 |
 |
| Plant Pathol J > Volume 33(4); 2017 > Article |
Abstract
Incidence rates of diseases in kiwiberry orchards were investigated monthly from late June to late September in Gwangyang and Boseong in 2015 and 2016. The impact of postharvest fruit rot was investigated during ripening after harvest. Bacterial canker was only observed on one single tree in 2015, but black rot, powdery mildew, leaf spot and blight, and postharvest fruit rot diseases were problematic throughout the study period in both 2015 and 2016. Incidence rates of the diseases varied with kiwiberry cultivar, region and sampling time. Incidence rates of powdery mildew, leaf spot and blight diseases increased significantly during the late growing stages near fruit harvest, while black rot peaked in late August. Incidence rate of postharvest fruit rot on fruit without fruit stalks was less than half of fruit with fruit stalks, regardless of kiwiberry cultivars. Among the four cultivars, Mansu was relatively resistant to black rot and postharvest fruit rot diseases. In our knowledge, this is the first report of various potential pathogens of kiwiberry in Korea.
The genus Actinidia consists of at least 70 species, most of which are native to eastern Asia (Ferguson and Huang, 2007). Two most important commercial species worldwide are A. deliciosa (green-fleshed kiwifruit) and A. chinensis (yellow-fleshed kiwifruit) but A. arguta (kiwiberry) is cultivated only in limited regions. While fuzzy fruits of green- and yellow-fleshed kiwifruits are inconvenient to eat, kiwiberries are very convenient for consumption because the grape-sized fruits can be eaten whole, without peeling (Cossio et al., 2015).
The most popular cultivars of green- and yellow-fleshed kiwifruits are Hayward and Hort16A, respectively, both of which were bred in New Zealand. Hayward was introduced to Korea in the late 1970s and Hort16A was introduced to Jeju Island, Korea, in 2004. Since then, many domestically bred cultivars of yellow-fleshed kiwifruit such as Haegeum, Zecy-gold and Halla-gold, as well as other imported yellow- and red-fleshed cultivars have been grown in Korea, and their cultivation area has recently been expanding rapidly due to a strong market demand for kiwifruits (Kim et al, 2016b). Kiwiberry is native to the Korean peninsula and has been commonly found in mountainous regions for thousands of years. Breeding and cultivation of new commercial kiwiberry cultivars have only recently begun in Korea.
Due to higher cultivation acreage, diseases affecting the cultivation of fuzzy kiwifruit have already been reported (Koh, 1995), but little information is available for kiwiberries to date. In this study, we surveyed the incidence rates of major diseases affecting the kiwiberry at two orchards located in Gwangyang and Boseong, Jeonnam Province in Korea in 2015 and 2016. Since only limited commercial kiwiberry cultivars are available, this survey was performed four accessible kiwiberry cultivars, Chiak (A. arguta), Haeyeon (A. arguta), Mansu (A. arguta), and Skinny-green (Actinidia hybrid), at Gwangyang orchard located in Bonggang-myeon, Gwangyang, Jeonnam Province and only two commercial cultivars, Chiak (A. arguta) and Mansu (A. arguta), at Boseong orchard located in Joseong-myeon, Boseong-gun, Jeonnam Province, Korea.
Monthly surveys were conducted to investigate the incidence rates of diseases at the two kiwiberry orchards from January to September in 2015 and 2016. Three trees per cultivar were randomly selected and 100 leaves per tree were carefully checked for any detectable disease symptoms. Ambiguous symptoms were collected and their causal microorganisms were isolated and identified in the laboratory. Disease incidences were scored by the percentages of diseased leaves.
There was no detectable disease on kiwiberry cultivars except for one bacterial canker observed at Gwangyang orchard in early June of 2015. Bacterial canker symptoms with reddish bacterial exudates appeared on the fruit-bearing branches of a single tree of Skinny-green at Gwangyang orchard in late April of 2015. A causal bacterium was consistently isolated from the lesions on the diseased branch and identified as biovar 3 (Psa3) of Pseudomonas syringae pv. actinidiae based on multiplex PCR assay for haplotype differentiation (Koh et al., 2014). Psa3 is responsible for the world-wide outbreaks of bacterial canker on fuzzy kiwifruits, especially on yellow- and red-fleshed cultivars (Butler et al., 2013; Chapman et al., 2012; McCann et al., 2013; Vanneste, 2012). Pollen contaminated with Psa3 turned out to be one of the causal inoculum for recent epidemics of bacterial canker on various cultivars of the fuzzy kiwifruits in Korea (Kim et al., 2016a, 2016b). Since the same kind of Psa3 was isolated from the diseased Skinny-green tree and extra pollen kept in a freezer at the farmerŌĆÖs orchard without epidemic history of bacterial canker, it was assumed that pollen contaminated with Psa3, which was imported from China and used for pollination in 2014, might have been responsible for the bacterial canker on the Skinny-green tree. This assumption was confirmed by random amplification of polymorphic DNA analyses described in a previous study (Kim et al., 2016a).
Several diseases began to appear on kiwiberry cultivars in mid-June. The first ratings were conducted at two orchards in Gwangyang and Boseong on 30th of June 2015, followed by further ratings at 30-day intervals and it was repeated again in 2016. Black rot, powdery mildew and leaf spot and blight diseases were most frequently observed during the growing seasons of kiwiberry in the field (Fig. 1).
The typical symptoms of black rot caused by Alternaria alternata (Kwon et al., 2011) appeared as black spots around the remnants of the calyx of the infected kiwiberry fruits and gradually extended to the inside of the fruits. Three trees per cultivar were selected and 100 remnant of calyx of the kiwiberry fruits were counted. The first recorded disease incidence rates (30th of June 2015) of black rot on cultivars Chiak, Mansu, Haeyeon, and Skinny-green in Gwangyang were 4.5%, 1.0%, 0.5%, and 2.0%, respectively. In Boseong, the initial disease incidence rates on Chiak and Mansu were 2.0% and 0%. Diseases incidence rates tended to increase as the kiwiberry fruits matured. However, they peaked in late August because some of the fruits that had been severely infected by black rot had fallen during the late growing stages. The final disease incidence rates recorded for the flowering season (30th September 2015) for Chiak, Mansu, Haeyeon, and Skinny-green in Gwangyang were 9.5%, 3.5%, 7.5%, and 5.0%, respectively. On the same day, disease incidence rate of 4.0% and 1.5% were recorded for cultivars Chiak and Mansu, respectively, in Boseong. The overall disease incidences of black rot in 2016 were lower than in 2015 (Fig. 2). The incidence rates of black rot varied with kiwiberry cultivars and cultivating regions. Chiak was the most susceptible, whereas Mansu was relatively resistant to black rot in both regions. Slightly higher incidence rates of black rot were observed in Gwangyang compared to Boseong.
Circular, whitish powdery mycelia and proliferous of conidia on leaves are the typical symptoms of powdery mildew caused by Uncinula actinidiae (Shin, 2000). On the 30th of June 2015, powdery mildew had an initial incidence rate of 0.5% only on cultivar Mansu in Gwangyang. Otherwise no powdery mildew symptoms were observed on other cultivars in either region on the first rating of the growing season. Incidence rates of powdery mildew increased significantly during the late growing stages of kiwiberry around the time of fruit harvest. The overall disease incidences of powdery mildew in 2016 were much higher than those in 2015 (Fig. 3). Powdery mildew occurs under dry conditions with high temperatures. The weather in the summer in 2016 was extraordinarily dry and hot compared to 2015. This may account for the severe powdery mildew infections during the kiwiberry growing season in 2016. All the cultivars showed susceptible reactions to powdery mildew.
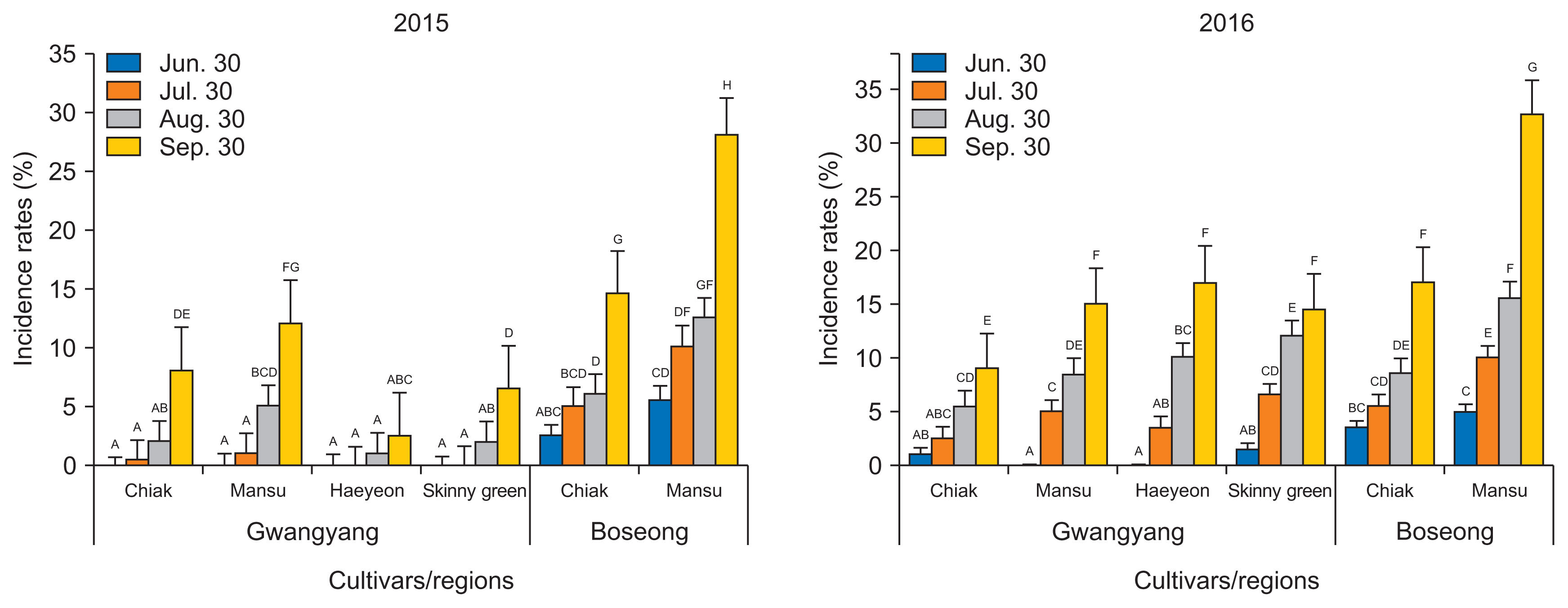
Leaf spot and blight diseases, which are mainly caused by Pestalotiopsis bicilia (Koh et al., 2006), occur as irregular brown spots or blight symptoms on the margins of the infected leaves. However, several other pathogenic fungi such as Glomerella cingulata, Alternaria sp., Phomopsis sp., and Phoma sp. were also isolated from the lesions. Since grayish ring spots, silvering gray leaf blights, and zonate leaf blights were frequently observed on the leaves of fuzzy kiwifruit in addition to brown spots and blights (Jeong et al., 2008), the causal agents of leaf spot and blight diseases on kiwiberry should be elucidated in the future. The incidences of leaf spot and blight diseases on Chiak and Mansu were 2.5% and 5.5%, respectively. In Boseong, but no spots and blight symptoms appeared on the four cultivars in Gwangyang at the first rating on the 30th of June 2015. The incidence rates of leaf spot and blight diseases significantly increased during the late growing stages of kiwiberry around the time of fruit harvest. This might be correlated with leaf aging during the late growing stages. The overall incidence of leaf spot and blight diseases in 2016 were higher than in 2015 (Fig. 4). The occurrence of leaf spot and blight diseases were slightly more frequent in Boseong, and particularly on the cultivar Mansu, which was found to be the most susceptible to leaf spot and blight diseases.
Postharvest fruit rot was investigated in harvested kiwiberry fruits from Gwangyang orchard on 30th of September in 2015 and 2016. Overall, 200 fruits per cultivar were randomly selected and picked with or without fruit stalks of the fruits. The harvested fruits were packed in a polyethylene bag for ripening at room temperature for 7-10 days, after which the incidence rates of postharvest fruit rot were investigated by observation of external and internal symptom development.
Postharvest fruit rot of fuzzy kiwifruits is usually caused by Botryosphaeria dothidea and Diaporthe actinidiae, with B. dothidea being the major pathogen responsible (Koh et al., 2003a, 2005). Ripe rot caused by B. dothidea is found in overripe fruits (Koh et al., 2003b, 2005). Clear external symptoms of ripe rot are sometimes absent from the surface of fruits, but a portion of the fruit surface collapses. Additionally, water-soaked flesh tissue can be seen on the sunken part when the skin of the collapsed portion is peeled back, and milky internal symptoms with dark green margins develops concentrically as the fruit ripens.
Symptoms of stem-end rot caused by D. actinidiae usually appear at the stem-end of the fruit as it ripens (Koh et al., 2005; Lee et al., 2001). The brown pubescent skin at the stem-end becomes soft and lighter in color than the adjacent firm healthy tissues, and a watery exudate and white mycelial mats frequently form at the stem-end, forming a water-drop stain down the sides on the dry brown healthy skin. The affected flesh tissue revealed by peeling the skin is usually water-soaked, disorganized, soft and lighter green than the healthy tissue.
Kiwiberry covered with hairless thin skin seemed to be more vulnerable to postharvest fruit rot than fuzzy kiwifruits, but ripe rot and stem-end rot on a postharvest diseased fruit were not easy to differentiate because of small lesion size and mixed, complex symptoms on kiwiberry. Although external symptoms usually co-occur with internal symptoms postharvest, a great amount of kiwiberries with a healthy exterior were found to be spoiled after the skin of the fruit was peeled back (Koh et al., 2003a).
The overall mean disease incidences of postharvest fruit rot on the fruits with fruit stalks and those without fruit stalks in 2015 and 2016 were 33.3% and 17.2%, respectively (Table 1). These findings indicated that fruit stalks might be sufficient sources of inoculum to double the incidence rate of fruit rot. Dead fruit stalks were reported to be one of the important sources of inoculum for bacterial blossom blight of fuzzy kiwifruits (Shin et al., 2004). Therefore, it is recommended that removing fruit stalks from the kiwiberry fruits during harvest will help to alleviate the burden of postharvest fruit rot.
The incidence rate of postharvest fruit rot in 2016 only showed a slight decrease from 2015. However, the incidence rates of postharvest fruit rot varied from cultivar to cultivar. Mansu fruits showed the lowest mean incidence rates of 12.9% when harvested with fruit stalks and 10.8% when harvest without, whereas Chiak had the highest mean incidence rates of 72.3% and 31.8% when harvested with and without fruit stalks, respectively. Among the four cultivars, Mansu was relatively resistant to black rot as well as postharvest fruit rot diseases. This is the first report of various potential pathogens of kiwiberry in Korea and would guide the recognition of disease characteristics and support the development of disease control strategies for the rapidly growing kiwiberry industry.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (114076-03-2-HD030).
Fig.┬Ā1
Symptoms of major diseases of kiwiberry. Chlorotic halo lesions (A) on a leaf and bacterial ooze (B) on a branch infected by bacterial canker. Black rot (C, D) on a fruit. Powdery mildew (E) on leaves. Brown spots (F) and blight (G) on a leaf. Postharvest fruit rot (H, I).
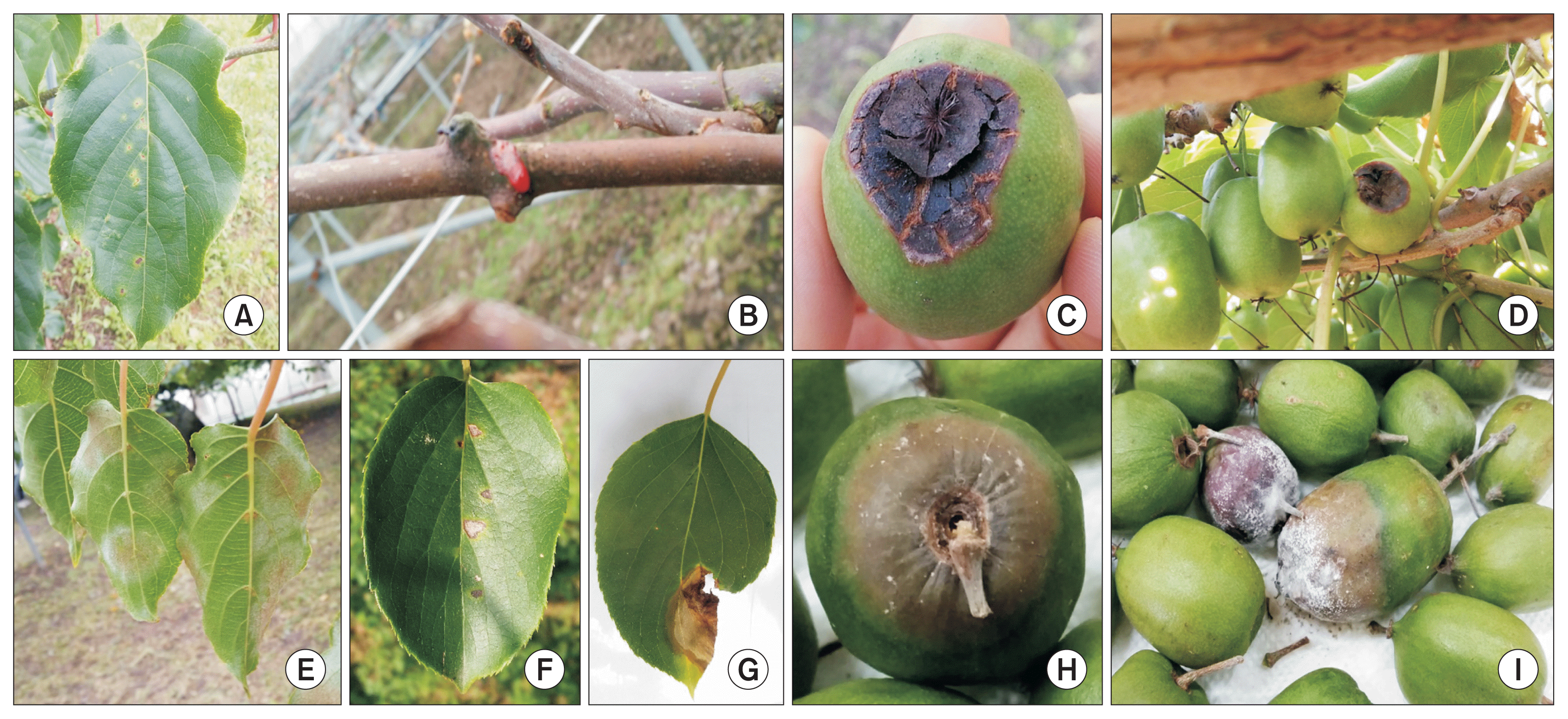
Fig.┬Ā2
Incidence rates of black rot on kiwiberry cultivars in Gwangyang and Boseong, Jeonnam Province, Korea during the growing season in 2015 and 2016. Error bars represent SD of the mean of three independent experiments. Bars with the same letters are not significantly different (TukeyŌĆÖs multiple comparisons test, P < 0.05).
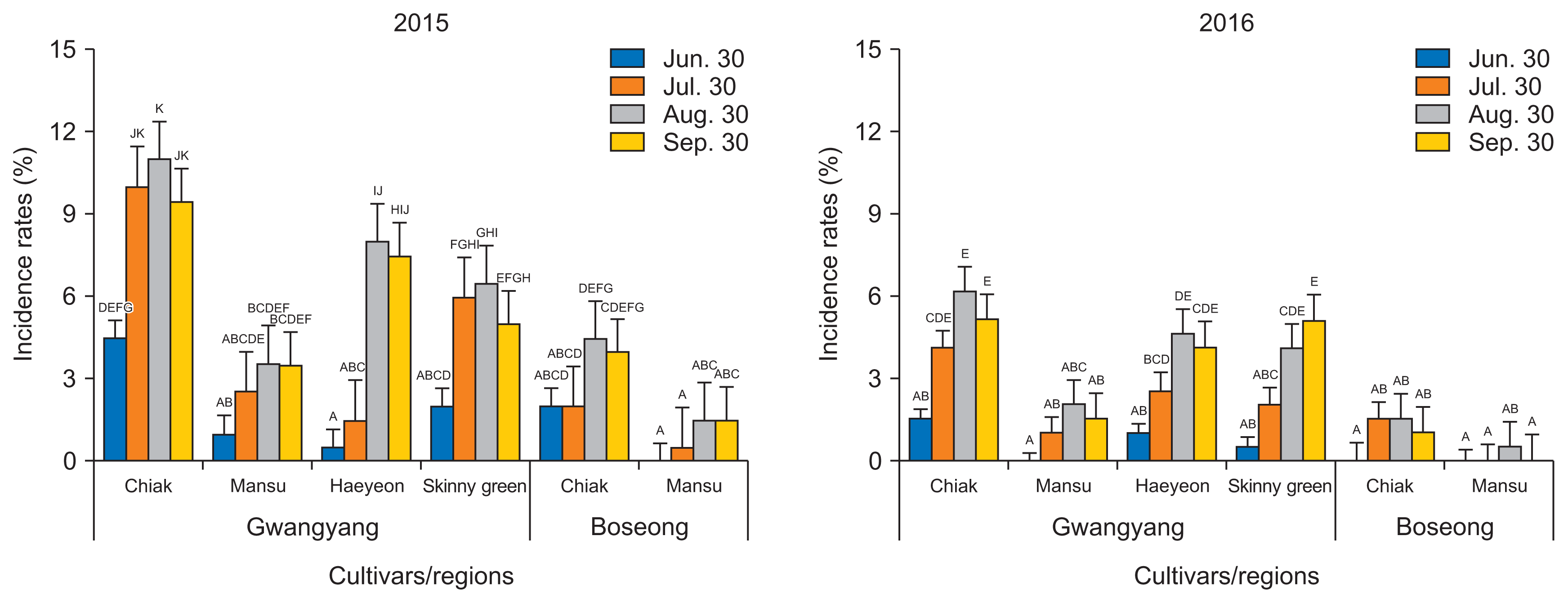
Fig.┬Ā3
Incidence rates of powdery mildew on kiwiberry cultivars in Gwangyang and Boseong, Jeonnam Province, Korea during the growing season in 2015 and 2016. Error bars represent SD of the mean of three independent experiments. Bars with the same letters are not significantly different (TukeyŌĆÖs multiple comparisons test, P < 0.05).

Fig.┬Ā4
Incidence rates of leaf spot and blight diseases on kiwiberry cultivars in Gwangyang and Boseong, Jeonnam Province, Korea during the growing season in 2015 and 2016. Error bars represent SD of the mean of three independent experiments. Bars with the same letters are not significantly different (TukeyŌĆÖs multiple comparisons test, P < 0.05).
Table┬Ā1
Incidence rates of postharvest fruit rot on four kiwiberry cultivars harvested in Gwangyang, Jeonnam Province, Korea in 2015 and 2016
| Storage methods | Cultivars | Disease incidences (%) | ||
|---|---|---|---|---|
|
|
||||
| 2015 | 2016 | Mean | ||
| Storage with fruit stalks | Mansu | 14.5BC a | 11.3AB | 12.9 |
| Skinny-green | 22.5D | 21D | 21.8 | |
| Haeyeon | 28.5E | 23.5D | 26 | |
| Chiak | 75.5H | 69G | 72.3 | |
| Mean | 35.3 | 31.2 | 33.3 | |
| Storage without fruit stalks | Mansu | 9.5A | 12AB | 10.8 |
| Skinny-green | 11AB | 10A | 10.5 | |
| Haeyeon | 17C | 14.5BC | 15.8 | |
| Chiak | 34F | 29.5E | 31.8 | |
| Mean | 17.9 | 16.5 | 17.2 | |
References
Butler, MI, Stockwell, PA, Black, MA, Day, RC, Lamont, IL and Poulter, RT 2013. Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PLoS ONE. 8:e57464



Chapman, JR, Taylor, RK, Weir, BS, Romberg, MK, Vanneste, JL, Luck, J and Alexander, BJ 2012. Phylogenetic relationships among global populations of Pseudomonas syringae pv. actinidiae. Phytopathology. 102:1034-1044.


Cossio, F, Debersaques, F and Latocha, P 2015. Kiwiberry (Actinidia arguta): New perspectives for a great future. Acta Hortic. 1096:423-434.

Ferguson, AR and Huang, H 2007. Genetic resources of kiwifruit: domestication and breeding. Hortic Rev. 33:1-121.


Jeong, IH, Lim, MT, Kim, GH, Han, TH, Kim, HC, Kim, MJ, Park, HS, Shin, SH, Hur, JS, Shin, JS and Koh, YJ 2008. Incidences of leaf spots and blights on kiwifruit in Korea. Plant Pathol J. 24:125-130.

Kim, GH, Choi, ED, Lee, YS, Jung, JS and Koh, YJ 2016a. Spread of bacterial canker of kiwifruit by secondary infection of Pseudomonas syringae pv. actinidiae biovar 3 in Gyeongnam in 2016. Res Plant Dis. 22:276-283.

Kim, GH, Kim, KH, Son, KI, Choi, ED, Lee, YS, Jung, JS and Koh, YJ 2016b. Outbreak and spread of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae biovar 3 in Korea. Plant Pathol J. 32:545-551.


Koh, YJ 1995. Economically important diseases of kiwifruit. Res Plant Dis. 1:3-13.
Koh, YJ, Hur, JS and Jung, JS 2005. Postharvest fruit rots of kiwifruit (Actinidia deliciosa) in Korea. N Z J Crop Hortic Sci. 33:303-310.

Koh, YJ, Hur, JS and Shin, HD 2006. Pestalotiopsis in Korea. National Institute of Agricultural Science and Technology, 101.
Koh, YJ, Lee, JG and Hur, JS 2003a. Incidences and causal agents of postharvest fruit rots in kiwifruits in Korea. Res Plant Dis. 9:196-200.

Koh, YJ, Lee, JG, Lee, DH and Hur, JS 2003b. Botrysphaeria dothidea, the causal organism of ripe rot of kiwifruit (Actinidia deliciosa) in Korea. Plant Pathol J. 19:227-230.

Koh, HS, Kim, GH, Lee, YS, Koh, YJ and Jung, JS 2014. Molecular characteristics of Pseudomonas syringae pv. actinidiae strains isolated in Korea and a multiplex PCR assay for haplotype differentiation. Plant Pathol J. 30:96-101.



Kwon, JH, Cheon, MG, Kim, J and Kwack, YB 2011. Black rot of kiwifruit caused by Alternaria alternata in Korea. Plant Pathol J. 27:298

Lee, JG, Lee, DH, Park, SY, Hur, JS and Koh, YJ 2001. First report of Diaporthe actinidiae, the causal organism of stem-end rot of kiwifruit in Korea. Plant Pathol J. 17:110-113.
McCann, HC, Rikkerink, EHA, Bertels, F, Fiers, M, Lu, A, ReesGeorge, J, Andersen, MT, Gleave, AP, Haubold, B, Wohlers, MW, Guttman, DS, Wang, PW, Straub, C, Vanneste, J, Rainey, PB and Templeton, MD 2013. Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathog. 9:e1003503



Shin, HD 2000. Erysiphaceae of Korea. National Institute of Agricultural Science and Technology, 320.
- TOOLS
-
METRICS

- Related articles
-
Incidences of Leaf Spots and Blights on Kiwifruit in Korea2008 June;24(2)
Survey on the Occurrence of Abiotic Diseases on Kiwifruit in Korea2007 December;23(4)
The Incidence of Virus Diseases on Melon in Jeonnam Province during 2000-20022007 September;23(3)
Incidence and Ecology of Major Diseases on Peach in Gyeongbuk Province1995 September;11(3)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



