 |
 |
| Plant Pathol J > Volume 34(1); 2018 > Article |
Abstract
Acknowledgments
Fig. 1
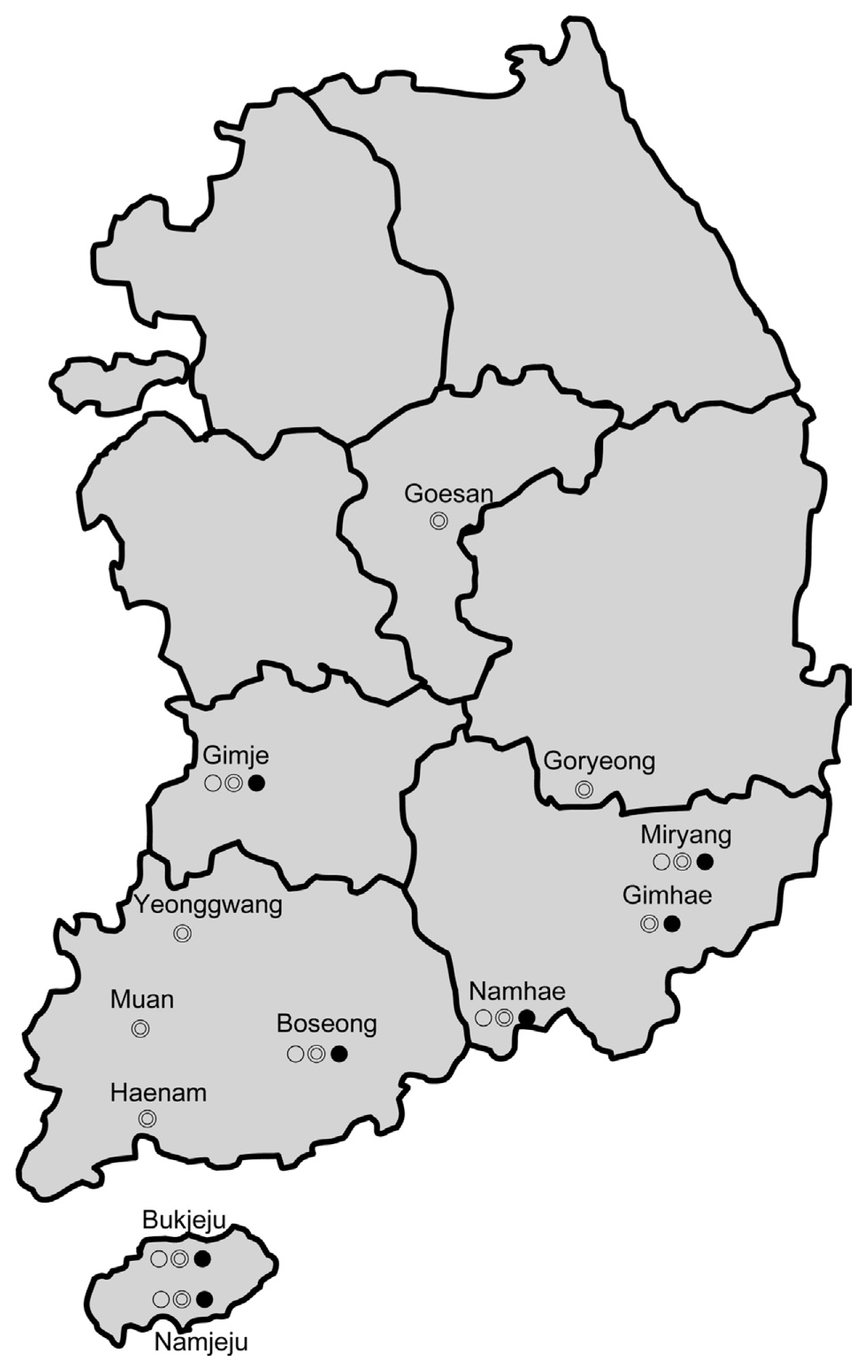
Fig. 2
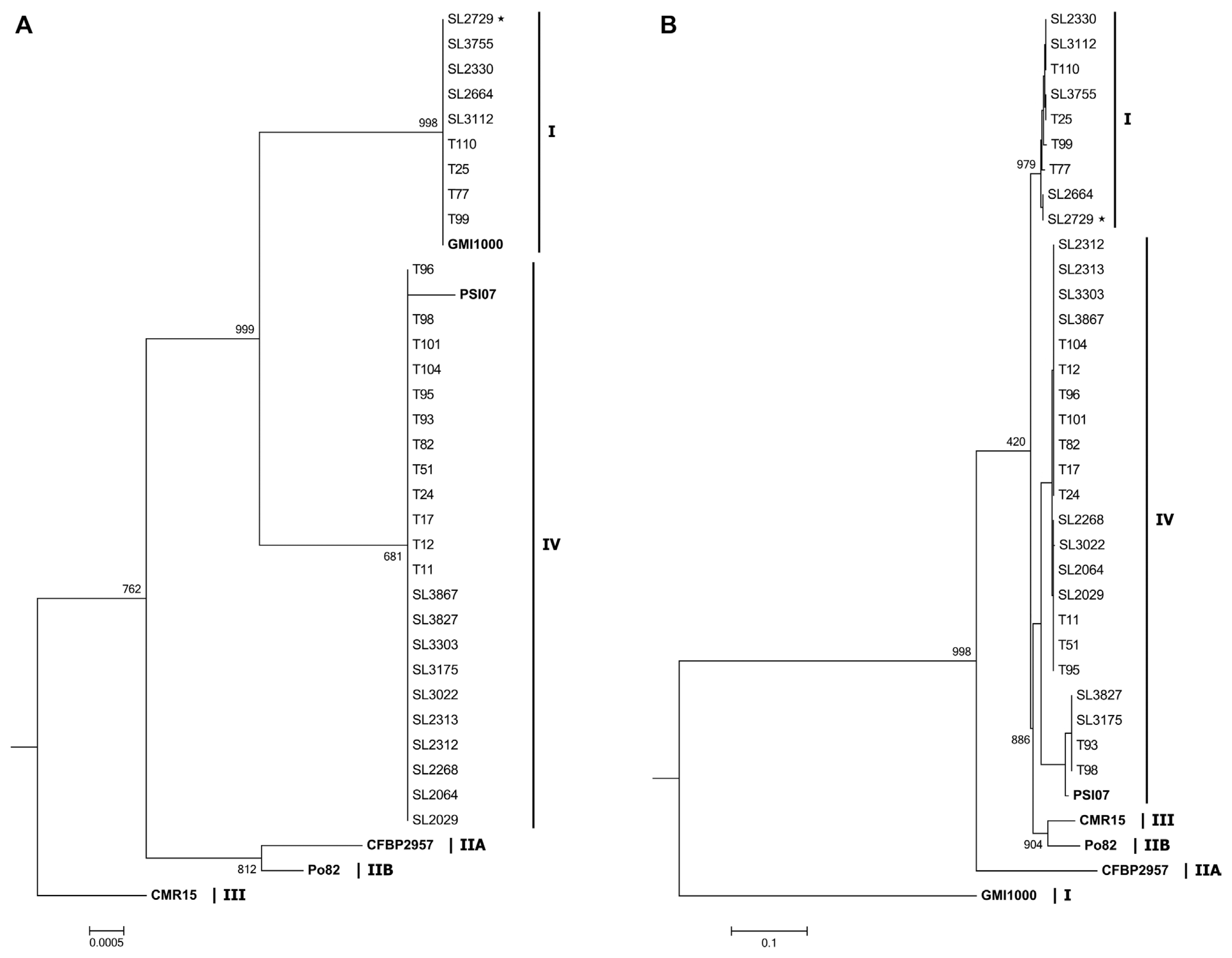
Fig. 3
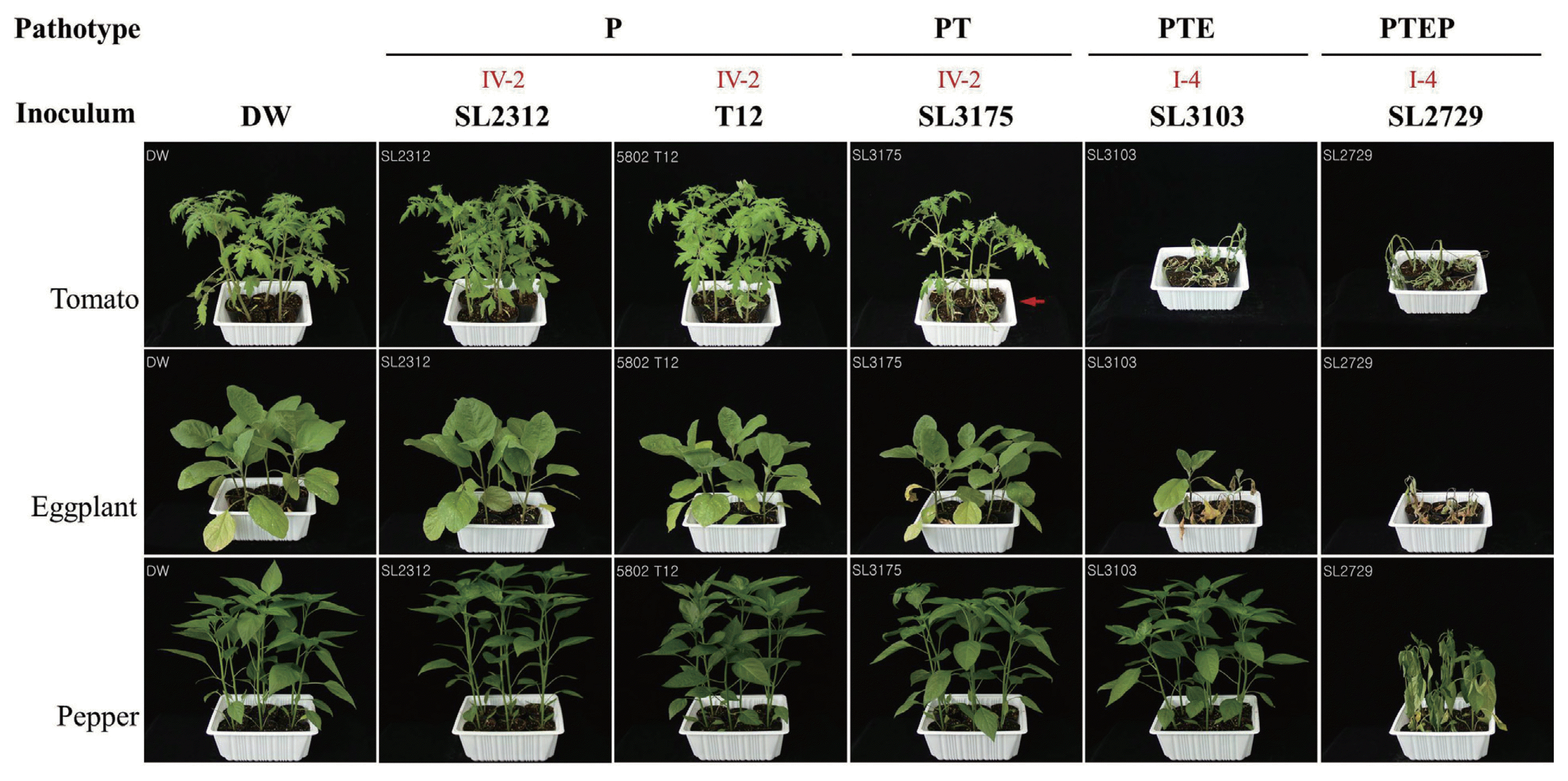
Table 1
| Isolateα | Host | Year | Geographical origin | Phylotype | Biovar | rsa1β | Pathogenicityδ | Pathotypeɛ | 16S rRNAζ | Endoglucanaseη | Source | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Potato | Tomato | Eggplant | Pepper | |||||||||||
| SL1870 | Potato | 1998 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119263 | KY313644 | This study |
| SL2029 | Potato | 1998 | Namjeju, Jeju-do | IV | 2 | + | +++ | + | ++ | − | PTE | KY119264 | KY313645 | Jeong 2007 |
| SL2064 | Potato | 1998 | Namjeju, Jeju-do | IV | 2 | + | +++ | +++ | +++ | − | PTE | KY119265 | KY313646 | Jeong 2007 |
| SL2230 | Potato | 1998 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119266 | KY313647 | This study |
| SL2268 | Potato | 1998 | Namjeju, Jeju-do | IV | 2 | + | ++ | ++ | ++ | − | PTE | KY119268 | KY313649 | Jeong 2007 |
| SL2313 | Potato | 1998 | Namhae, Gyeongsangnam-do | IV | 2 | + | +++ | − | − | − | P | KY119270 | KY313651 | Jeong 2007 |
| SL2312 | Potato | 1998 | Miryang, Gyeongsangnam-do | IV | 2 | + | +++ | − | − | − | P | KY119269 | KY313650 | This study |
| SL2264 | Potato | 1998 | Muan, Jeollanam-do | I | 4 | − | + | ++ | ++ | − | PTE | KY119267 | KY313648 | This study |
| SL2543 | Potato | 1999 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119272 | KY313653 | This study |
| SL3175 | Potato | 1999 | Namjeju, Jeju-do | IV | 2 | + | +++ | ++ | − | − | PT | KY119285 | KY313666 | This study |
| SL3150 | Potato | 1999 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119283 | KY313664 | This study |
| SL3166 | Potato | 1999 | Bukjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119284 | KY313665 | This study |
| SL3177 | Potato | 1999 | Bukjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119286 | KY313667 | This study |
| SL2330 | Potato | 1999 | Namhae, Gyeongsangnam-do | I | 3 | − | + | +++ | +++ | + | PTEP | KY119271 | KY313652 | This study |
| SL3112 | Potato | 1999 | Namhae, Gyeongsangnam-do | I | 3 | − | ++ | +++ | +++ | + | PTEP | KY119282 | KY313663 | This study |
| SL3022 | Potato | 1999 | Gimhae, Gyeongsangnam-do | IV | 2 | + | +++ | +++ | − | − | PT | KY119276 | KY313657 | This study |
| T11 | Potato | 1999 | Gimhae, Gyeongsangnam-do | IV | 2 | + | ++ | ++ | ++ | − | PTE | KY119311 | KY313692 | This study |
| T17 | Potato | 1999 | Gimhae, Gyeongsangnam-do | IV | 2 | + | +++ | − | − | − | P | KY119316 | KY313697 | This study |
| SL2729 | Potato | 1999 | Miryang, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119275 | KY313656 | This study |
| SL2664 | Potato | 1999 | Boseong, Jeollanam-do | I | 3 | − | ++ | +++ | + | + | PTEP | KY119274 | KY313655 | This study |
| SL3085 | Potato | 1999 | Boseong, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119279 | KY313660 | This study |
| SL3100 | Potato | 1999 | Boseong, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119280 | KY313661 | This study |
| SL2610 | Potato | 1999 | Yeonggwang, Jeollanam-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119273 | KY313654 | This study |
| SL3055 | Potato | 1999 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119277 | KY313658 | This study |
| SL3079 | Potato | 1999 | Muan, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119278 | KY313659 | This study |
| SL3103 | Potato | 1999 | Haenam, Jeollanam-do | I | 4 | − | + | +++ | +++ | − | PTE | KY119281 | KY313662 | This study |
| SL3300 | Potato | 2000 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119289 | KY313670 | This study |
| SL3303 | Potato | 2000 | Namjeju, Jeju-do | IV | 2 | + | +++ | − | − | − | P | KY119290 | KY313671 | This study |
| SL3827 | Potato | 2000 | Namjeju, Jeju-do | IV | 2 | + | +++ | +++ | − | − | PT | KY119302 | KY313683 | This study |
| SL3809 | Potato | 2000 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119300 | KY313681 | This study |
| SL3835 | Potato | 2000 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119303 | KY313684 | This study |
| T92 | Potato | 2000 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119340 | KY313721 | This study |
| T93 | Potato | 2000 | Namjeju, Jeju-do | IV | 2 | + | ++ | ++ | + | − | PTE | KY119341 | KY313722 | This study |
| SL3796 | Potato | 2000 | Bukjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119299 | KY313680 | This study |
| SL3822 | Potato | 2000 | Bukjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119301 | KY313682 | This study |
| SL3760 | Potato | 2000 | Namhae, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | ++ | PTEP | KY119295 | KY313676 | This study |
| T14 | Potato | 2000 | Namhae, Gyeongsangnam-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119313 | KY313694 | This study |
| T15 | Potato | 2000 | Namhae, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | + | PTEP | KY119314 | KY313695 | This study |
| SL3762 | Potato | 2000 | Gimhae, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | ++ | PTEP | KY119296 | KY313677 | This study |
| T2 | Potato | 2000 | Gimhae, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | + | PTEP | KY119309 | KY313690 | This study |
| SL3774 | Potato | 2000 | Miryang, Gyeongsangnam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119297 | KY313678 | This study |
| SL3781 | Potato | 2000 | Miryang, Gyeongsangnam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119298 | KY313679 | This study |
| SL3747 | Potato | 2000 | Boseong, Jeollanam-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119293 | KY313674 | This study |
| SL3755 | Potato | 2000 | Boseong, Jeollanam-do | I | 3 | − | + | +++ | + | + | PTEP | KY119294 | KY313675 | This study |
| SL3283 | Potato | 2000 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119288 | KY313669 | This study |
| SL3705 | Potato | 2000 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119291 | KY313672 | This study |
| SL3730 | Potato | 2000 | Muan, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119292 | KY313673 | This study |
| SL3282 | Potato | 2000 | Goryeong, Gyeongsangbuk-do | I | 4 | − | + | +++ | +++ | ++ | PTEP | KY119287 | KY313668 | This study |
| SL3873 | Potato | 2001 | Namjeju, Jeju-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119306 | KY313687 | This study |
| T85 | Potato | 2001 | Namjeju, Jeju-do | I | 4 | − | + | +++ | +++ | + | PTEP | KY119338 | KY313719 | This study |
| T87 | Potato | 2001 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | + | PTEP | KY119339 | KY313720 | This study |
| T111 | Potato | 2001 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119352 | KY313733 | This study |
| T112 | Potato | 2001 | Namjeju, Jeju-do | I | 4 | − | + | +++ | +++ | +++ | PTEP | KY119353 | KY313734 | This study |
| SL3867 | Potato | 2001 | Bukjeju, Jeju-do | IV | 2 | + | +++ | − | − | − | P | KY119304 | KY313685 | This study |
| SL3869 | Potato | 2001 | Bukjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119305 | KY313686 | This study |
| T104 | Potato | 2001 | Bukjeju, Jeju-do | IV | 2 | + | +++ | − | − | − | P | KY119348 | KY313729 | This study |
| T105 | Potato | 2001 | Bukjeju, Jeju-do | I | 4 | − | + | +++ | +++ | +++ | PTEP | KY119349 | KY313730 | This study |
| T5 | Potato | 2001 | Gimhae, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | ++ | PTEP | KY119310 | KY313691 | This study |
| T18 | Potato | 2001 | Miryang, Gyeongsangnam-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119317 | KY313698 | This study |
| T77 | Potato | 2001 | Gimje, Jeollabuk-do | I | 3 | − | + | +++ | +++ | − | PTE | KY119334 | KY313715 | This study |
| T78 | Potato | 2001 | Gimje, Jeollabuk-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119335 | KY313716 | This study |
| T80 | Potato | 2001 | Gimje, Jeollabuk-do | I | 4 | − | +++ | +++ | +++ | + | PTEP | KY119336 | KY313717 | This study |
| SL3879 | Potato | 2001 | Boseong, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119307 | KY313688 | This study |
| T56 | Potato | 2001 | Boseong, Jeollanam-do | I | 4 | − | + | +++ | +++ | − | PTE | KY119325 | KY313706 | This study |
| T57 | Potato | 2001 | Boseong, Jeollanam-do | I | 4 | − | + | +++ | +++ | ++ | PTEP | KY119326 | KY313707 | This study |
| T58 | Potato | 2001 | Boseong, Jeollanam-do | I | 4 | − | + | +++ | +++ | − | PTE | KY119327 | KY313708 | This study |
| T59 | Potato | 2001 | Boseong, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119328 | KY313709 | This study |
| T67 | Potato | 2001 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119331 | KY313712 | This study |
| T46 | Potato | 2001 | Muan, Jeollanam-do | I | 4 | − | ++ | +++ | +++ | ++ | PTEP | KY119322 | KY313703 | This study |
| SL3882 | Potato | 2001 | Haenam, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119308 | KY313689 | This study |
| T73 | Potato | 2001 | Haenam, Jeollanam-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119333 | KY313714 | This study |
| T34 | Potato | 2001 | Goryeong, Gyeongsangbuk-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119320 | KY313701 | This study |
| T115 | Potato | 2001 | Goesan, Chungcheongbuk-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119354 | KY313735 | This study |
| T117 | Potato | 2001 | Goesan, Chungcheongbuk-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119355 | KY313736 | This study |
| T95 | Potato | 2003 | Namjeju, Jeju-do | IV | 2 | + | +++ | + | +++ | − | PTE | KY119342 | KY313723 | This study |
| T96 | Potato | 2003 | Namjeju, Jeju-do | IV | 2 | + | +++ | − | − | − | P | KY119343 | KY313724 | This study |
| T98 | Potato | 2003 | Namjeju, Jeju-do | IV | 2 | + | +++ | +++ | − | − | PT | KY119344 | KY313725 | This study |
| T99 | Potato | 2003 | Namjeju, Jeju-do | I | 3 | − | + | +++ | +++ | + | PTEP | KY119345 | KY313726 | This study |
| T100 | Potato | 2003 | Namjeju, Jeju-do | I | 4 | − | ++ | +++ | +++ | +++ | PTEP | KY119346 | KY313727 | This study |
| T101 | Potato | 2003 | Namjeju, Jeju-do | IV | 2 | + | +++ | − | − | − | P | KY119347 | KY313728 | This study |
| T109 | Potato | 2003 | Bukjeju, Jeju-do | I | 4 | − | + | +++ | +++ | +++ | PTEP | KY119350 | KY313731 | This study |
| T110 | Potato | 2003 | Bukjeju, Jeju-do | I | 3 | − | + | +++ | ++ | + | PTEP | KY119351 | KY313732 | This study |
| T12 | Potato | 2003 | Namhae, Gyeongsangnam-do | IV | 2 | + | +++ | − | − | − | P | KY119312 | KY313693 | This study |
| T16 | Potato | 2003 | Namhae, Gyeongsangnam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119315 | KY313696 | This study |
| T24 | Potato | 2003 | Miryang, Gyeongsangnam-do | IV | 2 | + | ++ | − | − | − | P | KY119318 | KY313699 | This study |
| T25 | Potato | 2003 | Miryang, Gyeongsangnam-do | I | 3 | − | ++ | +++ | ++ | + | PTEP | KY119319 | KY313700 | This study |
| T82 | Potato | 2003 | Gimje, Jeollabuk-do | IV | 2 | + | +++ | − | − | − | P | KY119337 | KY313718 | This study |
| T50 | Potato | 2003 | Boseong, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119323 | KY313704 | This study |
| T51 | Potato | 2003 | Boseong, Jeollanam-do | IV | 2 | + | +++ | + | + | − | PTE | KY119324 | KY313705 | This study |
| T60 | Potato | 2003 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | +++ | PTEP | KY119329 | KY313710 | This study |
| T61 | Potato | 2003 | Yeonggwang, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | ++ | PTEP | KY119330 | KY313711 | This study |
| T42 | Potato | 2003 | Muan, Jeollanam-do | I | 4 | − | +++ | +++ | +++ | + | PTEP | KY119321 | KY313702 | This study |
| T70 | Potato | 2003 | Haenam, Jeollanam-do | I | 4 | − | + | +++ | +++ | ++ | PTEP | KY119332 | KY313713 | This study |
α Isolate: SL-numbering isolates were obtained from Dr. Seungdon Lee and T-numbering isolates were from Dr. Young Kee Lee.
β rsa1: PCR was carried out using two primer pairs; one pair produced a 720-bp fragment containing full rsa1 gene including promotor (720-rsa1F/720-rsa1R) at 70°C annealing temperature, and another produced a 315-bp fragment containing partial rsa1 ORF region (315-rsa1F/315-rsa1R) at 59°C annealing temperature.
δ Pathogenicity: Symptoms were recorded at 28 days post inoculation using the following scale: −, no symptoms; +, one to three leaves wilted; ++, four to six leaves wilted; and +++, seven more leaves or whole plant wilted.
ɛ Pathotype: (P) only pathogenic on potato and nonpathogenic on tomato, eggplant and pepper, (PT) pathogenic on potato and tomato, and nonpathogenic on eggplant and pepper, (PTE) pathogenic on potato, tomato, and eggplant, and nonpathogenic on pepper, and (PTEP) pathogenic on all tested crops - potato, tomato, eggplant, and pepper.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




