Pathological Interrelations of Soil-Borne Diseases in Cucurbits Caused by Fusarium Species and Meloidogyne incognita
Article information
Abstract
Pathological interrelations of two soil-borne diseases in cucurbits (watermelon, oriental melon, shintosa and cucumber) caused by Fusarium isolates (FI) and the root-knot nematode (RKN), Meloidogyne incognita were characterized by the fusarium disease severity index (DI), RKN gall index (GI) and eggmass index (EI) in inoculation tests using FI and RKN. Virulence of FI as determined by DI at 4 weeks after inoculation was mostly in the higher order of Fusarium proliferatum F6, F5 and Fusarium oxysporum f. sp. melonis or Fusarium oxysporum f. sp. niveum with no significant differential interactions among the cucurbits and RKN co-infection. Significant increases of DI due to RKN coinfection were noticed in watermelon and oriental melon infected with F. proliferatum isolates, suggesting the DI increase due to RKN coinfection may depend upon the virulence of FI relative to aggressiveness of RKN on the cucurbits. For the coinfection of FI and RKN, GI and EI were mostly reduced logarithmically with the increase of DI, largely more in EI than GI, in all cucurbits except for shintosa. Microscopic examination of the root tissues showed histopathological features characteristic to infection types; formation of fungal hyphae and/or spores and plant defense structures (tyloses and mucilage) in variable degrees and formation of giant cells at variable developmental stages and with variable cytoplasmic depletion or degeneration which were visualized in relations with the values of DI, GI and EI. These findings will be helpful to develop control strategies of the soil-borne disease complex based on their pathological characteristics.
Introduction
The cucurbits such as cucumber (Cucumis sativus L.), oriental melon (Cucumis melo var. makuwa Makino) and watermelon (Citrullus lanatus [Thumb] Matsum & Nakai) are important vegetable crops whose fruits are major products consumed in Korea. The total cultivation areas (annual yields) of these crops are 4,137 ha (271,040 tons) for cucumber, 5,438 ha (161,100 tons) for oriental melon and 15,185 ha (634,352 tons) for watermelon in 2015 (MAFRA, 2016). Especially for the cultivation of these crops, the greenhouse areas (ratios) and yields are 3,338 ha (80.7%) and 240,212 tons, 5305 ha (97.6%) and 158,528 tons, and 12,572 (82.8%) and 544,605 tons (MAFRA, 2016). This indicates their productivities (yields per 0.1 ha) are 7,196 kg for cucumber, 2,988 kg for oriental melon and 4,177 kg for watermelon, which are 1.87, 1.55 and 1.26 times higher than those from open fields, respectively. This may be the major reason that the growers prefer to cropping these vegetables continuously in limited areas under the greenhouse-environmental conditions.
However, continuous greenhouse cropping (CGC) of the cucurbits in limited areas has caused severe epidemics of soil borne diseases especially caused by Fusarium species and root-knot nematodes (RKNs) that favor warm temperature for their growth and reproduction, resulting in the rapid increase of their inoculum potentials even in harshly cold weather conditions during the winter season. These CGC of the cucurbits have caused recurring disease problems of the fusarium wilts and remarkable increases of the RKN populations for 20 years of CGC in Korea (Banihashemi and deZeeuw, 1975; Kim and Yeon, 2001; Kim et al., 2005; Park et al., 1995; Yeon et al., 2003).
In the cucurbits, it has long been practiced since 1920 that the vegetable production has relied on grafting of their seedlings to rootstock plants resistant to the fusarium wilts, which becomes a common practice in oriental melon cultivation (currently constituting about 96% of its total propagation) to increase its cold tolerance during the winter time and to prevent the fusarium wilt caused by Fusarium oxysporum f. sp. melonis (Kim et al., 2005; Lee, 1994; Lee and Oda, 2003). Several rootstocks for grafting the oriental melon have been developed for their resistance to the fusarium wilt, among which shintosa (Cucurbita maxima × Cu. moschata) has been widely used as a rootstock plant since it was firstly used in 1920 (Lee, 1994). However, the fusarium wilt still occurs prevalently in oriental melon greenhouses with little indication of the disease decrease despite the propagation of the cucurbit using the fusarium wilt-resistant cucurbit as rootstocks for grafting.
For the RKNs, there are several methods suggested to be effective in their control such as admixtures of soil and rotation of rice in paddy field conditions (Byeon et al., 2014; Kim and Choi, 2001; Kinloch and Hinson, 1972; Rhoades, 1976). However, these methods have been rarely implemented in the practical control of the nematodes in oriental melon greenhouses probably because of the exceeding disease pressure from enormous nematode population densities and no development of oriental melon cultivars resistant to the Korean race of the RKNs as yet (Freeman et al., 2002; Lee et al., 2015; Matsumoto et al., 2011).
Since the fusarium wilt/RKN disease complex of crops was firstly found in cotton, this type of disease complex has a synergistic interaction that shows the presence of the nematodes mostly resulting in earlier expression of wilt symptoms and increased wilt severity and incidences (Atkinson, 1892; Mai and Abawi, 1987; Powell, 1971). Unless the populations of Fusarium oxysporum f. sp. vasinfectum are very high, little or no fusarium wilt occurs on cotton in greenhouses and fields in the absence of the nematode (Garber et al., 1979; Jorgenson et al., 1978). Several studies have been conducted to examine the relationships between the fusarium wilts and RKNs on several cucurbitaceous crops such as muskmelon (Cucumis melo), summer squash (Cucurbita pepo), and watermelon (Citrullus lanatus var. lanatus) (Bergeson, 1975; Caperton et al., 1986). Considering these aspects in the relationships of the fusarium wilts and the RKN infestation, there should be a high probability of complex diseases in the cucurbitaceous greenhouse crops in Korea; however, little study has been conducted on the disease complex in the cucurbitaceous crops in CGC.
In this study, pathological aspects of the fusarium wilts and RKNs and their inter-relations in the complex disease were examined to reason out the current soil-borne disease problems in the greenhouse-grown cucurbitaceous vegetables in Korea.
Materials and Methods
Plants, pathogens and inoculum preparation
For inoculation experiments, four cucurbits such as watermelon (Citrullus lanatus cv. Wori-Ggul), oriental melon (Cucumis melo var. makuwa cv. Searon-Ggul), and cucumber (Cucumis sativus cv. Headong-baekdadagi) and shintosa (Cucurbita maxima × Cu. moschata) were used in this study. Four Fusarium isolates (FI) were used in this experiment. Two isolates of Fusarium oxysporum (F. oxysporum f. sp. melonis [FOM] and F. oxysporum f. sp. niveum [FON]) were provided from Rural Development Administration, Korea. Two Fusarium proliferatum isolates such as F5 and F6 were isolated from the oriental melon plants with wilt symptoms (Seo and Kim, 2017). Oat meal-sand medium (oat meal:sand:distilled water = 1:20:4, w/w/w) sterilized at 121°C for 20 min in an autoclave was used for preparing FI inocula. For this, mycelial plugs from FI cultures grown on potato dextrose agar (PDA) at 25°C for 7 days were inoculated into the oat meal medium and incubated at 25°C for 15 days to prepare the cultural medium (CM) used as the fungal inocula (Seo and Kim, 2017; Song et al., 2014).
For the inoculum preparation of the RKN, Meloidogyne incognita used in this study, it was cultured and maintained purely on chili pepper (Capsicum annuum cv. Bugang) in a greenhouse (Seo et al., 2014). The plants were uprooted and washed free of adhering soils in tap water, and egg masses were taken from the entire roots by the forceps with naked eyes. They were put on Baermann funnels and incubated for 3–5 days to obtain second-stage juveniles of RKN (J2) hatched out of eggs (Son et al., 2009; Southey, 1986), and diluted to nematode population density of about 400 J2 ml−1 in sterile distilled water (SDW), and used for the nematode inoculation.
Inoculation of FI and RKN for examination of the fusarium disease severity, RKN gall and eggmass formations
Fifteen day-old seedlings of the cucurbits were planted in plastic pots of 10-cm-diameter filled with 130 g sterilized mixtures (1:1) of sand and bed soil (composed of 64.9% coco-peat, 15% peat-moss, 7% zeolite, 10% perlite, 2.6% dolomite, 0.03% wetting agent, and 0.47% N-P-K common fertilizer) and were inoculated with either or both of FI and RKN inocula. For FI inoculation, the top soils in a depth of 5 cm were each mixed evenly with 10 g CM/pot (for lower inoculum density [LD]) and 20 g CM/pot (for higher inoculum density [HD]) of oat mealsand FI cultures with four replications for each treatment. For RKN inoculation, the plants were inoculated with the nematode by pouring 5 ml RKN suspension (containing about 2,000 J2) per pot with 4 replications for each treatment. All of the plants in pots were grown at 25 ± 2°C in a greenhouse, watering daily to field capacity, in a completely randomized design with three factors (plants, FI, and RKN) and plant-pathogen interactions.
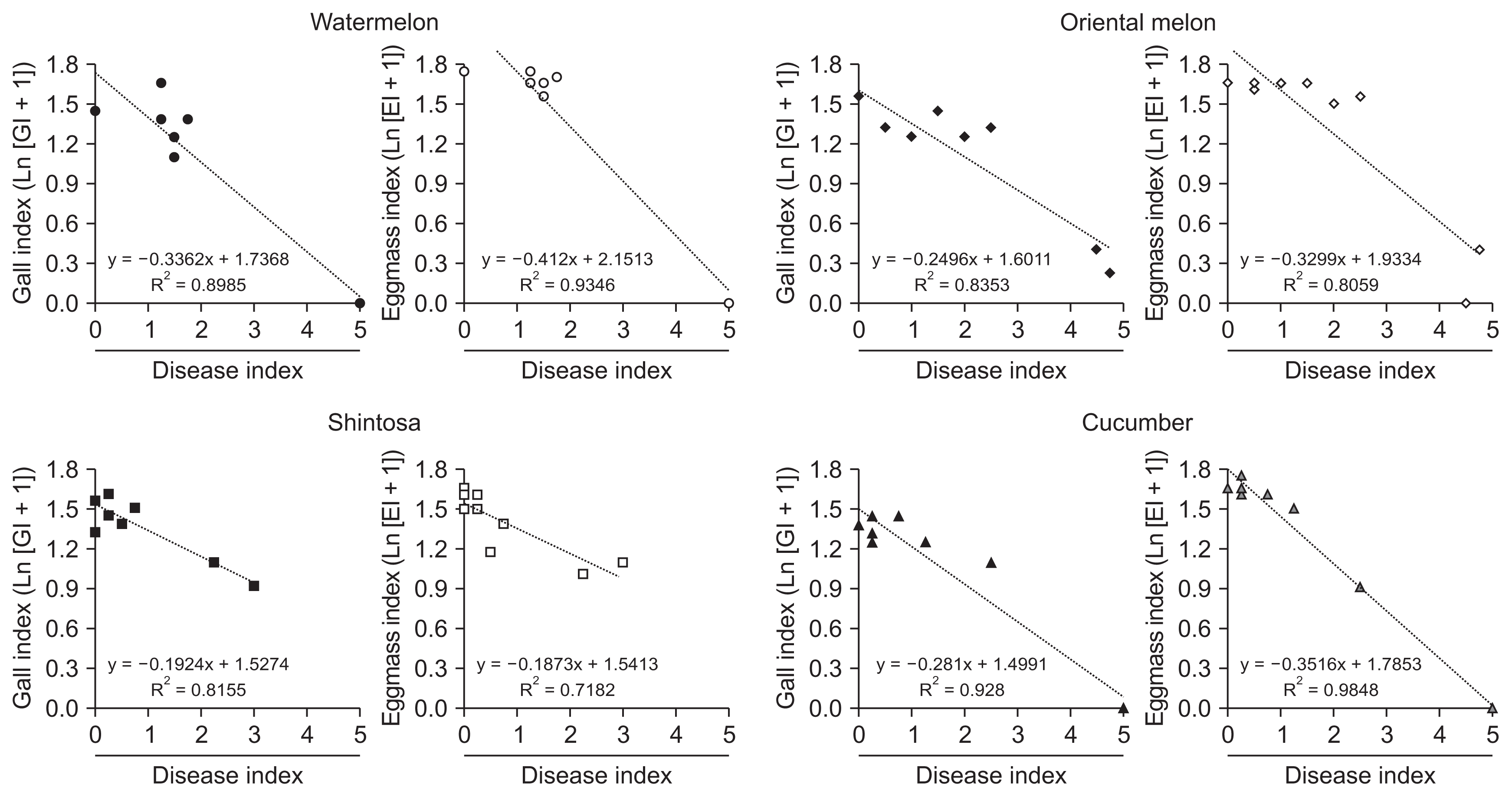
For the disease severity caused by FI, wilt or stem rot symptoms were observed at 4 weeks after inoculation and evaluated using the method modified from Bletsos (2005), which was based on vascular wilt severity index (disease index, DI) 0 to 5; 0 = no symptom; 1 = underground stem yellow-brownish discolored; 2 = < 30% aboveground stem brownish discolored; 3 = stem bottom region decayed; 4 = stem darkly discolored and split; 5 = whole plant dead, or those corresponding to these disease indices for others such as root or stem rot symptoms. For the diseases caused by RKN, gall and eggmass formations on the plant roots were examined at 4 weeks after inoculation. For this, plants were uprooted from the pots and washed free of soil with tap water, and formations of the root-knot galls and eggmasses were examined with naked eyes, which were evaluated by gall index (GI) and eggmass index (EI), respectively, with GI scored 0–5 assigned as 0 = 0–10%; 1 = 11–20%; 2 = 21–50%; 3 = 51–80%; 4 = 81–90%; 5 = 91–100% of galled roots (Barker, 1985); and with EI assigned to each eggmass number using a rating of 0 = no egg mass; 1 = 1–2; 2 = 3–10; 3 = 11–30; 4 = 31–100; and 5 = ≥ 100 egg masses per root system (Taylor and Sasser, 1978). Effects of the pathogen infections alone or in combination on DI, GI and EI were evaluated by comparing these indices from single or combined infections for synergistic effects. For analyzing correlations of GI or EI and with DI, trend graphs were generated in Microsoft Excel 2013 (Microsoft, Redmond, WA, USA) after GI and EI were normalized by transforming them to natural logarithmic values such as Ln (GI + 1) and Ln (EI +1) for data analysis and presentation, respectively.
Statistical analysis
For all of these experiments, SAS 9.3 (SAS Institute, Cary, NC, USA) was employed for the analyses of variance carried out, and Fisher’s least significant difference (LSD) was employed to test for significant differences among the factors at P ≤ 0.05 (two-tailed) from the critical values in the t-distribution table for all the results from the inoculation experiments.
Histopathological characteristics of cucurbits infected with FI and RKN alone or in combination
Plants were uprooted from pots 7 days after inoculation and root tissue samples were excised from the roots containing galls for RKN inoculation alone and in combination with FI and near maturation regions of the root for FI inoculation alone. The root segments were fixed with Karnovsky’s fixative consisting of 2% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.05 M cacodylate buffer (pH 7.2) for 4 h, and washed in 0.05 M cacodylate buffer (pH 7.2) three times for 15 min each. Then they were post-fixed in 1% osmium tetroxide in the same buffer at 4°C for 2 h in a refrigerator. They were washed briefly with distilled water 2 times and en block stained in 0.5% uranyl acetate overnight at 4°C in a refrigerator. The specimens were then dehydrated in an ethanol series of 30%, 50%, 80%, and 90%, and finally three times in 100% ethanol for 10 min each. The specimens were further treated with two changes of 100% propylene oxide each for 15 min and embedded in Spurr’s epoxy resin (Spurr, 1969), followed by polymerization at 70°C for 8 h for the specimen embedding. The embedded specimens were sectioned 800 nm in thicknesses with a glass knife on a MT-X ultramicrotome (RMC, Tucson, AZ, USA). The sections were then stained with 1% toluidine blue O in 2% sodium tetra-borate and observed under a compound light microscope (Axiophot; Carl Zeiss, Oberkochen, Germany). At least 30 root tissues were sectioned and observed for each treatment in this study.
Results
DI on the cucurbits infected by FI alone and in combination with RKN
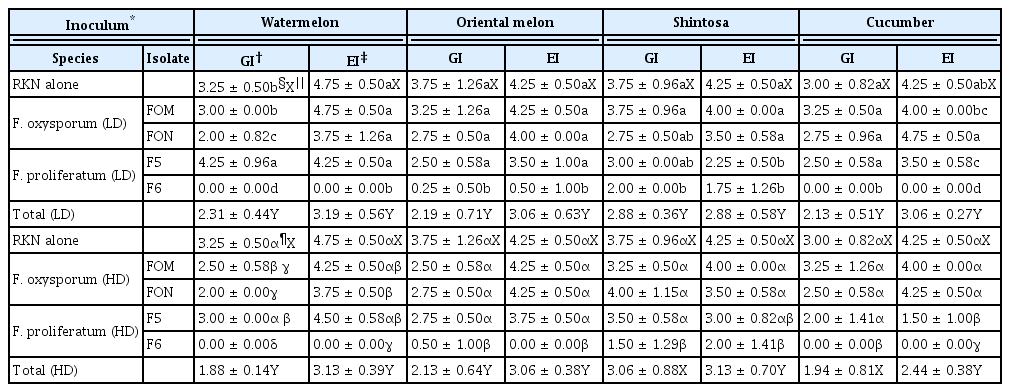
In the inoculation tests of FI alone and in combination with RKN on the cucurbits, DI varied depending on FI and cucurbits, while no disease occurred in all cucurbits without the inoculation of FI alone or in combination with RKN and in shintosa inoculated by two isolates (FOM and FON) of F. oxysporum with LD (Table 1). Virulence of FI determined by DI was mostly in the higher order of F. proliferatum F6 (DI of 2.0–5.0), F5 (DI of 0.5–2.5), and FOM or FON (DI of 0–1.5) in all the cucurbits except in watermelon infected by FI with LD alone, showing the lowest DI of 0.50 for F5. For F. oxysporum, there was no significant difference in DI between FOM and FON on all cucurbitaceous vegetables except in oriental melon co-infected by RKN; however, F. proliferatum F6 always induced severer symptoms with significantly higher DI than F. proliferatum F5 on all cucurbits tested except in oriental melon infected by FI with LD alone (Table 1). DIs for all FI were lower comparatively (with DI of 0.0–0.5 for FOM, FON, and F5) or insignificantly (with DI of 2.0–3.5 for F6) in shintosa than in the other cucurbits tested, regardless of RKN coinfection. DI was significantly increased in watermelon and oriental melon by the infection of FI with LD and RKN compared to FI with LD alone, which was attributed to significantly increased DI by the infection of F. proliferatum F5 (in watermelon) and F6 (in oriental melon) in combination with RKN (Table 1). However, no significant difference in DI was shown in all cucurbits infected by FI with HD alone and in combination with RKN, and shintosa and cucumber infected by FI even with LD alone and in combination with RKN.
GI and EI on cucurbits infected by RKN alone and in combination with FI
Root-knot galls and eggmasses were prominently formed with GI of 3.00–4.75 and EI of 4.25–4.75 on all cucurbits infected by RKN alone at 4 weeks after inoculation (Table 2). For the co-infection of FI and RKN, the degrees for the total GI and EI were significantly reduced in all cucurbits except for GI in shintosa and cucumber co-infected by RKN and FI with HD (Table 2, Fig. 1). In the statistical analysis for the effect of DI on gall and eggmass formations, both GI and EI were reduced logarithmically with the increase of DI by the coefficients of determination (R2) = 0.7182–0.9848 with steeper functional gradients for EI than GI in all cucurbits tested except shintosa that showed similar functional gradients for both GI and EI (Fig. 2). In the comparison of R2 values, GI had higher R2 with DI in oriental melon and shintosa, but EI, higher R2 with DI in watermelon and cucumber, respectively (Fig. 2).
Effects of fungal pathogen (Fusarium oxysporum and Fusarium proliferatum) inoculation on gall and eggmass formations of root-knot nematode (RKN) on cucurbits at 4 weeks after inoculation

Root-knot gall formations on cucurbits infected by the root-knot nematode (RKN) alone and in combination with Fusarium isolates (FI). FOM, Fusarium oxysporum f. sp. melonis; FON, Fusarium oxysporum f. sp. niveum; F5 and F6, Fusarium proliferatum isolates obtained from oriental melon greenhouses. Control, infection with neither RKN nor FI.
Histopathological characteristics of the cucurbits infected by FI and RKN alone or in combination
Giant cells were extensively formed in the roots of all cucurbits infected by RKN alone, occupying large portions of the stelar regions, and the root systems of the cucurbits infected by FI alone had mostly the intact stele containing functional xylem vessels (related to plant vascular tissue system) of variable diameters in cross sections, in which FI hyphae and/or spores were frequently distributed sometimes together with the formation of defense-related structures such as tyloses and mucilage (or phenolic compounds) in and around xylem vessels (Fig. 3–6). Giant cells induced by RKN infection alone were characterized by cellular hypertrophy with intact cytoplasm accompanying no necrotic responses; however, those infected by FI and RKN combined showed the formation of giant cells at varying developmental stages and cytoplasmic degeneration or depletion and sometimes with extensive necrotic responses in the root tissues of all cucurbits except in watermelon and oriental melon co-infected by FON with LD and in shintosa co-infected by F6 (Fig. 3–6). The frequency of defense-related structures such as tyloses and mucilage was reduced by the co-infection of FI and RKN compared to FI infection alone (Fig. 3–6).
Histopathological characteristics of watermelon root tissues infected with root-knot nematode (RKN) alone and in combination with Fusarium isolates (FOM, FON, F5) at 7 days after inoculation, showing the formation of immature (G0) and mature giant cells (G) with cytoplasmic depletion or degeneration (G+), replacing functional xylem vessels (X) in control and FI inoculation of lower (10 g cultural medium [CM]/pot) and higher (20 g CM/pot) inoculum densities alone. FOM, Fusarium oxysporum f. sp. melonis; FON, Fusarium oxysporum f. sp. niveum; F5, Fusarium proliferatum isolates obtained from oriental melon greenhouses. Asterisks, phenolic compounds; T, tyloses; M, mucilage; N, nematode; Nc, necrosis. Yellow solid circles contain fungal hyphae and/or spores. Scale bars = 50 μm.
Histopathological characteristics of oriental melon root tissues infected by root-knot nematode (RKN) alone and in combination with Fusarium isolates (FOM, FON, F5, F6) at 7 days after inoculation, showing the formation of immature (G0) and mature giant cells (G) with cytoplasmic depletion or degeneration (G+), replacing functional xylem vessels (X) in control and FI inoculation of lower (10 g cultural medium [CM]/pot) and higher (20 g CM/pot) inoculum densities alone. FOM, Fusarium oxysporum f. sp. melonis; FON, Fusarium oxysporum f. sp. niveum; F5 and F6, Fusarium proliferatum isolates obtained from oriental melon greenhouses. Asterisks, phenolic compounds; T, tyloses; M, mucilage; N, nematode; ETD, extensive tissue destruction. Yellow solid circles contain fungal hyphae and/or spores. Scale bars = 50 μm.

Histopathological characteristics of shintosa root tissues infected by root-knot nematode (RKN) alone and in combination with Fusarium isolates (FOM, FON, F5, F6) at 7 days after inoculation, showing the formation of immature (G0) and mature giant cells (G) with cytoplasmic depletion or degeneration (G+), replacing functional xylem vessels (X) in control and FI inoculation of lower (10 g cultural medium [CM]/pot) and higher (20 g CM/pot) inoculum densities alone. FOM, Fusarium oxysporum f. sp. melonis; FON, Fusarium oxysporum f. sp. niveum; F5 and F6, Fusarium proliferatum isolates obtained from oriental melon greenhouses. Asterisks, phenolic compounds; T, tyloses; M, mucilage; N, nematode. Yellow solid circles contain fungal hyphae and/or spores. White dotted circle indicates necrotized root tissues. Scale bars = 50 μm.
Histopathological characteristics of cucumber root tissues infected by root-knot nematode (RKN) alone and in combination with Fusarium isolates (FOM, FON, F5) at 7 days after inoculation, showing the formation of immature (G0) and mature giant cells (G) with cytoplasmic depletion or degeneration (G+), replacing functional xylem vessels (X) in control and FI inoculation of lower (10 g cultural medium [CM]/pot) and higher (20 g CM/pot) inoculum densities alone. FOM, Fusarium oxysporum f. sp. melonis; FON, Fusarium oxysporum f. sp. niveum; F5, Fusarium proliferatum isolates obtained from oriental melon greenhouses. Asterisks, phenolic compounds; T, tyloses; M, mucilage; N, nematode. Yellow solid circles contain fungal hyphae and/or spores. White dotted circle indicates necrotized root tissues. Scale bars = 50 μm.
Specifically, the production of profound mucilage and phenolic compounds and the formation of intact giant cells without these materials as those in RKN alone were observed in the stele of watermelon infected by F5 with LD alone and in combination with RKN, respectively (Fig. 3). Intact giant cells were also prominently formed in the stele of the other cucurbits as in watermelon with the coinfection of F5 with LD and in combination with RKN; however, mucilage and phenolic compounds were less prominent in the cucurbits infected by F5 with LD alone than in watermelon (Fig. 4–6). Prominent tyloses were formed in xylem vessels of cucumber infected by FOM with HD alone, but not by FOM (with HD) and RKN (Fig. 6). No giant cell was formed in the root tissues of oriental melon by the infection of RKN and F6, in which a large portion of the stele was extensively depleted by the coinfection of RKN and F6 (Fig. 4).
Discussion
Pathological characteristics and interrelations of soilborne diseases on cucurbits such as watermelon, oriental melon, shintosa and cucumber caused by FI and RKN were examined in this study.
The virulence of FI as determined by DI was in the higher order of F. proliferatum F6, F5 and FOM or FON to all cucurbits with no differential interactions between pathogen isolates and cucurbit species examined. Also there were no changes in the higher order of FI virulence due to the RKN co-infection, suggesting the virulence of FI may not be differentially influenced by the host factors, RKN infection and their interactions. No significant increases of DI due to RKN co-infection were shown in all cucurbits examined in this study except watermelon and oriental melon infected by RKN and F. proliferatum isolates (F5 and F6) with LD. This suggests DI increase in the disease complex (due to RKN co-infection) may depend upon cucurbit species, FI and their inoculum densities, all of which influence on the disease incidence and severity (Agrios, 2004).
Considering DI in the cucurbits infected by FI with both LD and HD, watermelon and oriental melon appeared to be mostly susceptible to all FI; however, shintosa and cucumber were more susceptible to F. proliferatum isolates than to F. oxysporum isolates, regardless of RKN co-infection. Especially, F. proliferatum F6 with both inoculum densities and F5 with HD induced susceptible symptoms on shintosa that has been used as root-stock for grafting of the cucurbits for their propagation because of its resistance to the fusarium wilt (Lee, 1994). This suggests that current prevalence of the fusarium wilt in oriental melon greenhouses may be derived from the misidentification of the growers on the fusarium diseases caused by nonauthentic Fusarium species such as F. proliferatum that causes rots of other plant parts including roots and stems (KSPP, 2009; Seo and Kim, 2017).
Histopathological features of the cucurbit root tissues in relation to DI were characterized by the formation of functional xylem vessels structurally intact, accompanying fungal structures (hyphae and/or spores) in the stele and cortex together with the formation of defense structures such as tyloses and xylem mucilage (or phenolic compounds) in variable degrees depending on the cucurbits infected by FI alone. However, the root tissues of the cucurbits infected by FI in combination with RKN showed the defense structures reduced, accompanied by formation of giant cells at variable development stages and with cytoplasmic depletion. Fusarium wilt pathogens begin their infection by penetrating root surfaces and invading into the vascular tissue in the stele, in which the disease development is determined by the time in the formation of the defense structures and their quantities (Crews et al., 2003; Egel and Martyn, 2007). This suggests the prevalence of fungal structures relative to defense structures in the stele may reflect the status of the disease development. Xylem mucilage (phenolic compounds) and tyloses were extensively formed in the root tissues of watermelon infected by F5 with LD and cucumber infected by FOM with HD for both with the low DI of 0.50, respectively. However, DI was significantly increased in these cucurbits by the RKN coinfection, for which the defense structures were diminished clearly, accompanied by the formation of giant cells that plug xylem vessels to enhance water deficiency in aboveground plant parts (Jones, 1981; Mitkowski and Abawi, 2003; Shepherd and Huck, 1989; Sijmons et al., 1994). A significant increase of the fusarium disease severities (DI 2.00–4.75) due to RKN coinfection was also noticed in oriental melon infected by F6 with LD; however, this DI increase might be derived from the extensive destruction of vascular tissues by the F6-RKN coinfection relative to the F6 infection alone, but not from the reduced formation of defense structures in watermelon infected by F5 and RKN as mentioned above.
All of the cucurbits examined in this study showed highly susceptible responses to RKN infection with GI ≥ 3.0 and with EI of 4.25–4.75 (Roberts et al., 1990; Sasser et al., 1984). The highest GI of 3.75 was shown in oriental melon and shintosa, and the lowest (GI = 3.00) in cucumber, while the higher EI of 4.75 was shown in watermelon than in the other cucurbits with EI of 4.25, which suggests the host vulnerability (determined by GI) and suitability for nematode growth and reproduction (determined by EI) may be different among the cucurbits. In this sense, oriental melon and shintosa may be more damaged by RKN infection, and watermelon may be more suitable host for RKN growth and reproduction than the other cucurbits examined in this study.
The histopathological features of the root tissues infected by RKN alone were characterized by the prominent formation of giant cells with intact cytoplasm, occupying a large portion of the stele and rarely accompanied by necrotic responses. The giant cell formation initiates at the inflectional stages that RKN J2 begin to feed after their establishment on the feeding sites, which make their surrounding root tissues become a gall via excessive mitotic cell divisions, suggesting GI and giant cell formations are closely inter-related (Hussey and Grundler, 1998; Sijmons et al., 1994). These giant cells and galls provide RKN with the energy the nematode needs for growth, especially in a large amount at later inflectional stages for the female nematodes to become rapidly swollen due to the production of ovaries and eggs in their body, suggesting EI is related to the persistence of the giant cells containing intact cytoplasmic contents as nutritional sources for the nematode (Jones, 1981; Mitkowski and Abawi, 2003; Shepherd and Huck, 1989; Sijmons et al., 1994). The giant cells formed in the cucurbits infected by RKN alone had all so similar structural features that they could be hardly related to the differences in GI and EI among the cucurbits. There may be other host factors governing the development and persistence of the giant cells. However, the general decreases of GI and EI by the RKN-FI coinfection relative to RKN infection alone could be visualized by the histopathological features showing mostly under-developed giant cells with cytoplasmic contents depleted in their coinfection compared to RKN infection alone. Also the decreases of specific GI and EI values in the cucurbits with the coinfection could be matched to the structural features induced by their coinfection relative to those induced by the RKN infection alone in this study. This strongly suggest the development of giant cells and their cytoplasmic features may be closely related to GI and EI, which determines host vulnerability and suitability, respectively.
There are numerous studies on the inter-relations of microbial pathogens and plant-parasitic nematodes to cause complex diseases in several crops including alfalfa (Griffin and Thyr, 1986), beans (France and Abawi, 1994), tomatoes (Abawi and Barker, 1984; Suleman et al., 1997), coffee (Bertrand et al., 2000), peas (Siddiqui et al., 1999) and bananas (Jonathan and Rajendran, 1998). The giant cells formed by RKN are nutrient-rich and can be utilized as substrate by the fungal pathogens for their growth and development, increasing the disease severity, although RKN infection alone induces no wilt and rot symptoms (Abawi and Chen, 1998; Khan and Muller, 1982; McLean and Lawrence, 1993; Meléndez and Powell, 1970; Seo and Kim, 2017). This suggests the fusarium disease severities in cucurbits infected by FI may be enhanced by the RKN co-infection. However, FI coinfection inhibited the development of giant cells and enhanced their degeneration with the cytoplasmic contents depleted, consequently reducing the fungal disease severities in return owing to the reduced contribution of RKN infection to enhancing DI as shown in this study. More specifically, a significant DI increase due to the RKN coinfection was shown in watermelon infected by F5 with LD (DI of 0.50), but not by F5 with HD (DI of 1.50) and F6 with both inoculum densities (DI of 5.00). Also RKN infection inhibited the formation of defense structures in response to the fungal infection in this study, attenuating plant resistance responses to FI infection (Beckman, 1987; Hall et al., 2011; Inch et al., 2012). All of these aspects suggest the inter-relations of RKN and FI for disease synergism may depend upon virulence of fungal pathogens, inoculum potentials, aggressiveness of the nematode and host vulnerability and suitability for the pathogen growth and development.
Considering the outcomes of inoculation tests in this study, RKN and Fusarium pathogens may not mutually benefit from each other, but the latter benefit more from the former, probably because the fungal pathogen is a necrotrophic parasite benefiting from the cells and tissues decayed by the nematode infection, but not vice versa because the fungal infection generally deteriorates niches and nutritional substrates for the biotrophic parasite, RKN. Therefore, the findings in this study would provide valuable information for understanding pathological characteristics of the soil-borne diseases and principles of their inter-relations in cucurbits that have been growing continuously in greenhouse conditions in Korea, which can be used for the development of their control strategies.
Acknowledgments
This study was conducted with a partial financial support from a project (No. 111042-05-5-SB010) of the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.