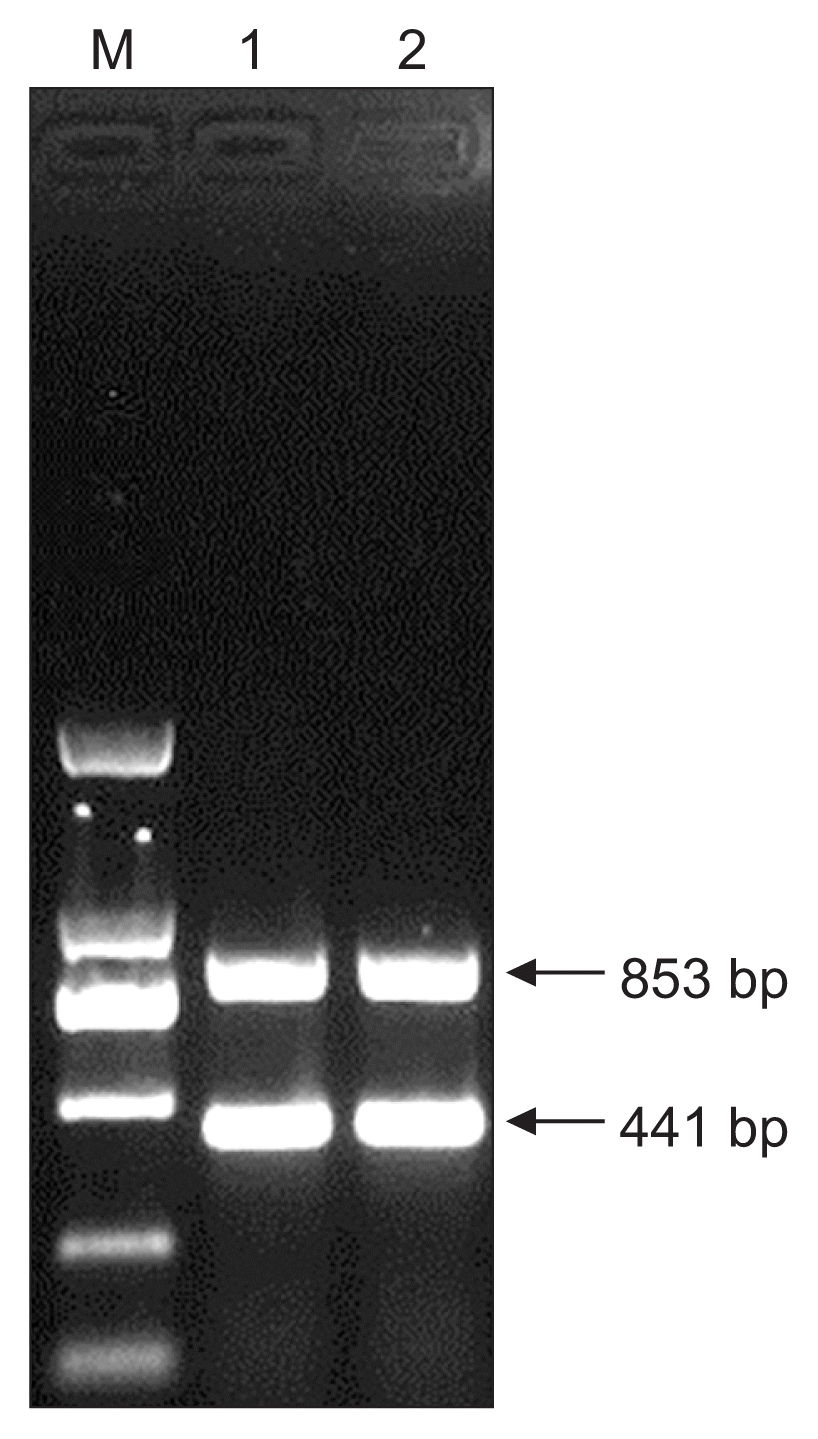
HLJ-C-44 isolates were amplified into two specific fragments through the multiplex RT-PCR at approximately 800 and 400 bp, respectively (Fig. 6). These were of concordant band size with the two specific 853 and 441 bp of the PVYN-Wi strain in the determination method reported by Ali et al (2010b). This result further confirms that the HLJ-C-44 isolates are of the PVYN-Wi strain.
Discussion
In this study, we characterized, at a biological and molecular level, a unique PVY isolate (HLJ-C-44) found infecting potatoes in Northeast China. Multiple sequence aligments and phylogenetic analysis indicate that this isolate (HLJ-C-44) clusters within the PVYN-Wi strain group (Fig. 4) and also shares the highest amino acid and nucleotide sequence similarity with members of the PVYN-Wi strain group, supporting its phylogenetic clustering.
Symptoms inducing between HLJ-C-44 and a PVYN-Wi isolate in some potatoes varieties and in N. tabacum were different, indicating that these isolates do not have identical biological properties. Furthermore, recombination analysis revealed that HLJ-C-44 has different recombination patters than those of other members of the PVYN-Wi strain group (SGS-AG and Mb112). Many studies show that changes in the amino acid sequence of viral encoded proteins can result in different symptoms. For example, specific point mutations within the 6K2 protein of Potato virus V have an impact on the symptoms generated in two Nicotiana species (N. tabacum and N. benthamiana). This difference in symptom expression was not correlated with different viral titers, but rather due to specific host plant interactions (Spetz and Valkonen, 2004). Furthermore, the P3-6K1 region of Plum pox virus is involved in the severity of chlorotic mottle symptoms in N. clevelandii (Riechmann et al., 1995) as well as in the ability to induce different symptoms in Pisum sativum (Sáenz et al., 2000). Thus, we can speculate that the biological difference observed in our study is due to the difference between P1 protein of HLJ-C-44 and other PVYN-Wi isolates, resulting from a recombination event. Indeed, it has been suggested that recombination events that occurred in one host may enable some recombinants to infect new hosts (Ohshima et al., 2002). However, to fully attribute this difference in symptom inducing to the P1 protein, construction of an infectious HLJ-C-44 cDNA clone, followed by mutation and replacement analysis is required. Nevertheless, the data in this study show that isolate HJL-C-44 is indeed a new recombinant that belongs to the PVYN-Wi strain group. Whether this isolate is just a sporadic case or widely distributed in China remains to be determined.
PVY is by far the most widely studies potato-infecting virus. Many studies on the molecular properties are available. In our study we have included 32 PVY isolates from which the complete coding region is available for the phylogenetic analysis, and the 15 most genetically distinct PVY isolates for sequence comparison. Our results confirm that PVY isolates group in four main clusters (N/NTN, N-Wi, O and C). However, recombination analysis shows that most of the isolates that group within the N/NTN and N-Wi cluster are not a homogenous genetic line, but rather composed of recombinants. With the current advances in meta-sequencing and decreasing sequencing prices, a much larger number of sequences are expected. It can also be expected that more PVY isolates of recombinant features would be discovered.