Characterization of the Lytic Bacteriophage phiEaP-8 Effective against Both Erwinia amylovora and Erwinia pyrifoliae Causing Severe Diseases in Apple and Pear
Article information
Abstract
Bacteriophages, bacteria-infecting viruses, have been recently reconsidered as a biological control tool for preventing bacterial pathogens. Erwinia amylovora and E. pyrifoliae cause fire blight and black shoot blight disease in apple and pear, respectively. In this study, the bacteriophage phiEaP-8 was isolated from apple orchard soil and could efficiently and specifically kill both E. amylovora and E. pyrifoliae. This bacteriophage belongs to the Podoviridae family. Whole genome analysis revealed that phiEaP-8 carries a 75,929 bp genomic DNA with 78 coding sequences and 5 tRNA genes. Genome comparison showed that phiEaP-8 has only 85% identity to known bacteriophages at the DNA level. PhiEaP-8 retained lytic activity up to 50°C, within a pH range from 5 to 10, and under 365 nm UV light. Based on these characteristics, the bacteriophage phiEaP-8 is novel and carries potential to control both E. amylovora and E. pyrifoliae in apple and pear.
Erwinia amylovora and E. pyrifoliae are Gram-negative bacterial pathogens that cause the devastating diseases, fire blight and bacterial black shoot blight, in apple and pear, respectively. Since E. amylovora was first discovered in 1780 in the United States, it has been reported globally in Europe, North America, the Middle East and central Asia, and New Zealand (Van der Zwet et al., 2012). In 2015, this disease was reported in apple and pear orchards in South Korea (Myung et al., 2016; Park et al., 2016). In the case of E. pyrifoliae, it was first reported in pear orchards in South Korea in 1995 (Kim et al., 1999). These two pathogens are quarantine pathogens in South Korea and have caused severe economic loss due to extensive host eradication and difficulty of fruit export (Park et al., 2017).
So far, antibiotics and copper compounds have been mostly used for the control of fire blight and bacterial black shoot blight in apple and pear. However, the appearance of bacteria resistant to these chemicals have limited their use in the field (Manulis et al., 1998). As an alternative, lytic bacteriophages have been reconsidered as a tool for biological control (Loc-Carrillo and Abedon, 2011). Bacteriophages infect very specific target bacteria, and their host ranges are very narrow unlike antibiotics and copper compounds. They have two different life cycles: the lytic and the lysogenic cycles. During the lytic cycle, a bacteriophage actively infects host bacteria, multiplies inside the host, and kills the host to release progeny (Orlova, 2012). Due to this feature, lytic bacteriophages have been used for phage therapy to control many bacterial pathogens causing disease in animals and plants (Buttimer et al., 2017; Doffkay et al., 2015).
Since the 1960’s, many bacteriophages effective against E. amylovora have been reported, and their genomic and physiological features have been determined (Born et al., 2011; Esplin et al., 2017; Gill et al., 2003; Meczker et al., 2014; Müller et al., 2011; Yagubi et al., 2014). Based on the morphology of these bacteriophages, they belong to either the Myoviridae or Podoviridae family. Some bacteriophages with a broad host range have been applied for phage therapy to control E. amylovora (Meczker et al., 2014) and some of them have been commercialized (Buttimer et al., 2017). However, no bacteriophages effective against E. pyrifoliae or both E. amylovora and E. pyrifoliae have yet been reported.
In this study, to isolate bacteriophages with effective host specificity to both E. amylovora and E. pyrifoliae, 18 soil samples from apple and pear orchards at Jecheon, Chungju, and Yongin, South Korea were collected. After mixing soil with SM (sodium chloride-magnesium sulfate) buffer [50 mM Tris-HCl (pH 7.5), 10 mM NaCl, 10 mM MgSO4] for 30 min, the mixed samples were centrifuged at 10,000 rpm, 4°C for 10 min and filtered with a 0.22 μm pore size filter (Sartorius, Gottingen, Germany). Then, bacteriophages were enriched through overnight incubation with 5ml of the supernatant, 10 ml of LB, and 500 μl of bacterial suspension (109 cfu/ml) of E. amylovora strain Ea-K1 isolated in South Korea at 26°C in a shaking incubator. The incubated samples were treated with chloroform (1% of the final volume) for 30 min, centrifuged at 3,000 g, 4°C for 15 min, and filtered with a 0.22 μm pore size filter. The presence of bacteriophages in the supernatant was confirmed with four E. amylovora strains and two E. pyrifoliae strains using a dotting assay (Kropinski et al., 2009; Yu et al., 2016). Then, in order to isolate individual bacteriophages, the overlay assay (Yu et al., 2016) was performed, and plaques with different sizes and shapes were picked separately. A total of 21 individual bacteriophages were picked based on their plaque sizes and shapes (Fig. 1). To determine whether isolated bacteriophages were separate isolates, the genomic DNAs from the 21 isolated bacteriophages were extracted using a phage DNA isolation kit (Norgen Biotek, Thorold, ON, Canada) and digested with restriction enzymes, EcoRI, BamHI or both. According to DNA patterns, isolated bacteriophages were categorized into three groups. The bacteriophage phiEaP-8 isolated from apple orchard soil in Yongin, South Korea, where no fire blight or bacterial black shoot blight has been reported, represents one of the three groups and this bacteriophage was used for further characterization.
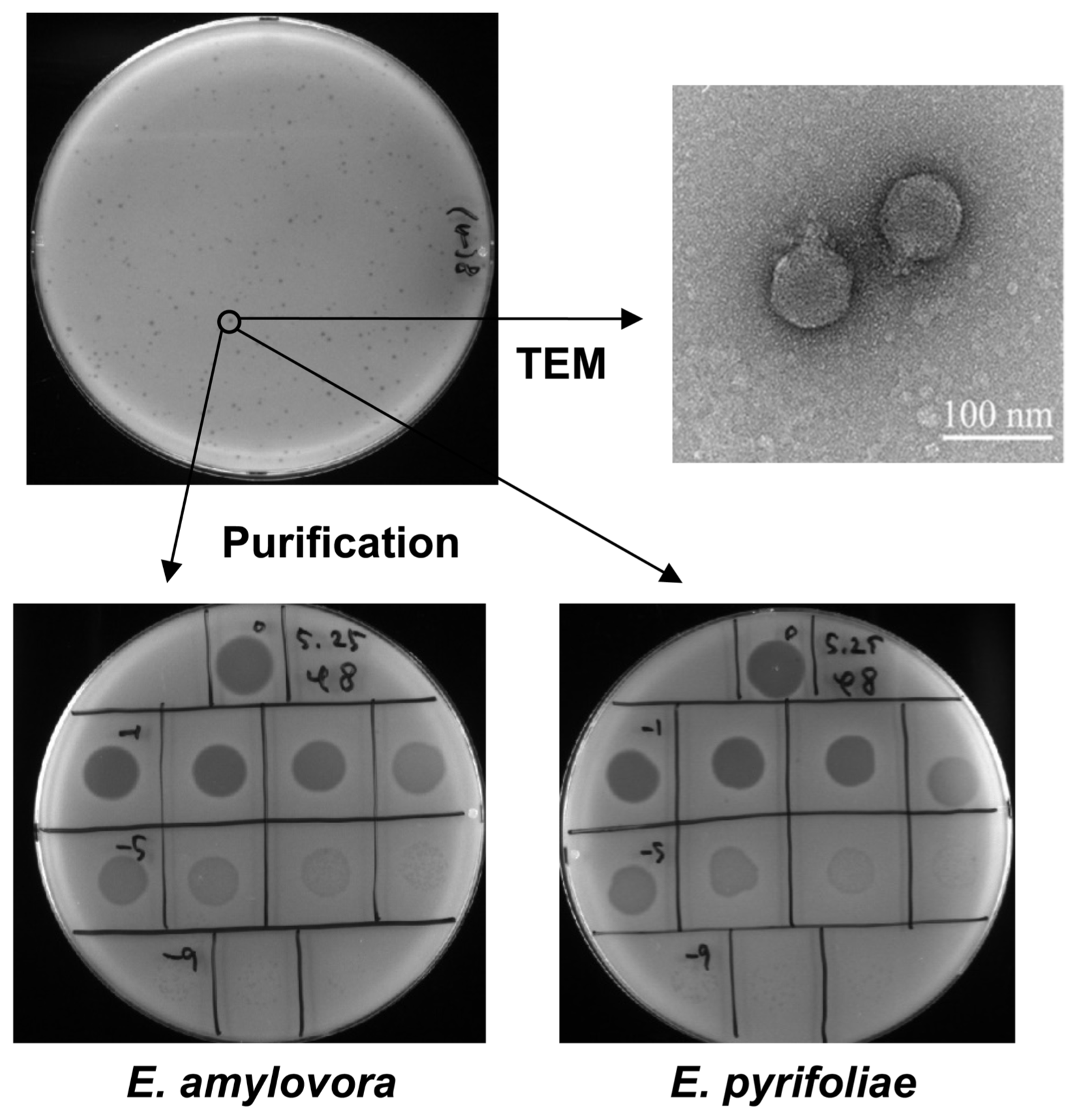
Plaques from the overlay assay after extracting from soil (top left) and the dotting assay (bottom) with purified serially diluted phiEaP-8 against E. amylovora and E. pyrifoliae. Morphology of the bacteriophage phiEaP-8 (top right) was determined by transmission electron microscopy (TEM). Photos were taken at the KBSI (Korea Basic Science Institute).
To determine the shape of bacteriophage phiEaP-8, it was propagated by incubating with the Ea-K1 strain (OD600 = 0.5–1.0), as described previously (Kim and Ryu, 2011), and was then purified using CsCl gradient ultracentrifugation (Lim et al., 2013). After ultracentrifugation at 25,000 rpm, 4°C for 2 h, bacteriophages were collected and purified through dialysis twice for 1 h in SM buffer. The purified bacteriophage phiEaP-8 was observed by transmission electron microscopy at 120 kV after negative straining with 2% aqueous uranyl acetate (pH 4.0) on carbon-coated copper grids (Ackermann and Heldal, 2010; Brum and Steward, 2010). The bacteriophage phiEaP-8 belonged to the Podoviridae family based on its morphology (Fig. 1). The total length and head size are 95 nm and 75 nm, respectively.
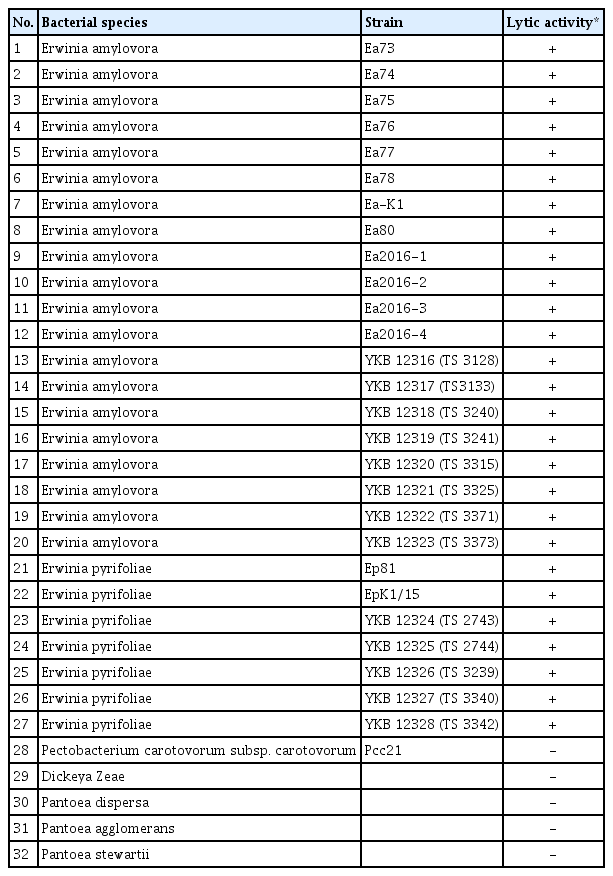
Next, the host range of the bacteriophage phiEaP-8 was determined by an overlay assay with twenty E. amylovora and seven E. pyrifoliae strains isolated in Korea as well as other related bacteria such as Pectobacterium carotovorum, Dickeya zeae and three Pantoea strains. The bacteriophage phiEaP-8 could efficiently kill all tested strains of both E. amylovora and E. pyrifoliae, but it was not effective against other related bacteria (Table 1), indicating that this bacteriophage is very likely specific to both E. amylovora and E. pyrifoliae.
To examine if the bacteriophage phiEaP-8 is homologous to known bacteriophages, whole genome sequencing was performed using the PacBio RS II platform (Pacific Biosciences, Menlo Park, CA, USA). Total genomic DNA of phiEaP-8 was isolated using a phage DNA isolation kit (Norgen Biotek, Thorold, ON, Canada), and 10 ug was used to generate a 10 kb SMRTbellTM template library. After sequencing, de novo assembly was performed using CANU v1.4 (Koren et al., 2017) and a single contig was generated. The bacteriophage phiEaP-8 carries a 75,929 bp genomic DNA (GenBank accession number, MH160392), and its G+C content is 46.8%. In order to compare the phiEaP-8 genome with other sequenced bacteriophages, which can infect E. amylovora, 42 sequenced genomes were obtained from GenBank database, and their genome were compared with BPGA (Bacterial Pan Genome Analysis) pipeline (Chaudhari et al., 2016) and USEARCH (Edgar, 2010). Specifically, 10 of them are Podoviridae, 29 of them are Myoviridae, and 3 bacteriophages are Siphoviridae. Based on a phylogenetic tree (Fig. 2, Supplementary Fig. 1), phiEaP-8 was closely related to five bacteriophages belonging to Podoviridae, which are vB_EamP_Rexella (GenBank accession number, KX098390), vB_EamP_Frozen (GenBank accession number, KX098389), Ea9-2 (GenBank accession number, KF806588), vB_EamP_Gutmeister (GenBank accession number, KX098391), and vB_EamP-S6 (GenBank accession number, HQ728266). At the DNA level, phiEaP-8 only has about 85% identity to these closely related bacteriophages.
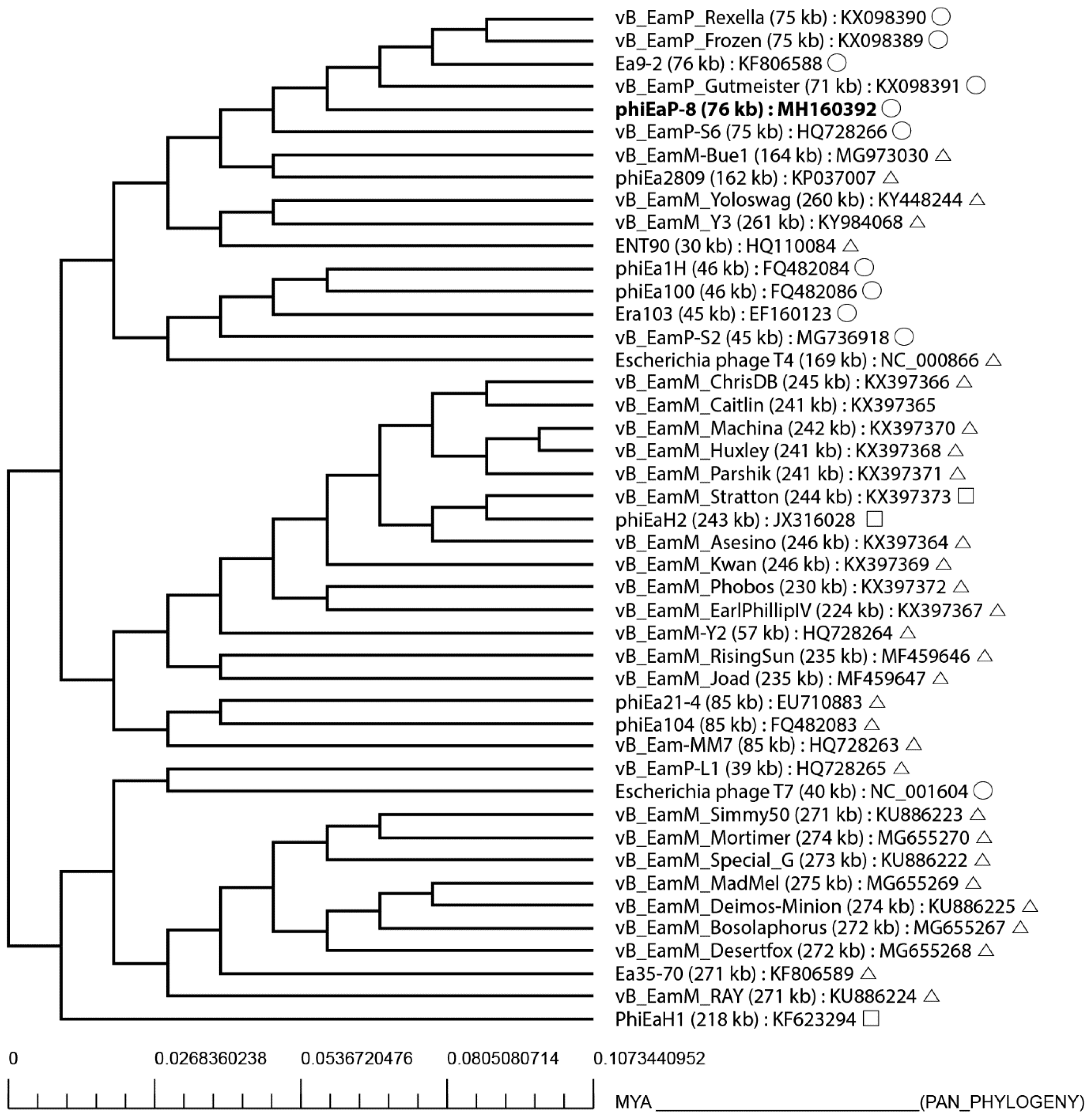
Phylogenetic tree with phiEaP-8 and other 42 Erwinia amylovora bacteriophages. The genome sequences were obtained from GenBank database, and their names, sizes, and accession numbers were stated in the figure. The tree was generated with BPGA pipeline and USEARCH tool. Escherichia phages, T4 and T7 bacteriophages, were used as an outgroup. Circle, Podoviridae; triangle, Myoviridae; square, Siphoviridae.
Gene annotation to find coding sequences (CDS), tRNA, and rRNA genes was performed using Prokka (v1.12b). Gene annotation showed that the genome contains 78 CDSs and 5 tRNA genes, but no rRNA genes (Supplementary Table 1). The bacteriophage phiEaP-8 genome carries genes encoding putative holin and Rz/Rz1 spanin proteins, indicating that it is a lytic bacteriophage. Interestingly, this bacteriophage carries a gene homologous to amsF responsible for amylovoran biosynthesis in E. amylovora and also a gene encoding serine protease highly homologous to one in Enterobacteriaceae like Escherichia coli and Salmonella enterica. These results indicate that this bacteriophage might have obtained these genes from host bacteria during infection and genome multiplication.
To determine if phiEaP-8 can be used for phage therapy against both E. amylovora and E. pyrifoliae, its lytic activity under diverse environmental conditions was examined. For this, 105 PFU/ml of bacteriophages were treated for 1 h at 30, 40, 50, and 60°C, at pH range 3 to 12, or under 365 nm UV light, and its lytic activity was measured by the dotting assay. This bacteriophage retained lytic activity stable against E. amylovora and E. pyrifoliae up to 50°C, within a pH range from 5 to 10, and under 365 nm UV light (Fig. 3).
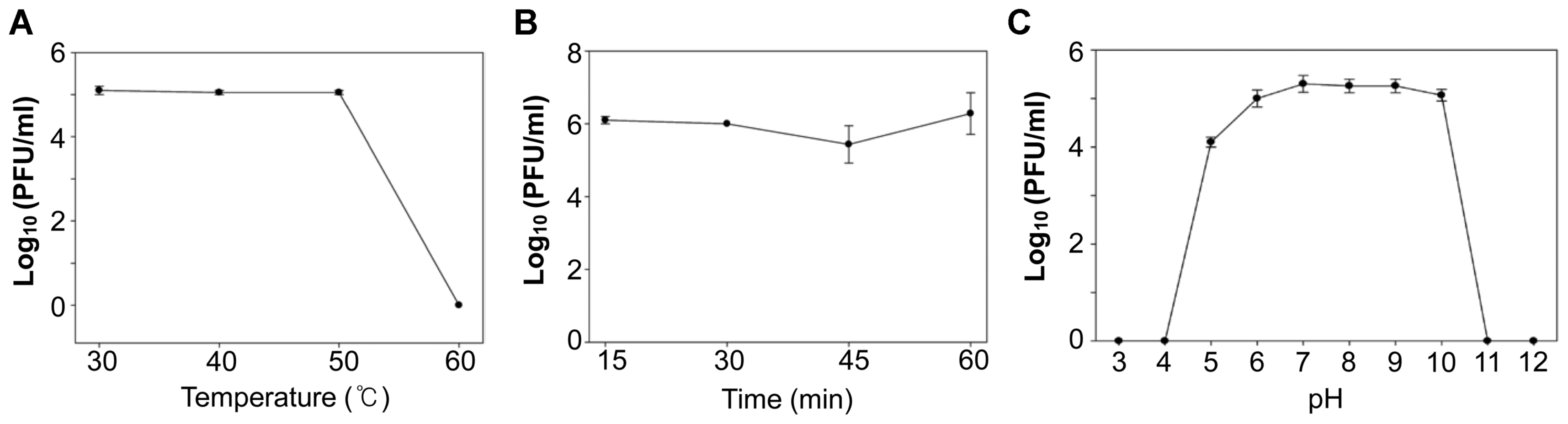
Stability of the bacteriophage phiEaP-8 under diverse environmental conditions. (A) Temperature. (B) 365 nm UV light. (C) pH. Bacteriophages were counted as PFU/ml by a dotting assay. All tests were repeated at least three times. Error bars indicate standard errors.
Bacteriophages are typically isolated from soil, water, and plants surrounding infected trees (Doffkay et al., 2015; Müller et al., 2011). Interestingly, the apple orchard in Yongin, South Korea where phiEaP-8 was isolated has never been infected with either E. amylovora or E. pyrifoliae. In a previous paper (Lagonenko et al., 2015), phiEa2809 was isolated from the leaves of an apple tree without fire blight symptoms in an apple orchard where fire blight was never detected. The genus Erwinia is classified to the Enterobacteriaceae family, which includes both pathogenic and non-pathogenic bacteria in genera such as Erwinia, Enterobacter, Pantoea, Pectobacterium, and Brenneria (Kado, 2006). Presence of bacteriophages in an apple orchard, in which fire blight or bacterial black shoot blight have never been detected, might be explained by the thought that bacteriophages could exist owing to the presence of some of these bacteria.
Born et al. (2011) reported eight bacteriophages effective against E. amylovora. Interestingly, some of them were effective against other related bacteria such as Erwinia billingiae, Pantoea agglomerans, and Pantoea ananatis, indicating the presence of wide host-range bacteriophages. The bacteriophage phiEaP-8 looks specific to both E. amylovora and E. pyrifoliae, which indicates that this is a somewhat narrow host-range bacteriophage. However, this bacteriophage could be very useful because both pathogenic bacteria can co-exist in apple and pear orchards in Korea.
Supplementary data
Acknowledgments
We thank Dr. Young-Gi Lee for providing YKB strains of Erwinia amylovora and Erwinia pyrifoliae and the Korea Basic Science Institute for TEM analysis. This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No: PJ0117582018)” of the Rural Development Administration, Republic of Korea and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 317012-4).