As a sessile organism, plant growth and response to environmental cues are largely governed by plant hormones, also called phytohormones. Phytohormones act spatially and temporally as endogenous signals at a very low dose to regulate various physiological functions. Unlike the animal systems producing hormones in specialized organs and transferring them to another parts via blood stream, each living plant cell can produce hormones on their own (Went and Thimann, 1937). To successfully survive under the biotic and abiotic stress conditions, plants evolved highly sophisticated crosstalk between different phytohormones. Fine-tuning of complex phytohormone network enables the balanced response of plants to developmental and environmental cues, thus minimizing defense-associated fitness costs. Harmony and/or disharmony of phytohormones, such as jasmonic acid (JA), ethylene (ET), abscisic acid (ABA), auxin (IAA), and salicylic acid (SA), result in specific response to specific stimuli.
Phytohormones JA and ET play a pivotal role in the regulation of plant immune response against necrotrophic pathogens and herbivory insect pathogens (Wasternack and Song, 2017). JA is biosynthesized from α-linolenic acid via octadecanoid pathway. During the activation of JA signaling, JA stimulates interaction of coronatine-insensitive 1 (COI1), which is a component of SCFCOI1 E3 ubiquitin ligase complex, with jasmonate-zim-domain (JAZ) proteins, a suppressor of JA-responsive transcription factors (TFs; e.g., MYC2). This JA-induced interaction between COI1 and JAZs facilitate degradation of JAZs through the 26S proteasome, thereby releasing downstream TFs to regulate gene expression and activate JA responses. ET, a gaseous plant hormone, is biosynthesized from S-adenosylmethionine via the action of two key enzymes ACC synthase and ACC oxidase (Wang et al., 2002). It regulates many different aspects of the plant physiology, including germination, senescence, abscission, fruit ripening, and response to biotic and abiotic stresses. Five ethylene receptor genes (ETHYLENE RESPONSE1 [ETR1], ETHYLENE RESPONSE SENSOR1 [ERS1], ETR2, ETHYLENE INSENSITIVE4 [EIN4], and ERS2) are identified from the Arabidopsis plants (Ju and Chang, 2015). Mutant plant lacks JA/ET signaling showed highly susceptible phenotype against infection by necrotrophic pathogens and infestation by herbivory insect pathogens.
ABA, a sesquiterpenoid hormone, is biosynthesized from C40 epoxycarotenoid precursors through an oxidative cleavage reaction in plastids (Xiong and Zhu, 2003). The C15 intermediate xanthoxin is converted to ABA by a two-step reaction via ABA-aldehyde oxidase in cytosol. ABA plays an important role in many cellular processes including seed development, dormancy, germination, and water stress responses (Ng et al., 2014). In the absence of ABA signaling, type 2C protein phosphatases (PP2Cs) constitutively dephosphorylate Snf1-related protein kinases 2 (SnRK2). In response to ABA signal, regulatory component of ABA receptor (RCAR)/pyrabactin resistance (PYR)/PYR1-like (PYLs) proteins, collectively termed PYLs, bind and inhibit PP2Cs. Inhibition PP2Cs in turn allows SnRK2 activation through autophosphorylation. Active SnRK2 mediate the downstream ABA response through the phosphorylation of target proteins (e.g., slow-type anion channel [SLAC1] and inward-rectifying potassium channel [KAT1]).
Both IAA and SA are synthesized from shikimate pathway (Pérez-Llorca et al., 2019). In the first step, shikimate is converted into chorismate by chorismate synthase. For IAA and SA biosynthesis, chorismate is further converted into either tryptophan (Trp) or isochorismate, respectively. Then, Trp is converted into IAA through several reaction steps, including the conversion of Trp to indole-3-pyruvic acid by the tryptophan aminotransferase. IAA plays an essential role in almost every aspect of plant growth and development processes, including the cell division and differentiation, as well as in biotic and abiotic stress responses (Korver et al., 2018; Pérez-Llorca et al., 2019). Perception of IAA via its cognate receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1), the F-box subunit of the ubiquitin ligase complex SCFTIR1, stabilizes the interaction between TIR1 and Aux/IAA proteins. This interaction results in Aux/IAA ubiquitination and subsequent degradation. Degradation of Aux/IAA led to release and activation of AUXIN RESPONSE FACTOR proteins for activation of downstream IAA signaling.
SA is biosynthesized from the chorismate via two independent pathways, isochorismate synthase- and phenylalanine ammonia-lyase-dependent pathways (Dempsey and Klessig, 2017). Role of SA in plant defense response has only become evident during the past 30 years. Before SA is recognized as an important plant hormone, it was assumed to be relatively unimportant products together with many other phenolic secondary metabolites. However, the findings regarding (1) enhanced resistance phenotype in SA-treated tobacco plants against Tobacco mosaic virus (TMV) infection, and (2) over 20-fold increase in endogenous SA level in TMV-infected resistant tobacco plants boost the researches on SA as a plant hormone regulating disease resistance (Klessig et al., 2018). Increasing reports suggest that SA plays important roles not only in regulating plant disease resistance, but also in thermogenesis, abiotic stress tolerance, DNA damage/repair, fruit yield, seed germination, and etc. (Dempsey and Klessig, 2017). In this review, we focused on the diverse effect of SA on different aspect of plant phenotypes in relation with other plant hormones. For SA-mediated defense signaling pathway at the molecular level, we recommend to read reviews by Lu (2009), Yan and Dong (2014), and Zhang and Li (2019).
SA and JA/ET in Plant Resistance to Biotic Stresses
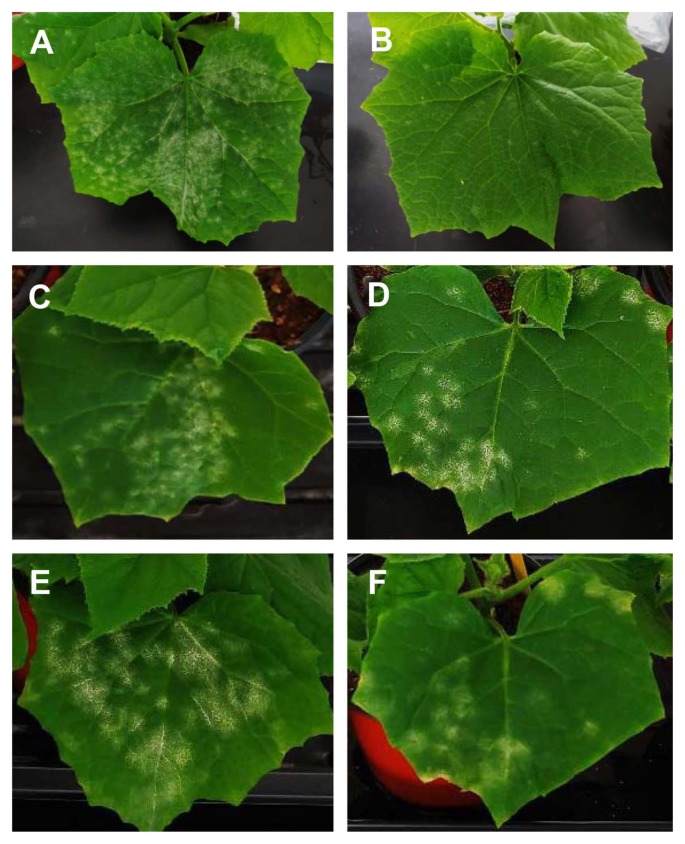
SA is a defense-related plant hormone that plays a key role in resistance to different microbial pathogens, such as virus, bacteria, fungi, and oomycetes (Kunkel and Brooks, 2002; Vlot et al., 2009). In plants, the positive correlation between endogenous levels of SA and resistance responses against biotrophic and hemibiotrophic pathogens are well established (Glazebrook, 2005). In addition, the exogenous SA application induces local and systemic acquired resistance in different plant species against various types of pathogens, including Fusarium oxysporum, Alternaria alternata, Magnaporthe grisea, Colletotrichum gloeosporides, Xanthomonas spp., different kinds of viruses and etc. (Table 1) (Daw et al., 2008; Esmailzadeh et al., 2008; Jendoubi et al., 2017; Kundu et al., 2011; Le Thanh et al., 2017; Mohan Babu et al., 2003; Radwan et al., 2007; Saikia et al., 2003; Wang and Liu, 2012; Wang et al., 2006). Notably, exogenous application of 1 mM SA almost completely suppressed powdery mildew disease development in cucumber plants (Fig. 1). However, SA’s roles in plant defense against necrotrophic pathogens are not fully understood yet, due to its complexity. JA and ET are known to be essential for plant resistant against necrotrophic pathogens (Erb et al., 2012; Wang et al., 2015a). Among different plants-necrotrophic pathogens interactions, a few cases of exogenous SA treatment-induced enhanced susceptibility was reported (Table 2). In broad bean, SA treatment compromised red light-induced resistance against the necrotrophic pathogen Botrytis cinerea; however, it does not further enhanced dark light-induced susceptibility (Khanam et al., 2005). Application of SA-induced enhanced susceptibility in tomato against B. cinerea in a dose-dependent manner. Controversially, the SA-induced enhanced resistance of tomato and Arabidopsis plants against B. cinerea is also reported (Ferrari et al., 2003; Li and Zou, 2017). Generally, SA-dependent defense singling is known to be antagonistic against JA-/ET-dependent defense signaling (Glazebrook, 2005). However, the hormone signaling pathways between SA and ET/JA are not exclusively antagonistic (Robert-Seilaniantz et al., 2011), thus it needs to be carefully analyzed in different plant-pathogen systems and field conditions.
SA and ABA in Plant Tolerance to Abiotic Stresses
Continuous cropping and climate change threaten plant production via multiple abiotic stresses induced by heavy metals, salinity, ozone, ultraviolet, temperature and drought (Connor, 2002). Intriguingly, SA is not only regulating the resistance to biotic stresses, but also the tolerance to various abiotic stresses (Horváth et al., 2007; Khan et al., 2015) (Table 3). The underlying mechanisms of SA-induced abiotic stress tolerance include that SA-mediated (1) accumulation of osmolytes, such as glycinebetaine, proline, soluble sugars and amines, which can help maintain osmotic homeostasis, (2) regulation of mineral nutrition uptake, (3) enhanced reactive oxygen species scavenging activity, (4) enhanced secondary metabolite production, such as terpenes, phenolics, and compounds with nitrogen (alkaloids, cyanogenic glucosides, non-protein amino acids) and sulfur (glutathione, glucosinolates, phytoalexins, thionins, defensins, and allinin), and (5) regulation of other hormone pathways (Horváth et al., 2007; Khan et al., 2015).
Exogenous SA treatment induces the expression of a set of pathogenesis-related (PR) genes, including PR1, PR2, and PR5 (Ali et al., 2018). Interestingly, transgenic overexpression of some PR genes not only enhanced the resistance to different pathogens, but also enhanced the tolerance to different abiotic stresses (Hong and Hwang, 2005; Sarowar et al., 2005; Wu et al., 2016). Transgenic tobacco overexpressing pepper PR-1 showed enhanced heavy metal tolerance (Sarowar et al., 2005). Overexpression of pepper PR-1 enhanced drought and salt stress tolerance in Arabidopsis plants (Hong and Hwang, 2005). However, the underlying molecular mechanisms on how these PR proteins enhancing the abiotic stress tolerance need further investigation.
ABA is known as a key plant hormone conferring abiotic stress tolerance, and it antagonistically regulates SA-mediated defense signaling (Robert-Seilaniantz et al., 2011). ABA-induced suppression of SA-dependent signaling pathway often led to enhanced disease susceptibility in different plant-pathogen interactions (Audenaert et al., 2002; Jiang et al., 2010; Ulferts et al., 2015). However, under the certain abiotic stress conditions, such as freezing and salt stresses, ABA and SA together seems to be able to positively regulate stress tolerance response (Horváth et al., 2015; Szalai et al., 2011; Wang et al., 2018). Exogenous SA pretreatment significantly induced freezing tolerance of wheat via enhancing biosynthesis of ABA (Wang et al., 2018). In tomato plants, exogenous SA treatment-induced ABA biosynthesis in a dose-dependent manner, and partially recovered lowered photosynthetic activity under salt stress condition (Horváth et al., 2015). Pretreatment of barley plants with SA increased the ABA content and reduced the damage induced by water deficit condition (Bandurska and Stroiński, 2005). Notably, an important cluster of genes that are similarly regulated by ABA and SA (28% of ABA-induced genes were also induced by SA, while 40% of ABA-repressed genes were also repressed by SA) is identified in Arabidopsis plants, suggesting plants equipped the common transcriptomic responses regulated by ABA and SA (Kalachova et al., 2016). Taken together, although ABA is antagonistically regulating the SA-mediated disease resistance, ABA and SA seem to be able to cooperate for developing abiotic stress tolerance.
SA and IAA in Plant Growth and Development
SA has controversial roles in plant growth and development depending on its concentration and plant growth conditions and developmental stages (Rivas-San Vicente and Plasencia, 2011). Generally, high levels of SA (It depends on the plant species, however, >1 mM SA considered as high concentration.) negatively regulate plant development and growth. Nevertheless, the application of optimal concentrations of SA showed beneficial effects on it. Depending on the experimental conditions, SA distinctly stimulated growth under both normal and different abiotic stress conditions in different plant species (Gunes et al., 2007; Gutiérrez-Coronado et al., 1998; Kováčik et al., 2009; Manzoor et al., 2015; Sakhabutdinova et al., 2003; Yildirim et al., 2008, 2015) (see also Table 3).
Exogenous SA application also showed different effects on plant development, including seed germination, budding, flowering, and fruit setting and ripening. In Finger Millet plants, SA stimulated flowering (Appu and Muthukrishnan, 2014). SA-induced enhanced fruit setting and weight were observed in strawberry (Kazemi, 2013), apple (Shaaban et al., 2011) and mango (Ngullie et al., 2014). Germination of barley and maize seeds imbibed in >3 mM SA were completely blocked (Guan and Scandalios, 1995; Xie et al., 2007). On the other hands, imbibing maize seeds in ~0.3 mM to ~0.9 mM SA showed higher germination speed, percentage and shoot length (Sallam and Ibrahim, 2015). Notably, ~0.43 mM SA exhibited the best germination stimulating effect, but its effect was decreased at the higher concentrations. Taken together, different concentrations of SA in different plants have either stimulating or blocking effects on plant development.
IAA influences plant growth and development, including tropic growth responses, vascular development, leaf and flower initiation, root growth, and lateral root formation. Recently, Pasternak et al. (2019) reported that SA regulates IAA biosynthesis and transport thereby changing the Arabidopsis root meristem patterning. Low-concentration SA (below 50 μM) promoted adventitious roots and altered architecture of the root apical meristem, whereas high-concentration SA (greater than 50 μM) inhibited overall growth processes in the root. Importantly, both SA and IAA are known to be biosynthesized from the shikimate pathway (Pérez-Llorca et al., 2019). This may suggest that higher biosynthetic activity of SA during the defense response may limit the resource required for the production of plant growth regulating hormone IAA, and vice versa, thereby fine-tuning the cost for the growth or defense depending on external and internal conditions.
SA and Plant Microbiome
Recently, researches on plant microbiome in relation with plant’s health are getting more attention from plant science community (Bulgarelli et al., 2012; Lundberg et al., 2012). In model plant Arabidopsis thaliana, the effect of SA on microbiome was analyzed after exogenous SA application or by using the mutants with altered endogenous SA levels (Lebeis et al., 2015). It revealed that SA application distinctly enriched [Flavobacterium sp. 40 (Bacteroidetes) and Terracoccus sp. 273 (Actinobacteria)] and depleted [Mitsuaria sp. 370 (β-Proteobacteria)] specific bacterial isolates from synthetic community (SynCom) experiment. In addition, population density of nine Actinobacteria and 12 Proteobacteria families were reduced and increased, respectively, in cpr5 mutants that constitutively produces SA. This suggests that soil (and/or rhizosphere) microbiome can be distinctly altered by SA. So far, SA’s effects on plants after soil drench application are majorly focused on induction of systemic immune response; however little is known about its effect on plant root or endophytic microbiomes. Thus further studies on SA’s effects on diverse soil or endophytic microbiome will shed the lights on how SA influences different aspect of plant physiology, including immunity, growth, development and etc.
Concluding Remarks and Perspectives
Increasing numbers of reports suggesting that use of SA and its derivatives, collectively termed salicylates, reduces the risk of multiple chronic diseases in humans, including heart attack, stroke, arthritis, diabetes, certain type of cancers and Alzheimer’s diseases (Castro-Torres et al., 2015; Chang et al., 2016; Rothwell et al., 2010; Steinberg et al., 2013; Tschanz et al., 2013). Given beneficial effects of SA on both plant and human health, SA and salicylates can be used as an efficient plant protector with very minor side effect on environments and humans. To use SA as an effective and environmentally friendly plant protector and/or plant growth regulator, following questions need to be addressed. First, the effective concentrations of SA for specific plants and/or purpose need to be determined. As mentioned above, high dose of SA (>2 mM) not only induces the enhanced disease resistance, but also has adverse effects on plant growth and productivity, which caused by undesirable balancing between cost and benefit of limited energy that plant can use. In humans, >2.5 mM SA in the plasma lead to acute toxicity (Choi et al., 2015a, 2015b, 2019). Thus, SA concentrations ranging from micromolar (~100 μM) to low milimolar (<2 mM) concentrations can be tested to screening the safe and effective concentrations for specific purpose. In addition, the use of high concentration of SA (~2 mM) as a plant growth regulator may also be considered, especially in case of growers need to reduce the growth rate of specific plants with disease control effect. Finally, natural SA derivatives, the amorfrutins, from the medicinal legume licorice Glycyrrhiza foetida, showed ~1,000 times stronger binding and inhibitory activities on selected SA-binding proteins (Choi et al., 2015b, 2019). Thus, the screening of salicylates with higher efficiency and specificity may warrant a novel plant protection method. Further studies on practical use of SA in different crop plants will contribute to developing the cost-effective and environmental friendly crop management system.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






