Characterization of Bacillus amyloliquefaciens DA12 Showing Potent Antifungal Activity against Mycotoxigenic Fusarium Species
Article information
Abstract
In an attempt to develop a biological control agent against mycotoxigenic Fusarium species, we isolated Bacillus amyloliquefaciens strain DA12 from soil and explored its antimicrobial activities. DA12 was active against the growth of mycotoxigenic F. asiaticum, F. graminearum, F. proliferatum, and F. verticillioides both in vitro and in planta (maize). Further screening using dual culture extended the activity range of strain DA12 against other fungal pathogens including Botrytis cinerea, Colletotrichum coccodes, Endothia parasitica, Fusarium oxysporum, Raffaelea quercus-mongolicae, and Rhizoctonia solani. The butanol extract of the culture filtrate of B. amyloliquefaciens DA12 highly inhibited the germination of F. graminearum macroconidia with inhibition rate 83% at a concentration of 31.3 μg/ml and 100% at a concentration of 250 μg/ml. The antifungal metabolite from the butanol extract was identified as iturin A by thin layer chromatography-bioautography. In addition, volatile organic compounds produced by DA12 were able to inhibit mycelial growth of various phytopathogenic fungi. The volatile compounds were identified as 2-heptanone, 5-methyl heptanone and 6-methyl heptanone by gas chromatography-mass spectrometry (GC-MS) analysis. These results indicate that the antagonistic activity of Bacillus amyloliquefaciens DA12 was attributable to iturin A and volatile heptanones, and the strain could be used as a biocontrol agent to reduce the development of Fusarium diseases and mycotoxin contamination of crops.
Introduction
Many Fusarium species are important plant pathogens causing serious diseases such as ear rot of maize, and scab (head blight) of cereals attributable to various mycotoxins, the most common of which are deoxynivalenol, zearalenone, and the fumonisins. Fusarium contaminates cereals worldwide, including wheat, barley, corn, and rice. Consumption of contaminated crops may seriously affect both human and animal health (Desjardins, 2006).
As control measures, contamination may either be prevented, or the contaminants removed. Prevention may feature chemical control, or the use of crop varieties resistant to fungal infections and the effects of mycotoxins. As fungal infections are often natural, fungicides are generally applied. Efforts are underway to breed crops resistant to (principally) the scab fungus Fusarium graminearum, but have not yet been successful. Mycotoxin removal is difficult because most such agents are chemically stable. Mycotoxin absorbents or binders (e.g., clays) have been developed, but their efficacies remain both variable and low (Jard et al., 2011). Enzymes and microbes that degrade toxins have been actively sought, but only a few commercial products are available.
Although chemical fungicides are widely used to control both disease and mycotoxin production, biological control methods are preferable, being both environmentally friendly and safe. Bacillus is well-known to produce a variety of antibiotics and serves as a biological agent targeting many phytopathogens. Numerous studies have found that Bacillus species effectively controlled several important plant pathogens including Podospora fusca (Romero et al., 2007), Xanthomonas campestris, Pectobacterium carotovorum (Zerlouh et al., 2011), F. graminearum (Crane et al., 2013; Zhao et al., 2014), and many others. Of the various antimicrobial compounds produced by Bacillus, cyclic lipopeptides including iturin, fengycin, and surfactin are known to effectively control plant disease and have been well-studied (Ongena and Jacques, 2008). Such compounds are produced by both B. subtilis and B. amyloliquefaciens.
In an attempt to develop a biological control agent against mycotoxigenic Fusarium species, we isolated a B. amyloliquefaciens strain and tested its antimicrobial activity. Therefore, the objectives of this study were 1) to examine the antagonistic activity of the B. amyloliquefaciens strain against mycotoxigenic Fusarium species in vitro and in planta, 2) to explore the range of antibiotic activity of the strain further targeting various fungal pathogens, and 3) to identify the substances responsible for the antimicrobial activity.
Materials and Methods
Microbial isolation
Microbes were isolated from soil in which tomatoes grew in a greenhouse in Buyeo-gun, Chungcheong Province. Soil (3 g) was suspended in 27 ml of peptone water (PW, Difco, USA), homogenized using a stomacher (Bagmixer 400VW, Interscience, France) for 2 min at low speed, and serially diluted (10-fold). The diluted soil suspensions (100 μl) were spread on tryptic soy agar (TSA) (Difco, USA) plates and incubated at 28°C for 16 h. Single bacterial colonies were transferred to fresh TSA plates.
Identification of DA12
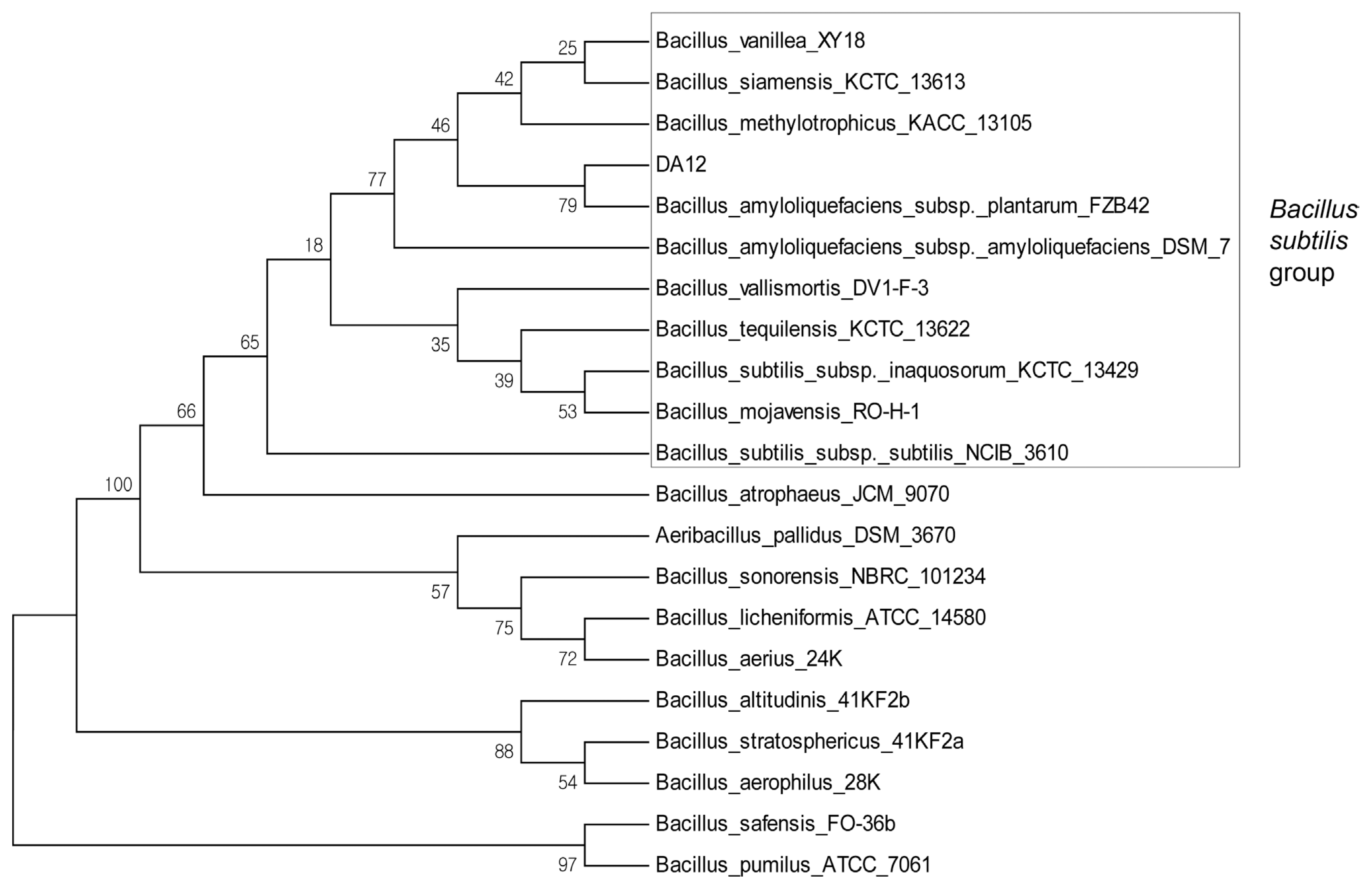
Bacterial identification was performed as previously described (Joo et al., 2015); 16S rRNA sequences were amplified using the primer pair 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Eden et al., 1991). For phylogenetic analysis, the 16S rRNA sequences (~1.4 kb) of various Bacillus species from the NCBI database were aligned using the Clustal W multiple alignment method and a maximum parsimony tree (1,000 bootstrap replicates) was constructed using MEGA version 6 software (Tamura et al., 2011).
Dual culture assay for antifungal activity
We screened microbial isolates, using the dual culture method, for activity against eight strains of mycotoxigenic Fusarium held in the culture collection of the National Institute of Agricultural Sciences. The four F. graminearum strains produced deoxynivalenol (DON) together with 3-acetyl DON (H7-4, H7-11) or 15-acetyl DON (Z-3639, H-11); the two F. asiaticum strains produced nivalenol (NIV) with 4-acetyl NIV (SCK04, R308); and the F. verticillioides (Fv) and F. proliferatum (Fp) strains were fumonisin producers (data not shown). Dual culture was performed as described previously (Joo et al., 2015) and the assay was repeated two times with three replicates. Additional 6 fungal pathogens including Botrytis cinerea, Colletotrichum coccodes, Endothia parasitica, Fusarium oxysporum f. sp. lycopersici, Raffaelea quercus-mongolicae, and Rhizoctonia solani were tested in a dual culture assay. These strains were held in the laboratory collection (maintained at –80°C) of Chonnam National University. The antifungal activity was calculated according to the following formula: Inhibition activity (%) = [(radius of fungal mycelia of untreated control − radius of fungal mycelia inhibited by DA12)/radius of fungal mycelia of untreated control] × 100.
In vivo assay of the effect of DA12 culture filtrate on maize
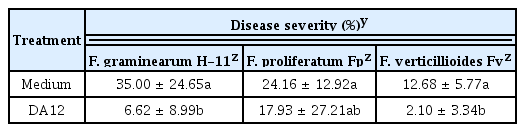
To explore whether DA12 exerted an anti-Fusarium activity in vivo, maize was used because it is a common host of mycotoxigenic F. graminearum, F. verticillioides and F. proliferatum. Full-grown maize ears (cv. Taihakchal) were purchased from a retail market and the assay was performed as described previously (Joo et al., 2015). Each ear was horizontally cut into three pieces and surface-sterilized with NaOCl (1%) for 2 min. The ear cuts were washed once with sterile water and blot dried. DA12 was grown in nutrient broth (NB) for 5 days and the culture was filtered through a 0.2 μm membrane (Advantec, Japan). Conidia (1 × 105/ml) of F. verticillioides (Fv), F. proliferatum (Fp), and F. graminearum (H-11) were prepared from 5 day carboxylmethylcellulose (CMC) medium culture. For inoculation, a spot between kernels in the middle of ear cut was pricked with 15 μl of DA12 culture filtrate or NB (for control). Then, the same volume of conidia suspension was injected into the inoculation spot. The inoculated cuts were placed on a rack in a plastic chamber (30 × 40 × 12 cm) layered with 200 ml of distilled water. The chamber was covered with plastic wrap then incubated at 25°C for 3 days. Ear rot (%) was calculated by counting the total number of kernels per cut and the number of kernels with fungal growth. Each treatment used six ear cuts and the data was analyzed by two-way ANOVA (SAS Institute Inc., USA).
Inhibitory activity against conidia germination of F. graminearum
The butanol extract of the culture supernatant of DA12 was tested for inhibitory activity against macroconidia germination of F. graminearum (Z-3639). A single DA12 colony from a TSA plate was inoculated into tryptic soy broth (TSB) and grown at 30°C (150 rpm) overnight. Then, a 1 ml amount of the culture was transferred into fresh TSB medium (100 ml) and incubated at 30°C (150 rpm) for 2 days. The culture was centrifuged at 7,000 rpm for 20 min and then 30 ml of the supernatant was extracted twice with equal volumes of butanol. The butanol extracts were pooled and concentrated to dryness on a rotary evaporator. The butanol extract was dissolved at 50 mg/ml in methanol and filtered through a 0.2-μm pore size membrane (Advantec) to remove the bacteria. The axenic butanol extract was tested at concentrations of 500, 250, 125, 62.5, and 31.25 μg/ml.
Macroconidia were obtained from F. graminearum (Z-3639) incubated in a CMC medium for 4 to 7 days (Witt et al., 1985). A macroconidia suspension was filtered through 4 layers of sterilized gauze to remove mycelium. The macroconidia suspension was adjusted to 1.0 × 106 macroconidia/ml by using haemocytometer. Methanol (1%) was used as a negative control. The final volume of the conidia suspension treated with the butanol extract of DA12 was 200 μl in each well in 48-well plate. The plates were cultured at 25°C with agitation (200 rpm). The germination percentage of conidia was counted when the conidia in the control wells germinated 100%. One hundred conidia for each experiment were observed by microscopy. Macroconidia were considered germinated if the germ tube length was greater than equal to the greatest dimension of the swollen spore (Dantigny et al., 2006).
The inhibitory activity of macroconidia germination was calculated according to the following formula: Inhibition activity (%) = [(germination rate of control − germination rate of treatment)/germination rate of control] × 100. The experiment was repeated twice with three replicates.
Characterization of agar-diffusible antifungal metabolites
In order to determine the agar diffusible antifungal metabolite using thin layer chromatography-direct bioautography, the butanol extract solution of DA12 (12 μl) was spotted onto a thin layer chromatography (TLC) plate (Kiegel 60, 0.25 mm thick, 1 cm × 8 cm; Merck, Germany) and developed in chloroform:methanol:water (14:6:1, v/v/v). After drying, the plate was sterilized under UV light on a clean bench for 10 min. Then the plate was placed in a Petri dish and then spread with PDA containing conidia of F. graminearum (Z-3639, 106 spores/ml), followed by incubation at 25°C until mycelia grew. The experiment was repeated two times with two replicates. When microbial growth was retarded, the activity in that region was compared with that of iturin A (Sigma-Aldrich, USA) to determine if the inhibitory substance was in fact iturin A. After spotting the butanol extract and iturin A standard, The TLC plate was developed in the same solvent system described above. Then, the TLC plate was dried and sprayed with distilled water to detect cycliclipopeptides.
Antimicrobial activities of volatiles
To explore if strain DA12 produced volatiles exhibiting antifungal activity, I-plate petri dishes with two separate sections (SPL Life Sciences, Korea) were used. One compartment received TSA (7 ml), while the other received TSA (7 ml, for bacteria) or potato dextrose agar (PDA, for fungi). For antifungal activity, PDA culture plugs (5 mm in diameter) of B. cinerea, E. parasitica, R. quecus-mongolicae, and F. graminearum were placed in the middle of PDA compartment. The TSA compartment was spread with 50 μl of DA12 culture suspension as described above and the plates were incubated at 25°C for 3–7 days. The experiment was repeated twice with three replicates.
Gas chromatography-mass spectrometry (GC-MS) analysis of volatiles produced by DA12
A single DA12 colony grown in TSA was inoculated into 5 ml of TSB and grown at 30°C with aeration and shaking (150 rpm) for 24 h. The culture was then inoculated into 50 ml of TSB in a 500 ml flask and further incubated for 72 h. The culture (5 ml) was homogenized in 10 ml of distilled water (sterile TSB served as the control). For GC-MS analysis, a QP-2010 Ultra (Shimadzu Co., Japan) instrument equipped with an Rtx-5ms column (30 m × 0.25 mm ID, 0.25 μm film thickness) through which helium flowed at 1 ml/min was used. The TSB culture (1 ml) was diluted with 4 ml of distilled water and placed in a vial. The diluted sample was heated at 60°C for 30 min and 1 ml of the headspace was injected using a gas syringe (Shioma and Shibamoto, 1990). The column was maintained at 40°C for 2 min, then at 250°C for 13 min after the temperature had been increased at 6°C per min. The injection port and interface were at 240°C and 200°C, respectively. The mass spectra of various compounds were compared with those in the NIST/EPA/NIH MS Library (version 2.0).
Results
Antifungal activities of DA12
Initial screening using the dual culture system revealed that DA12 exhibited antifungal activities against eight strains of toxigenic Fusarium spp. DA12 inhibited the mycelial growth of all strains tested that produced NIV, DON (and its acetylated derivatives), and fumonisin (Table 1). Growth inhibition ranged from 57.1% to 74.0%. To explore the range of antifungal activity, we included various fungal phytopathogens in further screening. DA12 also inhibited the growth of six other pathogens including Botrytis cinerea (cucumber grey mould), Colletotrichum coccodes (pepper anthracnose), Endothia parasitica (chestnut blight), F. oxysporum f. sp. lycopersici (tomato wilt), Raffaelea quercus-mongolicae (oak wilt), and Rhizoctonia solani (rice sheath blight) (Table 1). Growth inhibition ranged from 31.6% to 95.5%. F. oxysporum f. sp. lycopersici was the most insensitive, whereas B. cinerea was the most sensitive.
Phylogenetic analysis of DA12
Phylogenetic analysis identified DA12 as Bacillus amyloliquefaciens of the B. subtilis group (Fig. 1). The strain has been deposited in the KACC (Korean Agricultural Culture Collection, Jeonju, Korea) as KACC 92144P and the 16S rRNA sequence has been deposited in Genbank (accession no. MF326683).
In vivo maize assay of the DA12 culture filtrate
DA12 culture filtrate effectively inhibited fungal growth in maize ears. When the culture filtrate and Fusarium conidia were co-inoculated, growth of all strains tested was reduced by day 3 (Fig. 2). ANOVA showed that DA12 significantly suppressed the growth of both F. verticillioides and F. graminearum (Table 2), and that of F. proliferatum to a lesser extent.
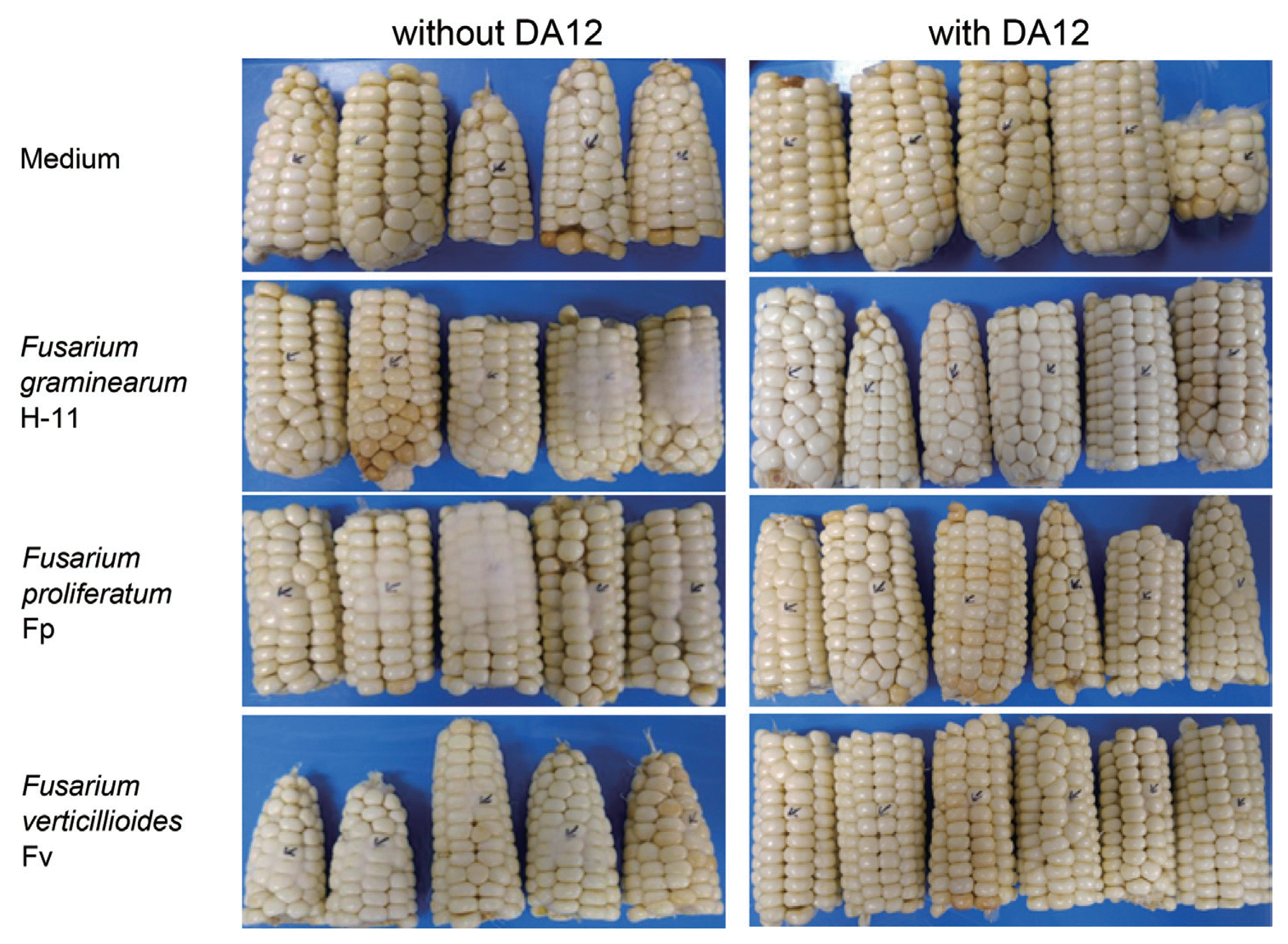
In vivo anti-Fusarium activity exhibited by DA12 culture filtrate on maize. The left panel (−DA12) shows medium only control and ear rot caused by three Fusarium species. The right panel (+DA12) shows control (DA12 culture filtrate only) and ears treated with the culture filtrate plus respective fungal conidia. Arrowheads indicate the points of inoculation. The photographs were taken 3 days after inoculation.
Inhibitory activity of DA12 against conidia germination
DA12 inhibited conidia germination of F. graminearum. When cell-free culture medium of DA12 was added to conidia at a range of 0.625% to 10% [v/v], conidial germination was completely inhibited at 10% and the extent of inhibition was dose-dependent (data not shown). The butanol extract of DA12 culture filtrate also efficiently inhibited germination of F. graminearum macroconidia. At a concentration of 31.3 μg/ml, 83% of the conidia did not germinate. At 250 μg/ml, the germination of F. graminearum macroconidia was completely inhibited (data not shown).
Identification of antifungal metabolites from DA12
Bioautography using TLC revealed a clear zone (at Rf = 0.34) on a TLC plate (Fig. 3A), indicating the presence of antifungal substances. On TLC analysis with iturin A standard, the active metabolite was identified as iturin A (Fig. 3B).
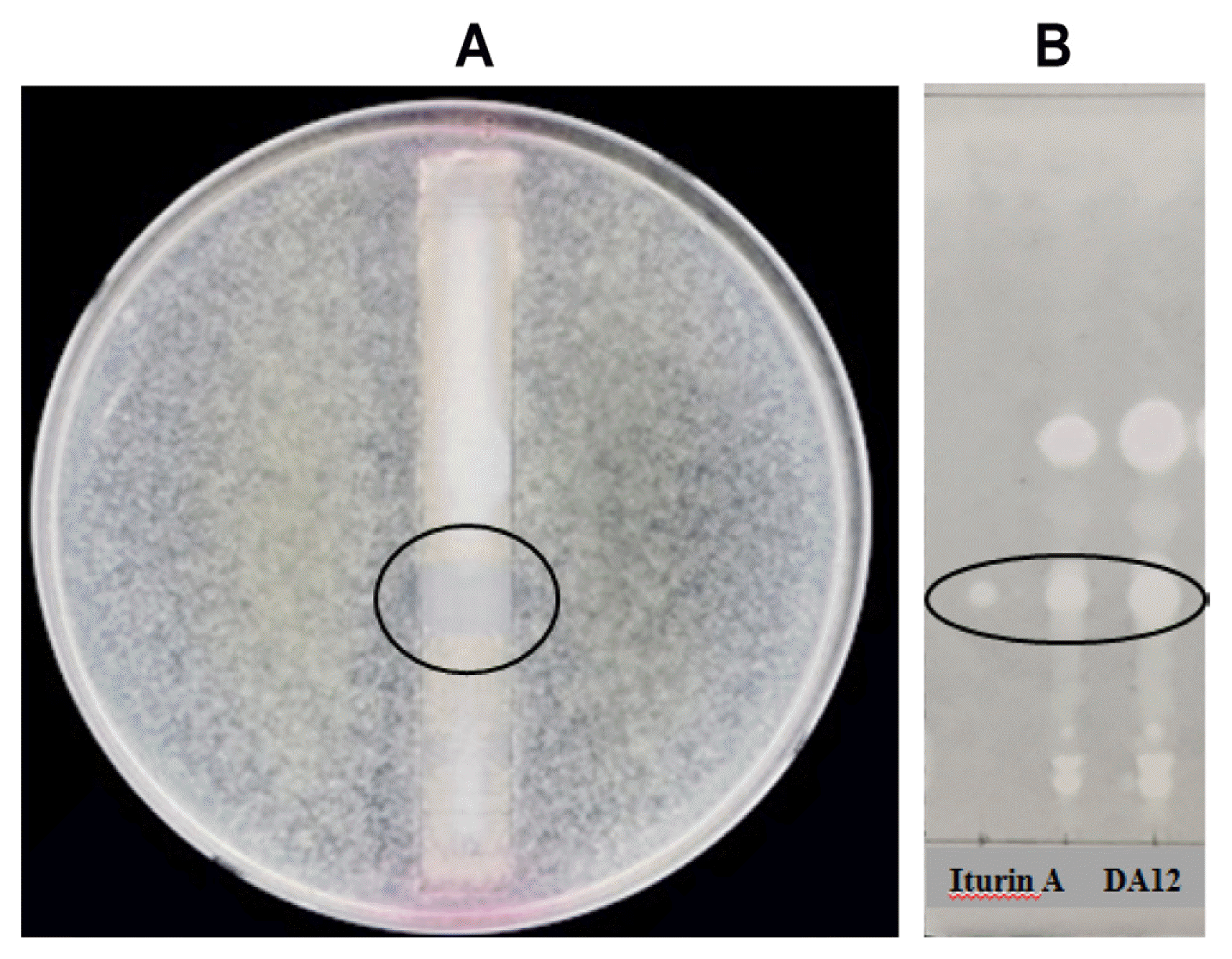
TLC bioautography of the butanol extract of DA12 against F. graminearum (A) and TLC analysis of the butanol extract of DA12 with iturin A (B). The TLC plates were developed in a solvent system of chloroform:methanol:water (14:6:1, v/v/v). The circle in panel A indicates a clear zone on the TLC plate.
The volatile organic compounds (VOCs) produced by DA12 also inhibited the growth of B. cinerea, E. parasitica and R. quercus-mongolicae, but not the growth of F. graminearum (Table 3). In addition, the VOCs inhibited the growth of phytopathogenic bacteria such as Agrobacterium tumefaciens, Burkholderia glumae, Clavibacter michiganensis subsp. michiganensis, Pseudomonas syringae pv. actinidiae, Xanthomonas arboricola pv. pruni, Ralstonia solanacearum (data not shown).
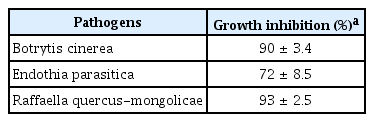
Inhibitory activity of volatile compounds produced by DA12 agianst mycelial growth of phytopathogenic fungi
GC-MS analysis of the DA12 volatiles revealed three peaks eluting between 6 and 9 min; the volatiles were 2-heptanone (6.51 min), 6-methyl-2-heptanone (8.17 min), and 5-methyl-2-heptanone (8.43 min) (Fig. 4).
Discussion
Bacillus amyloliquefaciens is well-known to produce antimicrobial compounds and DA12 exhibited antimicrobial activities against diverse fungal plant pathogens, including four mycotoxigenic fungi (F. asiaticum, F. graminearum, F. proliferatum, and F. verticillioides) and six other phytopathogenic species (B. cinerea, C. coccodes, E. parasitica, F. oxysporum f. sp. lycopersici, R. quercus-mongolicae, and R. solani). It was earlier found that several B. amyloliquefaciens strains were active against Colletotrichum dematium, Glomerella cingulata, Pyricularia oryzae, Rosellinia necatrix (Yoshida et al., 2001), and R. solani (Yu et al., 2002). Fusarium graminearum (Gong et al., 2015) and seven citrus pathogens including Alternaria citri, Botrysphaeria sp., Colletotrichum gloeosporioides, Fusicoccum aromaticum, Lasiodiplodia theobromae, Penicillium crustosum, and Phomopsis persea (Arrebola et al., 2009) were also inhibited by B. amyloliquefaciens.
The antifungal activities of B. amyloliquefaciens have received the most attention but less is known about its antibacterial activities. Bacillus amyloliquefaciens was previously reported to be active against Agrobacterium tumefaciens, Clavibacter michiganensis subsp. michiganensis, Pectobacterium carotovorum subsp. carotovorum, and X. campestris pv. campestris (Yoshida et al., 2001). We found that DA12 was also active against all the bacterial pathogens listed above and four additional bacterial species including Burkholderia glumae, P. syringae pv. actinidiae, Xanthomonas arboricola pv. pruni, and Ralstonia solanacearum (data not shown). Further extension of the antimicrobial range may be possbile as B. amyloliquefaciens was reported to inhibit human pathogens including Candida albicans (Song et al., 2013) and Vibrio spp. (Xu et al., 2014).
The antimicrobial activities of B. amyloliquefaciens are largely attributable to cyclic lipopeptides including iturin, fengycin, and surfactin (Ongena and Jacques, 2008). Both B. subtilis and B. amyloliquefaciens produce these lipopeptides (Ongena and Jacques, 2008; Wang et al., 2007; Yoshida et al., 2001); most antimicrobial activity is attributed to iturin and fengycin. Iturin is also produced by other Bacillus species including B. licheniformis (Kong et al., 2010) and B. megaterium (Dey et al., 2015). Iturins A and C, and bacillomycin, are heptapeptides linked to a β-amino fatty acid chain that is variable in length and the antimicrobial activity is attributable to osmotic perturbation caused upon formation of ion-conducting pores (Ongena and Jacques, 2008). In addition, iturin A inhibited the proliferation of, and induced apoptosis in, breast cancer cells (Dey et al., 2015). The fungicidal activity of iturin A against F. graminearum was attributable to hyphal distortion and aggregation, and inhibition of branch formation (Gong et al., 2015). We showed directly, in the TLC assay, that iturin A produced by DA12 was principally responsible for fungal inhibition.
In addition to iturin A, several volatiles inhibiting both fungi and bacteria were produced by DA12. The volatile 2-heptanone is a methyl n-amyl ketone acting as both a pheromone and an anesthetic (https://en.wikipedia.org/wiki/2-Heptanone/). We did not explore the mechanism of action in the present study, but found that heptanone could also act as an antibiotic. Previously, 2-heptanone was reported to be toxic to both insect and plant fungal pathogens such as Pythium irregular, Rhizoctonia solani, and Fusarium oxysporum as the compound inhibited radial colony growth and spore germination of the fungi (Cole and Blum, 1975). Bacillus atrophaeus was earlier reported to produce volatile antifungal agents and antifungal lipopeptides (Zhang et al., 2013). Additional nonvolatile antimicrobial metabolites were produced by DA12, warranting further study.
Many fungal and bacterial strains are used to control mycotoxigenic Fusarium species. The bacteria include Brevibacillus sp. (Joo et al., 2015), Bacillus pumilus (Munimbazi and Bullerman, 1998), B. amyloliquefaciens (Crane et al., 2013; Gong et al., 2015), B. subtilis (Zhao et al., 2014), Spirulina sp. (Pagnussatt et al., 2014), Sphingomonas (Wachowska et al., 2013), and Streptomyces sp. (Jung et al., 2013). The fungi include Clonostachys rosea (Xue et al., 2014), Trichoderma (Matarese et al., 2012), and yeast (Matic et al., 2014). However, most of these strains target only F. graminearum which causes cereal head blight and produces only DON. There is one report of a B. subtilis strain active against F. verticillioides which causes maize ear rot and produces fumonisins (Cavaglieri et al., 2005). Previously, we isolated Brevibacillus species targeting F. asiaticum, F. graminearum, F. proliferatum, and F. verticillioides that produce NIV, DON, zearalenone, and fumonisin, respectively. These fungi are often found in barley, corn, rice, and wheat growing in Korea (Joo et al., 2015). As is true of Brevibacillus, DA12 may be a versatile biological agent, targeting not only four mycotoxigenic species but also 13 fungal and bacterial phytopathogens. DA12 can also be a valuable biocontrol agent for maize as DA12 effectively controlled F. verticillioides and F. graminearum, the two principal ear rot pathogens of maize. Effective inhibition of mycotoxigenic fungal strains by using DA12 can reduce production of mycotoxins.
Acknowledgments
This study was carried out with the support of “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ010856)”, National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.