 |
 |
| Plant Pathol J > Volume 36(5); 2020 > Article |
|
Abstract
An understanding of the contribution of secondary metabolites (SMs) to the antagonistic and biocontrol activities of bacterial biocontrol agents serves to improve biocontrol potential of the strain. In this study, to evaluate the contribution of each SM produced by Pseudomonas fluorescens NBC275 (Pf275) to its antifungal and biocontrol activity, we combined in silico analysis of the genome with our previous study of transposon (Tn) mutants. Thirteen Tn mutants, which belonged to 6 biosynthetic gene clusters (BGCs) of a total 14 BGCs predicted by the antiSMASH tool were identified by the reduction of antifungal activity. The biocontrol performance of Pf275 was significantly dependent on 2,4-diacetylphloroglucinol and pyoverdine. The clusters that encode for arylpolyene and an unidentified small linear lipopeptide influenced antifungal and biocontrol activities. To our knowledge, our study identified the contribution of SMs, such as a small linear lipopeptide and arylpolyene, to biocontrol efficacy for the first time.
Microbial secondary metabolites (SMs) are small organic compounds that play important roles in the survival of the microbes in their ecological niches. Biocontrol agents (BCAs) produce myriad biologically active SMs that are essential for their biocontrol performance. Genes in the biosynthetic gene clusters (BGCs) are responsible for the biosynthesis of SMs and are located within a single locus in the chromosome and plasmid (Bibb, 2005). These clusters mediate the coordinated expression of biosynthetic, regulatory, and transporter-related genes necessary for the metabolites to perform their functions.
Many species of Pseudomonas are potential BCAs (Haas and Keel, 2003) and produce a large number of bioactive metabolites, including cyclic lipopeptides (CLPs), quinolones, siderophores, phenazine, phloroglucinol, hydrogen cyanide, and rhamnolipids (Masschelein et al., 2017). There are diversities and differences in SM production even between strains that belong to the same species of Pseudomonas (Gross and Loper, 2009; Zhao et al., 2019). Identification of SM profiles and understanding their contribution to biocontrol efficacy will increase the possibility of identifying potent BCAs. Moreover, this knowledge will help in strain improvement through genetic modification.
In our previous studies, P. fluorescens NBC275 (Pf275) showed strong antagonism against several plant fungal pathogens (Dutta et al., 2019b) and biocontrol activity against gray mold in pepper fruit. In addition, overall analysis of Tn-mutant library of Pf275 revealed that production of antifungal SMs contributes to the strainŌĆÖs biocontrol performance (Dutta et al., 2019a). However, the functional roles of the genes and SMs towards antagonism and biocontrol performance remain unclear. In this study, to understand the contribution of SMs to overall antifungal and biocontrol activities of Pf275, we analyzed BGCs encoding antagonistic SMs using bioinformatics tools and compared the clusters with other Pseudomonas strains. Furthermore, we combined the in silico analysis of the genome with Tn-mutant analysis and evaluated the contribution and functional role of each SM towards antagonism and biocontrol performance of Pf275.
The antiSMASH (Antibiotics & SM Analysis Shell), a tool for the identification of potential SMs from genome sequences has been used to predict BGCs in many studies (Blin et al., 2019). In this study, antiSMASH v.5.0 program with the ŌĆśextra featureŌĆÖ setting was used to mine the genome of Pf275 (accession no. CP031648) for the presence of BGCs that encode SMs. The identified BGCs were further confirmed using NPSearcher predictions (Li et al., 2009). The domains of non-ribosomal peptide synthase (NRPS) genes was analyzed using PRISM3 (PRediction Informatics for Secondary Metabolomes) (Skinnider et al., 2015).
In this study, antiSMASH identified 14 gene clusters in Pf275 (Table 1, Supplementary Fig. 1), 4 of which were NRPS-type gene clusters, predicted for the biosynthesis of pyoverdines, cupriachelin, and an unknown compound (hereafter, a small linear lipopeptide). The genes in the small linear lipopeptide cluster were predicted to have 100% similarity to genes in the BGCs for syringopeptin production, based on the Minimum Information on Biosynthetic Gene Cluster repository interconnected with antiSMASH. Furthermore, one cluster was designated as the NRPS-like mangotoxin gene cluster based on the identification of atypical NRPSs that lacked the C-AT module architecture (Blin et al., 2019). One type III polyketide synthase cluster was found encoding 2,4-diacetylphloroglucinol (2,4-DAPG). Three clusters were predicted to synthesize bacteriocins and each one cluster for N-acetylglutaminylglutamine amide, an arylpolyene (APE Vf), a lanthipeptide, a beta-lactone (13% of genes in this BGC showed similarity to BGCs for fengycin production in Bacillus sp.), and a butyrolactone was identified (Table 1).
BLAST tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and genome-based distance matrix analysis (Rodriguez and Konstantinidis, 2016) revealed that the proteins encoded by the identified BGCs in Pf275 were highly homologous with those of the BCAs P. fluorescens 2P24 (2P24, NCBI genome accession no. CP025542) (Zhang et al., 2014) and P. kilonensis F113 (F113, accession no. CP003150) (Vacheron et al., 2018), previously known as P. fluorescens (Redondo-Nieto et al., 2013). Therefore, we identified BGCs in 2P24 and F113 and compared with Pf275. BGC analysis indicated that Pf275 belongs to the group II of Pseudomonas strains, which produce DAPG but not pyoluteorin, pyrrolnitrin, or CLPs (Zhao et al., 2019). Comparative analysis revealed that Pf275 has two additional NRPS clusters, which produce a small linear lipopeptide and cupriachelin along with two additional clusters for bacteriocin compared to F113 (Supplementary Table 1). In contrast, an NRPS cluster for chejuenolide A/B was exclusively found in strain F113. Comparison with 2P24 revealed Pf275 to have an additional cluster for bacteriocin and lanthipeptide production. A PKS-like cluster for the antibacterial mupirocin production was detected in 2P24 but not in Pf275. Our results indicate that there are wide differences in the SM production in various Pseudomonas strains.
Furthermore, the BGCs of Pf275 were compared with those of other BCAs in P. fluorescens complex, including Pseudomonas brassicacearum LBUM300 (accession no. CP012680), P. fluorescens Q2-87 (accession no. CM001558), and Pseudomonas protegens CHA0 (accession no. CP003190) and Pf5 (accession no. CP032358) (Zhao et al., 2019). The genomes of the strains were analyzed for the BGCs for SM production using antiSMASH (Supplementary Table 2). The BGC of a small linear lipopeptide was compared with the similar cluster of 2P24 and Pseudomonas sp. 11K1 (accession no. CP035088), derived from BLAST analysis. The amino acids of the BGC were also compared with those of syringomycin of Pseudomonas syringae pv. syringae B728a (accession no. CP000075). The genes of pyoverdine BGC were compared to the genes of P. fluorescens FW300-N2C3 (accession no. CP012831) and P. thivervalensis BS3779 (accession no. LT629691). The BGCs for lanthipeptide and arylpolyene were common between P. fluorescens Q2-87 and Pf275. However, Pf275 has a BGC for NRPS-like mangotoxin, which was not found in P. fluorescens Q2-87 (Supplementary Table 2). Three BGCs for bacteriocin were found in Pf275 compared to one BGC in the non-CLP producing strain P. brassicacearum LBUM300, which also had no BGCs for mangotoxin and arylpolyene. The CLP-producing strains, P. protegens CHA0, P. protegens Pf5, and Pseudomonas sp. 11K1 have additional BGCs producing pyoluteorin and pyrrolnitrin as well as DAPG-producing clusters. Pf275 has no BGCs encoding CLPs, pyoluteorin, or pyrrolnitrin. Overall, the analyses indicate that the strong antagonism of Pf275 could be primarily due to the production of 2,4-DAPG and pyoverdines.
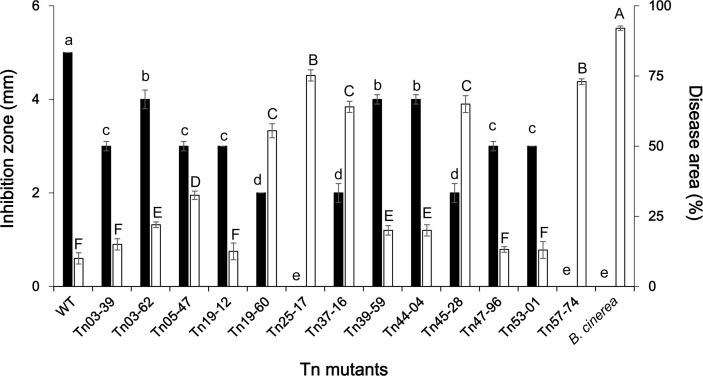
Tn insertion mutant analysis identified 13 mutants that resulted in the suppression of antifungal activity; the genes belonged to 6 BGCs of the 14 BGCs predicted by antiSMASH (Supplementary Table 3). In this study, to evaluate the contribution of each BGC to the overall biocontrol performance of Pf275, 13 mutants were assessed for the biocontrol efficacy against gray mold following Dutta et al. (2019a). The average disease area per pepper fruit was recorded from 3 replications (10 fruits per replication) (Fig. 1, Supplementary Fig. 2). Four mutants had mutation in the genes for 2,4-DAPG production, including two core biosynthetic genes phlD (Tn57-74) and phlC (Tn25-17), a gene mexB_2 (Tn45-28) encoding a multidrug efflux RND transporter permease subunit, and a gene citN_1 (Tn03-62) for a citrate transporter. The mutants impaired in phlD and phlC completely lost the antifungal activity and their biocontrol ability was also drastically reduced (Dutta et al., 2019a). The BGC for 2,4-DAPG production is composed of six structural (phlA, phlC, phlB, phlD, phlE, and phlI) and three regulatory (phlF, phlG, and phlH) genes (Mandryk-Litvinkovich et al., 2017) (Supplementary Figs. 2 and 3). In another report, Pseudomonas deficient in phlC and phlD showed reduced antifungal activity (Almario et al., 2017), which corresponds to our results. Mutant impaired in mexB_2 (Tn45-28), which is predicted as a transportrelated gene in the 2,4-DAPG cluster (Supplementary Fig. 3) showed significantly lower antifungal and biocontrol activities than Pf275. The MexAB-OprM efflux system implicated in the secretion of siderophore pyoverdine in P. aeruginosa K437 (Li et al., 1995). A similar multidrug efflux pump EmhR-EmhABC in P. fluorescens 2P24 indirectly affected 2,4-DAPG production (Tian et al., 2010). Taken together, the mexB_2 of Pf275 might influence the production of 2,4-DAPG and pyoverdine, consequently affecting the biocontrol performance.
An NRPS BGC (cluster 7), which is predicted to encode a small linear lipopeptide was identified from Pf275 (Table 1). The mutant Tn39-59 showed reduced antifungal and biocontrol activities, indicating the contribution of this compound on the biocontrol activity. Although antiSMASH predicted genes of this cluster to be similar to some genes of syringopeptin in P. syringae pv. syringae, the compound produced by this cluster expected to be linear comprising of eight amino acids (Val-Ala-Gln-Ala-Val-Ala-Pro-Thr), whereas syringopeptins are CL with 22 or 25 amino acids (Grgurina et al., 2005). Similar small linear lipopeptides were identified in 2P24 and Pseudomonas sp. 11K1 and predicted by antiSMASH to produce syringomycin and syringopeptin, respectively (Supplementary Fig. 4). In addition, our analysis revealed that the amino acid composition of the lipopeptide from Pf275 as well as 2P24 and Pseudomonas sp. 11K1 is different from that of syringomycin (Ser-Ser-Dab-Dab-Arg-Phe-zDhb-Asp-Thr) (Fukuchi et al., 1992). Jordan et al. (2005) reported that amino acids are lost or gained during protein evolution. However, there is no concrete evidence to assume that the BGC identified in Pf275 is a result of amino acid loss. To our knowledge, this study for the first time confirmed its role in the biocontrol ability. The underlying mechanisms as well as the structure and characteristics of the compound remain to be revealed.
We previously confirmed the role of pyoverdine in antagonism and biocontrol efficacy of Pf275 with mutants in genes pvdI and pvdD (Dutta et al., 2019a). In this study, antiSMASH identified two BGCs for pyoverdine (clusters 3 and 11) in Pf275 genome with conserved chromophore biosynthetic gene pvdL (Gross and Loper, 2009) in both clusters. The amino acid sequence of the two genes in Pf275 showed more than 90% similarity with PvdI and PvdD in P. fluorescens FW300-N2C3 and P. thivervalensis BS3779. The cluster also contains other pyoverdine-related genes such as peptide synthetase (pvdL), ABC transporter (pvdE), periplasmic enzymes (pvdM, pvdN, pvdO, and pvdP), efflux pump (pvdRT-opmQ), outer membrane transporter (fvpA), and alternative sigma factor (fvpI) (Supplementary Fig. 5) (Schalk and Guillon, 2013). While the well-known BCAs P. protegens CHA0, P. protegens Pf5, and Pseudomonas sp. 11K1 had additional BGCs for pyoluteorin and pyrrolnitrin, Pf275 has no BGCs for the siderophores. Multiple BGCs for pyoverdine in fluorescent pseudomonads conferred an evolutionary advantage to the microbes (Ravel and Cornelis, 2003). Overall, it is evident that pyoverdine production is essential for the biocontrol performance of Pf275.
AntiSMASH identified three clusters of bacteriocin in the Pf275 genome. In our previous study a mutant Tn03-39, impaired in ydaP, which predicted as an additional biosynthetic gene in bacteriocin BGC (Cluster 6) by antiSMASH, showed reduced antifungal activity (Dutta et al., 2019a). Bacteriocin are predominantly antibacterial compounds (Mataragas et al., 2002), but bacteriocins from the Lactobacillus spp. have antifungal activity (Magnusson and Schn├╝rer, 2001). However, the contribution of this compound to the biocontrol performance of Pf275 may not be essential because the biocontrol activity of the mutant was similar to that of Pf275. Further investigations are needed to understand the role of ydaP in bacteriocin production and antifungal activity.
Tn mutants in the gene cyst_2 (Tn19-12) and fhuA_6 (Tn53-01), showed reduced antifungal activity but similar biocontrol activity compared to wild-type. Both genes are predicted by antiSMASH to be involved in facilitating the transport of the NRPS-like mangotoxin. The ferrichrome transporter fhuA is a TonB-dependent transporter transporting siderophore and structurally-related or -unrelated antibiotics (albomycin or rifamycin, respectively) across the outer membrane (Noinaj et al., 2010). The cyst_2 and fhu_6 might influence the secretion of mangotoxin and other antibiotic compounds leading to the suppression of antifungal activity.
APE BGCs are the largest family of gene clusters abundant and divergent among Gram-negative bacteria. Three mutants impaired in, gbpA (Tn05-47), benM_2 (Tn44-04), and dmlR_23 (Tn47-96), which are predicted by antiSMASH as genes of arylpolyene BGC in Pf275, showed a reduction in the antifungal activity, but biocontrol activity was reduced only in mutant benM_2 (Dutta et al., 2019a). We previously reported that gbpA gene might facilitate attachment of Pf275 to the fungal cell wall owing to its chitin-binding ability. The antiSMASH predicted benM_2 and dmlR_23 to code for LysR-type transcriptional regulators, indicating their role in APE production. Our results for the first time indicated that APE Vf in Pf275 influence the antifungal and biocontrol activities, which needs further study.
Overall, in this study, the comparative analysis with other well-known BCAs indicated variation in the SM profile of Pf275, which consequently influencing its biocontrol ability. The in silico analysis combined with Tn-mutant analysis suggested that the antagonistic and biocontrol activities of Pf275 are predominantly mediated by 2,4-DAPG and pyoverdine. In addition, the cumulative effect of other BGCs, such as a small linear lipopeptide NRPS, APE Vf, and mangotoxin-type NRPS, cannot be ruled out. To our knowledge, our study revealed and highlighted for the first time the contribution of a small linear lipopeptide and APE Vf to the biocontrol performance of the Pseudomonas. Biological functions of some of the metabolites and their role in biocontrol activity need to be investigated in near future. Our study suggests that combination of in silico and Tnmutant analysis can provide profound insights that can aid in selecting genes for genetic modification to improve the performance of the BCAs.
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Acknowledgments
We gratefully acknowledge a grant from Forest Bioresource Material Development Research Program (Project No. 2020205A00-2022-BA01) funded by Forest Service. This research was also supported by National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (Project No.2017R1A2B2002221), Republic of Korea.
REFERENCES
Almario, J., Bruto, M., Vacheron, J., Prigent-Combaret, C., Moënne-Loccoz, Y. and Muller, D. 2017. Distribution of 2,4-diacetylphloroglucinol biosynthetic genes among the Pseudomonas spp. reveals unexpected polyphyletism. Front. Microbiol. 8:1218



Bibb, M. J. 2005. Regulation of secondary metabolism in Streptomyces. Curr. Opin. Microbiol. 8:208-215.


Blin, K., Shaw, S., Steinke, K., Villebro, R., Ziemert, N., Lee, S. Y., Medema, M. H. and Weber, T. 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47:W81-W87.



Dutta, S., Yu, S.-M., Jeong, S. C. and Lee, Y. H. 2019a. Highthroughput analysis of genes involved in biocontrol performance of Pseudomonas fluorescens NBC275 against gray mold. J. Appl. Microbiol. 128:265-279.


Dutta, S., Yu, S.-M., Nagendran, R., Jeong, S. C. and Lee, Y. H. 2019b. Complete genome sequencing of Pseudomonas fluorescens NBC275, a biocontrol agent against fungal pathogens of plants and insects. Korean J. Microbiol. 55:157-159.
Fukuchi, N., Isogai, A., Nakayama, J., Takayama, S., Yamashita, S., Suyama, K., Takemoto, J. Y. and Suzuki, A. 1992. Structures and stereochemistry of three phytotoxins, syringomycin, syringotoxin and syringostatin, produced by Pseudomonas syringae pv. syringae. J. Chem. Soc. Perkin Trans. 1:1149-1157.
Gross, H. and Loper, J. E. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 26:1408-1446.


Grgurina, I., Bensaci, M., Pocsfalvi, G., Mannina, L., Cruciani, O., Fiore, A., Fogliano, V., Sorensen, K. N. and Takemoto, J. Y. 2005. Novel cyclic lipodepsipeptide from Pseudomonas syringae pv. lachrymans strain 508 and syringopeptin antimicrobial activities. Antimicrob. Agents Chemother. 49:5037-5045.



Haas, D. and Keel, C. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41:117-153.


Jordan, I. K., Kondrashov, F. A., Adzhubei, I. A., Wolf, Y. I., Koonin, E. V., Kondrashov, A. S. and Sunyaev, S. 2005. A universal trend of amino acid gain and loss in protein evolution. Nature. 433:633-638.


Li, M. H. T., Ung, P. M. U., Zajkowski, J., Garneau-Tsodikova, S. and Sherman, D. H. 2009. Automated genome mining for natural products. BMC Bioinformatics. 10:185



Li, X.-Z., Nikaido, H. and Poole, K. 1995. Role of mexA-mexBoprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1948-1953.



Magnusson, J. and Schn├╝rer, J. 2001. Lactobacillus coryneformis subsp. coryneformis strains Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1-5.

Mandryk-Litvinkovich, M. N., Muratova, A. A., Nosonova, T. L., Evdokimova, O. V., Valentovich, L. N., Titok, M. A. and Kolomiets, E. I. 2017. Molecular genetic analysis of determinants defining synthesis of 2,4-diacetylphloroglucinol by Pseudomonas brassicacearum BIM B-446 bacteria. Appl. Biochem. Microbiol. 53:31-39.

Masschelein, J., Jenner, M. and Challis, G. L. 2017. Antibiotics from Gram-negative bacteria: a comprehensive overview and selected biosynthetic highlights. Nat. Prod. Rep. 34:712-783.


Mataragas, M., Metaxopoulous, J. and Drosinos, E. H. 2002. Characterization of two bacteriocins produced by Leuconostocmes enterioides L124 and Lactobacillus curvatus L442, isolated from dry fermented sausages. World J. Microbiol. Biotechnol. 18:847-856.

Noinaj, N., Guillier, M., Barnard, T. J. and Buchanan, S. K. 2010. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64:43-60.



Ravel, J. and Cornelis, P. 2003. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 11:195-200.


Redondo-Nieto, M., Barret, M., Morrissey, J., Germaine, K., Mart├Łnez-Granero, F., Barahona, E., Navazo, A., S├Īnchez-Contreras, M., Moynihan, J. A., Muriel, C., Dowling, D., O'Gara, F., Mart├Łn, M. and Rivilla, R. 2013. Genome sequence reveals that Pseudomonas fluorescens F113 possesses a large and diverse array of systems for rhizosphere function and host interaction. BMC Genomics. 14:54



Rodriguez, R. L. M. and Konstantinidis, K. T. 2016. The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Preprints. 4:e1900v1

Schalk, I. J. and Guillon, L. 2013. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: implications for metal homeostasis. Environ. Microbiol. 15:1661-1673.


Skinnider, M. A., Dejong, C. A., Rees, P. N., Johnston, C. W., Li, H., Webster, A. L. H., Wyatt, M. A. and Magarvey, N. A. 2015. Genomes to natural products Prediction Informatics for Secondary Metabolomes (PRISM). Nucleic Acids Res. 43:9645-9662.



Tian, T., Wu, X.-G., Duan, H.-M. and Zhang, L.-Q. 2010. The resistance-nodulation-division efflux pump EmhABC influences the production of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Microbiology. 156:39-48.


Vacheron, J., Desbrosses, G., Renoud, S., Padilla, R., Walker, V., Muller, D. and Prigent-Combaret, C. 2018. Differential contribution of plant-beneficial functions from Pseudomonas kilonensis F113 to root system architecture alterations in Arabidopsis thaliana and Zea mays. Mol. Plant-Microbe Interact. 31:212-223.


Fig.┬Ā1
Effect of transposon insertion mutations in genes belonging to biosynthetic gene cluster of secondary metabolites on antifungal activity and biocontrol efficacy of Pseudomonas fluorescens NBC275. The overlay technique was conducted to test the antifungal activity of each strain against the mycelial growth of Botrytis cinerea (black bar). The biocontrol activity against gray mold was assayed using pepper fruits (white bar). Data is presented as the mean ┬▒ standard deviation. Significant differences in the means of each treatment were determined using the least significant difference test at P = 0.05. Bars with the same letter(s) do not differ significantly at P = 0.05 (a-e for inhibition zone and A-F for disease area). Note that antifungal activity and biocontrol efficacy was assessed for all of the mutant strains that are not presented in previous study.
Table┬Ā1
Antibiotic secondary metabolite biosynthetic gene clusters in Pseudomonas fluorescens NBC275 as predicted by antiSMASH
| Clusters | Type | Most similar known cluster with MIBiG | MIBIG-BGC-ID (Gene location predicted by antiSMASH) |
|---|---|---|---|
| Cluster 1 | Bacteriocin | N.D. |
-a (322774-331524) |
| Cluster 2 | NAGGN | N-acetylglutaminylglutamine amide |
- (730798-745688) |
| Cluster 3 | NRPS |
Pyoverdine biosynthetic gene cluster (10% of genes show similarity)b BGC0000413 (871654-923725) |
|
| Cluster 4 |
Betalactone Fengycin biosynthetic gene cluster (13% of genes show similarity) |
BGC0001095 (1146428-1169662) |
|
| Cluster 5 | Bacteriocin | N.D. |
- (1184534-1193819) |
| Cluster 6 | Bacteriocin | N.D. |
- (1762091-1772936) |
| Cluster 7 |
NRPS A small linear lipopeptide gene cluster (100% of genes show similarity to some genes for syringopeptin) |
- (2194062-2260115) |
|
| Cluster 8 | Butyrolactone | N.D. (2646084-2659503) | |
| Cluster 9 | NRPS |
Cupriachelin biosynthetic gene cluster (11% of genes show similarity) |
BGC0000330 (2670678-2745704) |
| Cluster 10 | T3PKS |
2,4-DAPG biosynthetic gene cluster (100% of genes show similarity) |
BGC0000281 (2794756-2835805) |
| Cluster 11 | NRPS |
Pyoverdine biosynthetic gene cluster (18% of genes show similarity) |
BGC0000413 (3427563-3503750) |
| Cluster 12 | Lanthipeptide | N.D. |
- (4576049-4599123) |
| Cluster 13 | NRPS-like |
Mangotoxin biosynthetic gene cluster (71% of genes show similarity) |
BGC0000387 (5755901-5799431) |
| Cluster 14 | Arylpolyene |
APE Vf biosynthetic gene cluster (40% of genes show similarity) |
BGC0000837 (6066772-6110383) |
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




