 |
 |
| Plant Pathol J > Volume 35(4); 2019 > Article |
Abstract
The present study was undertaken to evaluate the integrated effect of zinc (Zn) with other nutrients in managing early blight (EB) disease in tomato. A pot experiment was carried out with basal application of the recommended level of macronutrients [nitrogen, phosphorus and potassium (NPK)] and micronutrients [magnesium (Mg) and boron (B)] in bilateral combination with Zn (2.5 and 5.0 mg/kg) in a completely randomized deigned in replicates. Results revealed that interactive effect of Zn with Mg or B was often futile and in some cases synergistic. Zn with NPK yield synergistic outcome, therefore EB disease was managed significantly (disease incidence: 25% and percent severity index: 13%), which resulted in an efficient signaling network that reciprocally controls nutrient acquisition and uses with improved growth and development in a tomato plant. Thus, crosstalk and convergence of mechanisms in metabolic pathways resulted in induction of resistance in tomato plant against a pathogen which significantly improved photosynthetic pigment, total phenolics, total protein content and defense-related enzymes [superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL)]. The tremendous increase in total phenolics and PAL activity suggesting their additive effect on salicylic acid which may help the plant to systemically induce resistance against pathogen attack. It was concluded that interactive effect of Zn (5.0 mg/kg) with NPK significantly managed EB disease and showed positive effect on growth, physiological and biochemical attributes therefor use of Zn + NPK is simple and credible efforts to combat Alternaria stress in tomato plants.
Sustainable agriculture farming is dire need of time to feed the population in the future without the degradation of natural resources. However, plant diseases are a serious major yield-limiting factor in crop production system worldwide. Early blight (EB) disease is one of the notorious diseases of tomato caused by Alternaria solani (Ellis and Martin) that can cause 80 Ā± 5% losses in warm and semiarid regions of the world (Gannibal et al., 2014). Many other reports reveal, this disease has spread epidemically on large of an area and caused massive yield losses (Handiyanti et al., 2018). The pathogen proliferates rapidly under warm weather with abundant moisture and heavy dew. EB disease is characterized by brown to dark brown necrotic lesions with concentric rings on leaves, stem and fruits (Cheng et al., 2014). Whereas disease affects photosynthetic activity and sometimes causes defoliation due to reduced size and surface area of leaves (Song et al., 2011). Due to inappropriate measures such as agrochemicals (fungicides) to control EB disease, but continuous use of chemicals have raised serious concerns about food safety, soil degradation and pathogens resistance against pesticides have led the need for alternative disease management methods that would be supportive for sustainable agriculture without disturbing environment (Adhikari et al., 2017; Nashwa and Abo-elyousr, 2012).
For EB management nutrient application could prove effective in reducing disease incidence, improving plant tolerance against disease by inducing resistance in the plant as well as improving plant growth and yield (Dordas, 2008). However, nutrients application has been given a little attention to disease control in a sustainable agriculture system to obtain higher yield. Inorganic macronutrients such as nitrogen (N), phosphorus (P) and potassium (K) are involved in many enzymatic and biological activities as well as in energy transfer (Mona et al., 2012; Sten et al., 2017). Furthermore, each nutrient has its specific role like N is essential for the synthesis of many cellular components and reduced the severity of the infection on tomato plant against A. solani (Kant et al., 2011). After N, P is important nutrient and a major constituent of ribonucleic acids (RNA) nucleotides, as well as it plays vital role energy transfer, protein metabolism, and other many functions like in biochemical and physiological processes (Sharma et al., 2013). K also has its importance in parallel to other macronutrients that contribute in various biochemical and physiological functions (Hasanuzzaman et al., 2018; Liljeroth et al., 2016) Like macronutrients, the micronutrients have also been reported to enhance the plant disease resistance by reducing disease severity. In plant metabolism, a key role is played by zinc (Zn) contributes in various reaction as enzyme co-factor like energy transfer, redox reactions, and stabilization of ribosomal fractions for protein synthesis (Mathpal et al., 2015; Noulas et al., 2018). Numerous researches suggested that the proper application of Zn enhances the growth and productivity in a diverse range of crops including beans, chickpea, maize, rice, cotton and wheat (Khan et al., 2018; Khattak et al., 2016; Rehman et al., 2018). Furthermore, disease incidence and severity in numerous crops has been reported to be reduced by using Zn based supplements that induces resistance and elicits various defenses enzymes like peroxidase (POX), catalase (CAT), polyphenol oxidase (PPO) and superoxide dismutase (SOD) against pathogen (Khan et al., 2018). In comparison to the chemical fungicides, the use of Zn could be an alternative strong, economic and self-maintaining disease control approach (Dimkpa and Bindraban, 2016).
Like Zn, the role of boron (B) has been documented in managing disease, through its direct involvement in cell wall structural integrity, stability and cell rigidity as well as its role in metabolic activities i.e., phenolics or lignin to resistor the permeability of cell membranes (Dordas, 2008; Ilyas et al., 2015). B also help in activation of systemic acquired resistance (SAR) mechanisms by releasing Ca2+ cations from cell walls, as well as B can restrict spore germination and active mycelial growth of fungal pathogen (Fu and Dong, 2013). Furthermore, the adequate supply of boron (B) and zinc (Zn) can improve the defense mechanism against fungal infection by showing positive impact on plant growth and the severity of potato early blight disease (Machado et al., 2018).
Likewise, Magnesium (Mg) acts as a central atom of chlorophyll in plant photosynthesis and transferring the phloem export of photosynthates. Mg role also has been reported in disease management of many crops like rice, poppy, wheat citrus, potato, and beans (Senbayram et al., 2015). Therefore, B and Mg application may help in activating self-defense mechanism in tomato plants against early blight pathogen (Huber et al., 2012). Although macro (NPK) and other essential micro-nutrients (B and Mg) have been found effective, a combined effect of Zn with these nutrients can improve its benefits by altering plant-defense mechanism (Anees et al., 2016; Dewdar and Rady, 2013; Khan et al., 2018).
Exploring the role of Zn in combination with NPK, Mg and B could provide a better option to mitigate EB disease incidence, inducing resistant, boosting plant growth and increasing yield. Therefore, the present study was designed to evaluate the role of the precision application of macro- and micro- nutrients in the activation of defense mechanism of tomato plants against early blight disease.
The research was conducted at the experimental area of the Institute of Agricultural Sciences (IAGS), University of the Punjab Lahore, located at 1Ā° 30ā 15ā N and 74Ā° 18ā 23ā E. The pot trial was carried out between August-September 2017 at temperature ranged from 27-32Ā°C and humidity 60-70%, both conditions are favorable for EB disease in tomato.
Tomato seeds of susceptible germline (Miracle) were surface sterilized with 1% sodium hypochlorite solution and used for nursery preparation (Awan et al., 2018). The pot experiment consisted of 15 treatments [T1-T5 (without Zn 0.0 mg/kg), T6-T10 (with Zn 2.5 mg/kg) and T11-T15 (with Zn 5.0 mg/kg)] with three replicates and was laid out in a completely randomized design (Table 1). Fertilizers like the amount of zinc sulphate (Zn), urea (N), diammonium phosphate (N & P), sulphate of potash (K), boric acid (B) and magnesium sulphate (Mg) were calculated on hectare to pot basis. Two different doses of Zn (recommended: 2.5 mg/kg and double: 5.0 mg/kg) alone and in combination with recommended dose of NPK (64 kg N/ha, 20 kg P/ha and 50 kg K/ha), B (3 kg/acre) and Mg (12 kg/acre) were applied in potted soil.
Four tomato seedlings were transplanted in each sterilized potted soil (12 kg/pot). Ten days after seedling transplanting, nutrients were applied in potted soil as per requirement for the treatment. A. solani (FCBP-1401) cultural suspension (3 Ć 104 conidia/ml) was sprayed on 15 days old-seedling in treatments (T2-T15), while negative control (T1) received distilled water. The plants were covered with plastic bags after pathogen inoculation to maintain humidity (70-80%). The plants were regularly monitored to measure the progression of disease in terms of disease intensity (disease incidence and disease severity).
Early blight disease incidence (DI) was assessed after 15 days pathogen inoculation as the percentage of leaves with visual symptoms of the disease in relation to all the leaves of the plant (Khan et al., 2016). The disease severity was assessed in terms of percent severity index (PSI) using 0-5 scoring scale (Pandey et al., 2003) (Table 2). PSI in each pot was calculated as:
Different physiological and biochemical alteration in tomato leaves from all treatments were assessed 5 days of pathogen inoculation. Total chlorophyll contents and carotenoids were assessed in leaf ethanolic extract (0.2 g/5 ml of 80% ethanol) (Minocha et al., 2009). Total phenolics were assessed in ethanolic plant extract mixed with 20% Na2CO3 and 0.025 mL of Folin-Ciocalteatouās reagent and after incubation absorbance (765 nm) was recorded (Vongsak et al., 2013).
The total protein content and enzyme activities were estimated in leaf extract prepared in chilled sodium phosphate buffer (0.1 M, pH 6.8). A modified method of Lowry (Pomory, 2008) was used for estimation of total protein content by recording absorbance of samples at 650 nm against bovine serum albumin as standard. Activities of SOD (Sugawara and Nikaido, 2014); CAT (Weydert and Cullen, 2010); POX (Sinsabaugh, 2010); PPO (Cheema and Sommerhalter, 2015) and PAL (Shi et al., 2013) were assessed and data were analyzed statistically.
Attributes related shoot and root (length, fresh and dry weight) were taken 30 days after inoculation. Dry biomass of plant material (shoot and root) was recorded by drying samples at 65Ā°C.
The normality of the data related to disease, growth, physiological and biochemical attributes of tomato plant was tested by the Kolmogorov-Smirnov test and then the data were submitted to analysis of variance (ANOVA) and means compared by the LSD (least significant difference test) at level P ā¤ 0.05 by using computer software Statistix 8.1. Two-way Factorial ANOVA was used to analyze the individual and interactive effects of zinc with other nutrients on disease intensity and physiology.
Inoculation of tomato plants with A. solani resulted in the maximum disease incidence (DI: 100%) and percent severity index (PSI: 70%) in the positive control treatment (T2) as compared to un-inoculated treatments (T1) (Table 3). Basal application of either NPK, Mg or B (T2-T5) resulted in still high DI (67-83%) and PSI (25-35%) (lower case) as compared to positive control. Conversely, the disease was significantly suppressed to variable extents, when Zn (2.5 and 5.0 mg/kg) was given alone or in combination with these nutrients. Consequently, DI and PSI were significantly decreased to 42% and 20%, respectively due to Zn (2.5 mg/kg) + AS, and foresaid parameters were further decreased significantly to 25% and 10%, respectively with Zn (5.0 mg/kg) + AS as compared to positive control. Pairwise comparisons using bilateral interactions indicated that the Zn along with NPK proved most effective in alleviating EB disease intensity (DI: 25 Ā± 8%; PSI: 10 Ā± 5%), but with the statistical insignificance differences between adjacent means (lower case) of Zn + AS. Furthermore, Mg and B remained ineffective inefficiently manage DI (70 Ā± 5%) and PSI and (29 Ā± 3%) when compared with a mean value (upper case in the column) of infected treatment (T2) as well as from the other combinations of nutrients (Table 3).
Zn application (2.5 and 5.0 mg/kg) significantly increased shoot related parameters (length, fresh and dry weight) by 23-48% in negative control treatments (T6 & T11) as compared to relevant control (T1). In positive control treatment (T2), the shoot growth attributes decreased significantly by 22-35% over negative control. Solitary application of nutrients showed variable results, only NPK + AS (T3) and Zn [T7 (2.5 mg/kg) and T12 (5.0 mg/kg)] + AS significantly improved shoot growth by 66-83% and 36-53%, respectively as compared to positive control. Moreover, NPK along with Zn [T8 (2.5 mg/kg) and T13 (5.0 mg/kg)] caused the maximum improvement in shoot attributes by 110-133% in inoculated treatments. Interactive application of B (T10 and T15) found less effective as compared to Mg (T9 and T14) in bilateral interactions with Zn in elevating growth attributes by 30-62% under EB stress over positive control. However, application of Mg in combination with 5.0 mg/kg Zn showed improvement in only shoot length and fresh weight by 57% and 31%, respectively as compared to positive control (Fig. 1).
A higher dose of Zn (5.0 mg/kg) significantly improved root fresh and dry weight by 15-25% as compared to relevant control (T1: healthy plants). Pathogen infected plants inoculated with A. solani (T2) exhibited a significant reduction in root attributes (length, fresh and dry weight) by 20-37% over negative control (T1). When infected plants were treated with Zn (5.0 mg/kg) alone, root length and fresh weight were increased significantly by 23-29%, and dry weight was significantly improved up to two folds with both doses of Zn over positive control. Like shoot attributes, NPK fertilizer alone (T3) and in combination with doses of Zn (T8: 2.5 and T13: 5.0 mg/kg) effectively and significantly improved the root attributes by 30-52% and 48-168%, respectively over T2 plants. In contrast, the infected plants supplemented with Mg and B, alone and in combination with Zn dose (2.5 mg/kg) have insignificant effect on the root attributes, but when applied with 5.0 mg/kg Zn, the root length and weight (fresh and dry) were significantly improved by 40-72% as compared to positive control (Fig. 2).
Quantitative assessments of physiological and biochemical attributes of A. solani infected tomato plants revealed that the pathogen affected tomato defense system to variable extents. Disease management through fertilization influenced plant-pathogen interactions and promoted resistance against a pathogen by fortifying the plant with improved physiological and biochemical attributes. In negative control (T1), total chlorophyll content (TCC), carotenoids (CARO), total phenolic content (PHE) and total protein content (TPC) were found as 2.55, 5.34, 7.89 and 0.27 mg/g, respectively, while the activities of antioxidants i.e. CAT, SOD, POX, PPO and PAL were 8.25, 0.32, 62, 0.53 and 2.11 Units/min/mg of protein, respectively. In infected plants (T2), TCC, CARO and PAL significantly decreased by 20-40%, whereas, PHE and antioxidant enzymes activities significantly increased by 30-50% and TPC by 70% as compared to negative control treatment (T1).
Among different fertilizers, basal application of Zn was profoundly influenced on all physiological and biochemical markers, where other nutrients like NPK, B and Mg showed the maximum enzyme activities in combination with Zn. Both doses of Zn (T7 & T12) were found equally stimulatory in pathogen-inoculated plants for TCC, CAR, PHE and TPP by 55%, 26%, 30% and 85%, respectively as compared to positive control (Table 4). Though, enzymes activities increased almost two-folds from 30 to 60% with the increase in Zn dose from 2.5 to 5.0 mg/kg (Table 5). Likewise, health and stress markers were noted to be higher and reached a maximum with a greater supply of Zn in bilateral interaction with NPK (T8 & T13) (Table 4-5). Consequently, in T8 & T13, the TCC, CAR and TPC increased significantly by 120-140%, 55-84% and 97-115%, respectively, whereas antioxidant enzymes improved significantly by 40-90%, and polyphenol synthesizing enzyme (PAL) by 96-126% with respect to the positive control. Application of Mg (T4) showed considerable enhancement in TCC by 55%, and the effect of Mg increased up to twofold in combination with Zn (T9 & T14). When B was given alone (T5), it failed to improve TCC, however, in combination with Zn (T10 & T15), the significant increase of 60-90% was observed in infected plants with respect to the positive control. Mg and B also improved CC by 55% and 35%, respectively when combined with Zn. Likewise, Zn + B and Zn + Mg showed significant enhancement of 30-70% and 20-50%, repetitively in the rest of the investigated attributes of inoculated plants over positive control (Table 4-5). Furthermore, the activity of polyphenol synthesizing enzyme boosted up tremendously by 70-87% in either B or Mg along with Zn.
Mean values (upper case) in a row displayed the effect of a higher dose of Zn either alone or in combination with other nutrients was significantly greater in negative and positive control as compared to treatments without Zn. Mean values (upper case) of the investigated attributes in column exhibited the interactive effect of Zn with NPK proved highly effective in stimulating defense response for resistance in tomato plants against early blight pathogen.
Plant disease management through nutrients application can modify hostās defense response in terms of susceptibility and resistance to disease, so maintenance of the plant nutrition is one of the fundamental strategies for sustaining plant health and suppressing plant diseases. The present investigation was concerned with the evaluation of the most promising interactive combination of Zn (2.5 and 5.0 mg/kg) with other plant nutrients (NPK, Mg and B) in managing EB disease of susceptible tomato germline.
Results revealed that EB infected plants (T2) showed the substantial increase in disease intensity (DI: 100% and PSI: 70%) with a significant reduction in growth attributes by 22-37% when compared with healthy plants (T1). The infected plants showed typical early blight symptoms (dark brown to black spots or irregular lesions surrounded by a chlorotic halo with concentric rings leading to blighting of leaves) under pathogenic stress (Awan et al., 2018). Application of macro- and micro- nutrients (NPK, Zn, Mg & B) improved the plant resistance/tolerance against pathogen to a variable degree by reducing the disease severity (Ghorbani et al., 2008; Potarzycki 2011; Sten et al., 2017). Basal application of Zn (2.5 & 5.0 mg/kg) alone and in combination with other nutrients (NPK, Mg & B) exhibited the excellent impact on the reduction of EB disease intensity, along with improvement in health and stress markers, however, 5.0 mg/kg Zn dose showed more promising results. Zn as a micronutrient helps to induce resistance in the plant against A. solani because it is a critical nutrient for several vital biological functions in plants like photosynthesis, growth regulation, sugar formation, seed production and defense mechanisms (Khan et al., 2018). Zn may induce its toxic effect on A. solani through effecting its metabolism and enzymatic activity as reported earlier on other microorganisms by Zn nanoparticles (Chai et al., 2015; Shen et al., 2015). Collin-Hansen et al. (2005) studies indicated that Zn toxicity occurred through oxidative damages to DNA and lipids in fungus Boletus edulis. On the basis of results obtained, it could be predicted that Zn in the soil after penetrating plant cell wall, cell membrane and cortical region of root accompanied by a complex series of events to enter plant vascular bundle (xylem) and move to the stele. Through xylem, Zn finds its way to distribute and translocate in the main organs of photosynthesis and transpiration (mainly in older leaves) mainly through active transport (Arruda et al., 2015). So, Zn might alleviated EB disease stress through (i) disrupting growth, reproduction and physiology of the pathogen (ii) changing plantās redox status, the level of signal molecules and antioxidant enzyme activities (iii) inducing synthesis of gene encoding enzymes for stress-related protein (PR proteins) and flavonoid biosynthesis pathway (Martos et al., 2016). The net outcome of Zn fertilization, therefore, might result in a faster and stronger induction of basal defense mechanisms through genetic or biochemical modifications in tomato plant upon exposure to A. solani. Likewise, Khan et al. (2018) suggested that Zn (2.5 and 5.0 mg/kg) fertilization significantly reduced charcoal rot disease and improved biological attributes in mung bean through altering physiological and biochemical attributes.
Basal fertilization with the recommended dose of NPK alone did not prove much effective in managing EB, it also increased the vegetative growth of the tomato plant. It seems that A. solani infection affected membrane permeability, impaired nutrient translocation and plant utilization, thus imbalance of NPK inside plant might affect plant tolerance to disease (Huber et al., 2012). The balance between N and K affects disease susceptibility of plants (Sten et al., 2017) due to the important role of K in photosynthesis, plant respiration, transpiration and nutrients translocation. P is involved in energy transfer compounds, cell membranes and phosphoproteins, whereas soils deficient in adequate P may also induce the severity of diseases in plants suffering from P starvation (Wissuwa, 2003). Likewise, in general application of either Mg or B proved inefficient in significantly reducing disease and improving growth attributes. Mg is an essential nutrient involved in chlorophyll formation, organic compound synthesis, protein synthesis, uptake and migration of phosphorus in plants (Aghofack-Nguemezi et al., 2014). B plays an important role in the expression of pathogenesis-related and induction of SAR in the host (Reguera et al., 2010).
The effect of Zn was uniform in combination with other nutrients including NPK, B and Mg. However, the interactive effect of NPK with both doses of Zn was the most effective in reducing disease intensity (DI: 25% and PSI: 13%) and improving growth attributes (50-168%) in pathogen-inoculated treatments over positive control that might be result of balance between required amount of nutrient for plant to cope with disease.
The positive impact of nutrients [(NPK, Mg and B) alone and in combination with Zn doses] on plant resistance to EB disease can be assessed by evaluating the alteration in health markers (photosynthetic pigments) and in the activities of various stress markers (PHE, TPC, SOD, CAT, POX, PPO, and PAL). Reduction in photosynthetic pigments (TCC and CARO) showed that toxins of A. solani damaged photosynthetic machinery by disturbing photosystems (Dehgahi et al., 2015) that might cause disruption in the food manufacturing system and also could be associated with the EB symptoms of necrosis and chlorosis (Sarkar et al., 2017). In response to infection caused by A. solani, the plant immune system possibly responds through induction of transient, low-amplitude and the first phase of ROS production suggesting a role for ROS in the establishment of the plant defenses thus resulted in 30-50% increase in activities of antioxidant enzymes (SOD, CAT, POX and PPO). Yet, due to acidification of host tissue through the production of oxalic acid by a pathogen, the calcium ion would be sequestered from the host cell walls which cause suppression of oxidative burst of the host plant (Anderson et al., 2010) (Fig. 3). Due to disruption in regulatory functions of ROS, the function of other associated plant signaling molecules like salicylic acid (SA) also disrupted. SA is essential for SAR and it synthesized from cinnamate produced by the activity of PAL (Kim and Hwang, 2014). Therefore, reduction in activity of PAL also showed failure in plant defense mechanism to induce resistance systemically through SA accumulation and plants suffered from blight disease (Chen et al., 2009).
When nutrients especially Zn was applied, activities of physiological and biochemical attributes increased significantly by 30-90%. Nevertheless, health and stress markers increased tremendously by 60-140% and reached a maximum with a greater supply of Zn in bilateral interaction with NPK. Triggering in defense response after nutrients application likely to occur sequentially through changes in Ca2+ influx that may results in alteration in extracellular pH, membrane potentials (Kurusu et al., 2005), along with biphasic ROS accumulation with a low-amplitude, transient first phase, followed by a sustained phase of much higher magnitude (Sharma et al., 2012; Tripathy and OelmĆ¼ller, 2012). SA would be interplay with ROS, whereas SA and ROS signals to the transcriptional control of defense genes for induction of resistance in tomato plant against disease, ultimately ROS-scavenging systems help to finally tuned ROS level (Khokon et al., 2011) (Fig. 3).
The current study revealed that synergistic crosstalk between interactive combination of Zn with NPK, Mg or B bestowed varying degree of resistance in tomato plant against EB disease through modifying activities of key players (defensives enzymes) and total phenolics which resulted in progressive effects on SA-responsive genes and defense-related proteins to elicit HR, SIR and SAR. Hence, infected plant developed sophisticated mechanisms of defense to alleviate EB stress of disease in tomato particularly through the interactive effect of Zn with NPK.
Fig.Ā 1
Effect of zinc [Zn (2.5 and 5.0 mg/kg)] with other plant nutrients on shoot attributes of tomato plants under the stress of Alternaria solani (AS) after 30 days of pathogen inoculation. Error bars indicate standard errors of the mean of three replicates. Values with different letters show a significant difference (P ā¤ 0.05) as determined by LSD-test.
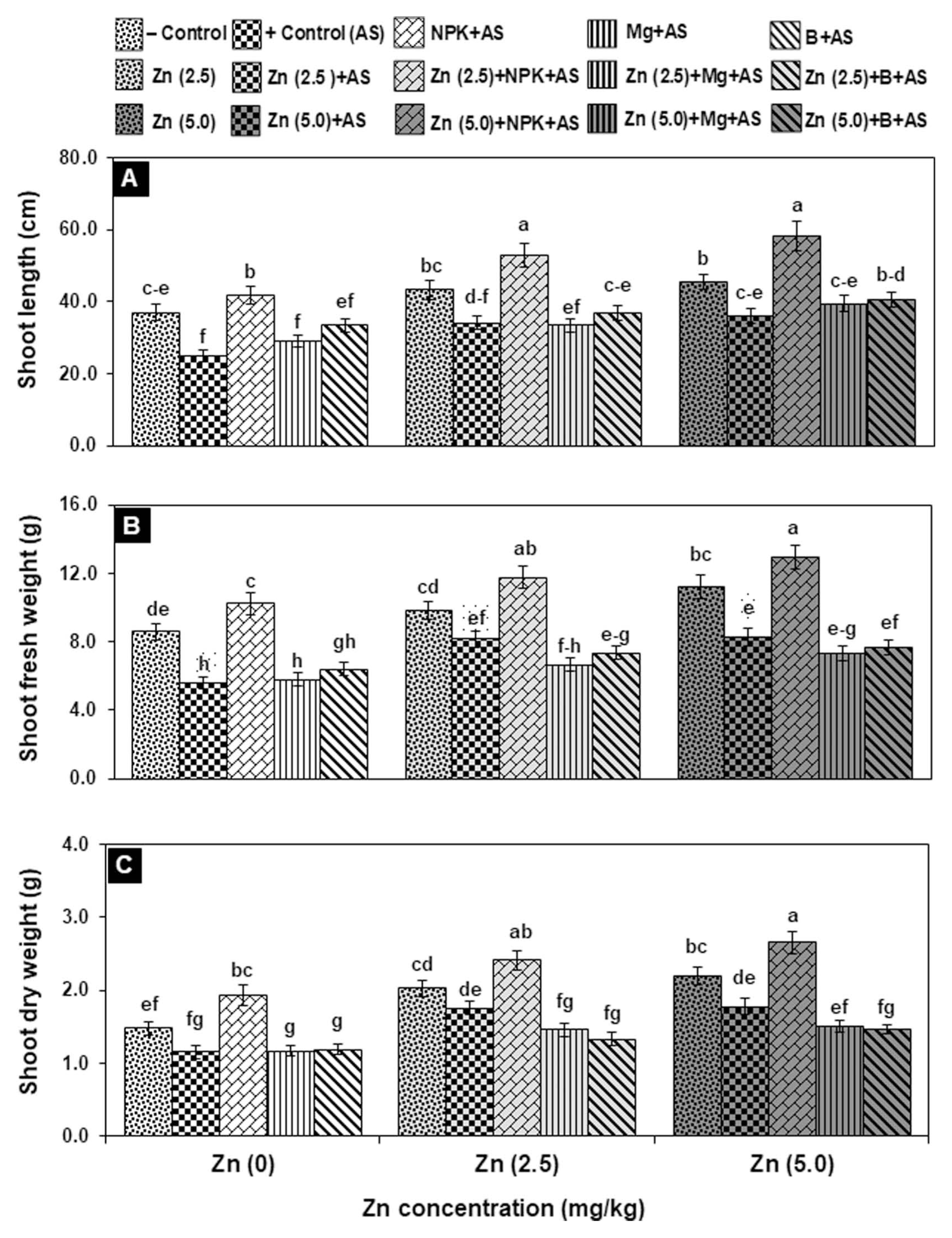
Fig.Ā 2
Effect of zinc [Zn (2.5 & 5.0 mg/kg)] in combination with plant nutrients on root attributes of tomato plants under the stress of Alternaria solani (AS) after 30 days of pathogen inoculation. Error bars indicate standard errors of the mean of three replicates. Values with different letters show a significant difference (P ā¤ 0.05) as determined by LSD-test.

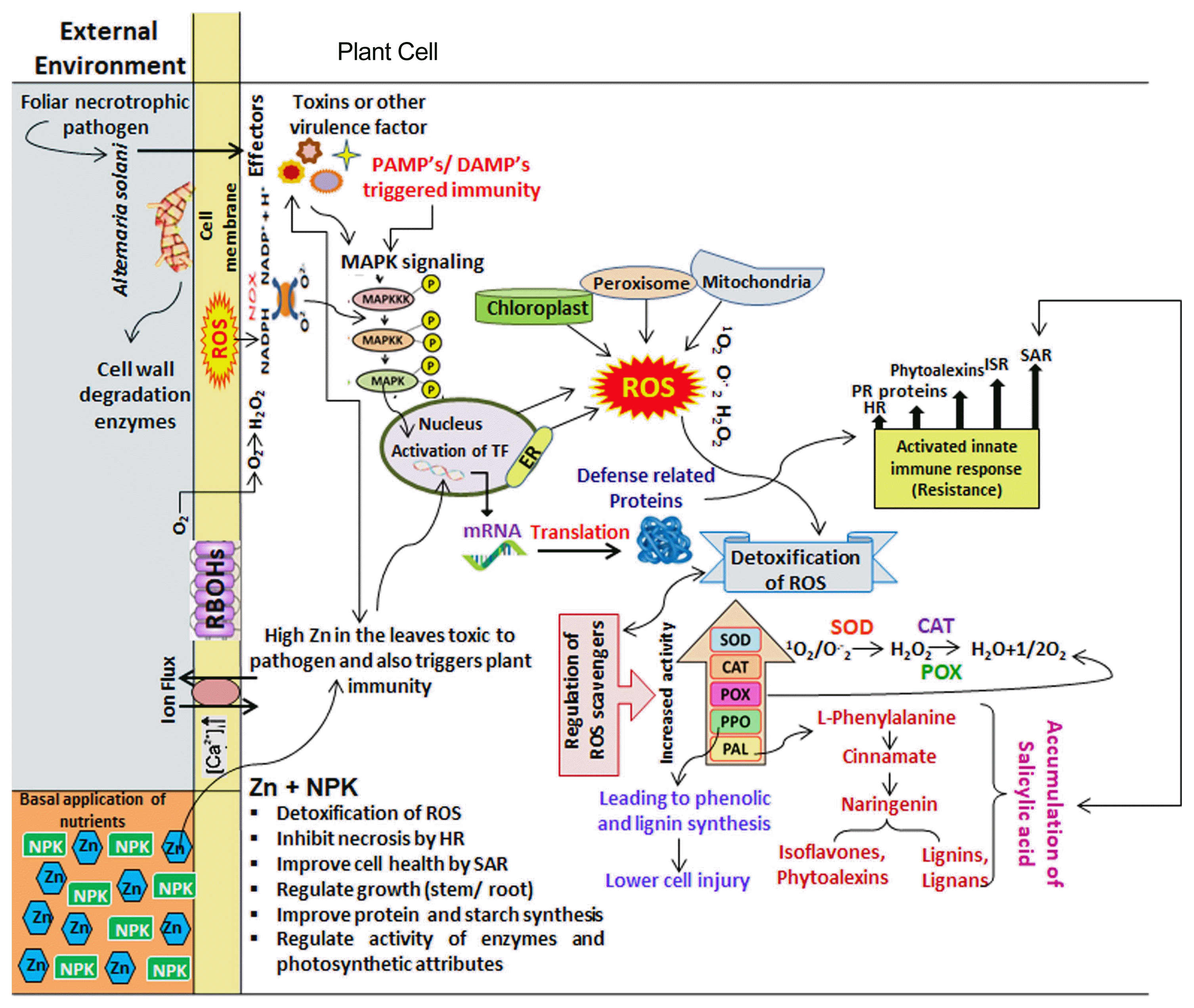
Fig.Ā 3
A schematic depiction of the interface and overlapping signaling pathways at the cellular level in tomato plants due to the basal application of Zn + NPK under Alternaria solani stress. To fight off pathogens, defense response triggers a battery of reactions including enhanced ROS production through downstream signaling by RBOHs localized at the plasma membrane, cytosolic MAPKs and others. ROS accumulation (H2O2) will diffuse into the cytosol and activate several plant defense responses including SA signaling and other phytohormones such as GA, JA & ET have a significant role during biotic stress tolerance in a tomato plant. However, ROS-scavenging systems (anti-oxidants) help to finally tune the ROS level. ROS, reactive oxygen species; SA, salicylic acid, GA, Gibberellic acid; JA, Jasmonic acid; ET, Ethylene; MAPKs, mitogen-activated protein kinase.
TableĀ 1
Treatments designed for the experiment
TableĀ 2
Disease rating scale for the assessment of early blight of tomato
TableĀ 3
Bilateral effect of Zn (2.5 and 5.0 mg/kg) with plant nutrients on disease incidence (DI) and percent severity index (PSI) in tomato plants inoculated with Alternaria solani (AS) at the 45th day after transplantation
TableĀ 4
Bilateral effect of zinc (Zn) levels with plant nutrients on physiological attributes in tomato plants inoculated with Alternaria solani (AS) after 15-D of transplantation
TableĀ 5
Bilateral effect of zinc (Zn) levels with plant nutrients on defense-related enzymes in tomato plants inoculated with Alternaria solani (AS) after 15-D of transplantation
References
Adhikari, P, Oh, Y and Panthee, DR 2017. Current status of early blight resistance in tomato: An update. Int J Mol Sci. 18:E2019

Aghofack-Nguemezi, J, Noumbo, GT and Nkumbe, CN 2014. Influence of calcium and magnesium based fertilizers on fungal diseases, plant growth parameters and fruit quality of three varieties of tomato (Solanum lycopersicum). J Sci Technol. 34:9-20.

Anderson, JP, Gleason, CA, Foley, RC, Thrall, PH, Burdon, JB and Singh, KB 2010. Plants versus pathogens: an evolutionary arms race. Funct Plant Biol. 37:499-512.



Anees, MA, Ali, A, Shakoor, U, Ahmed, F, Hasnain, Z and Hussain, A 2016. Foliar applied potassium and zinc enhances growth and yield performance of maize under rainfed conditions. Int J Agric Biol. 18:1025-1032.

Arruda, SCC, Silva, ALD, Galazzi, RM, Azevedo, RA and Arruda, MAZ 2015. Nanoparticles applied to plant science: a review. Talanta. 131:693-705.


Awan, ZA, Shoaib, A and Khan, KA 2018. Variations in total phenolics and antioxidant enzymes cause phenotypic variability and differential resistant response in tomato genotypes against early blight disease. Sci Hortic. 239:216-223.

Chai, H, Yao, J, Sun, J, Zhang, C, Liu, W, Zhu, M and Ceccanti, B 2015. The Effect of Metal Oxide Nanoparticles on Functional Bacteria and Metabolic Profiles in Agricultural Soil. Bull Environ Contam Toxicol. 94:490-495.


Cheema, S and Sommerhalter, M 2015. Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chem. 171:382-387.


Chen, Z, Zheng, Z, Huang, J, Lai, Z and Fan, B 2009. Biosynthesis of salicylic acid in plants. Plant Signal Behav. 4:493-496.



Cheng, S-X, Xie, C-Q, Wang, Q-N, He, Y and Shao, Y-N 2014 Different wavelengths selection methods for identification of early blight on tomato leaves by using hyperspectral imaging technique. Spectrosc Spect Anal. 34:1362-1366 (in Chinese).
Collin-Hansen, C, Andersen, RA and Steinnes, E 2005. Damage to DNA and lipids in Boletus edulis exposed to heavy metals. Mycol Res. 109:1386-1396.


Dehgahi, R, Subramaniam, S, Zakaria, L, Joniyas, A, Firouzjahi, FB, Haghnama, K and Razinataj, M 2015. Review of research on fungal pathogen attack and plant defense mechanism against pathogen. Int J Sci Res Agric Sci. 2:197-208.

Dewdar, MDH and Rady, MM 2013. Influence of soil and foliar applications of potassium fertilization on growth, yield and fiber quality traits in two Gossypium barbadense L. varieties. Afr J Agric Res. 8:2211-2215.

Dimkpa, CO and Bindraban, PS 2016. Fortification of micronutrients for efficient agronomic production: a review. Agron Sustain Dev. 36:7

Dordas, C 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron Sustain Dev. 28:33-46.

Fu, ZQ and Dong, X 2013. Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol. 64:839-863.


Gannibal, PB, Orina, AS, Mironenko, NV and Levitin, MM 2014. Differentiation of the closely related species. Alternaria solani and A. tomatophila, by molecular and morphological features and aggressiveness. Eur J Plant Pathol. 139:609-623.

Ghorbani, R, Wilcockson, S, Koocheki, A and Leifert, C 2008. Soil management for sustainable crop disease control: a review. Environ Chem Lett. 6:149-162.

Handiyanti, M, Subandiyah, S and Joko, T 2018 Molecular detection of Burkholderia glumae, a causal agent of bacterial panicle blight disease. J Perlindungan Tanam Indonesia. 22:98-107 (in Indonesian).
Hasanuzzaman, M, Bhuyan, MHMB, Nahar, K, Hossain, MS, Al Mahmud, J, Hossen, MS, Masud, AAC, Moumita, and and Fujita, M 2018. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy. 8:31

Huber, D, Rƶmheld, V and Weinmann, M 2012. Relationship between nutrition, plant diseases and pests. In: Marschnerās mineral nutrition of higher plants, eds. by P Marschner, 283-298. Academic Press, London, UK.

Ilyas, A, Ashraf, MY, Hussain, M, Ashraf, M, Ahmed, R and Kamal, A 2015. Effect of micronutrients (Zn, Cu and B) on photosynthetic and fruit yield attributes of citrus reticulata Blanco var. kinnow. Pak J Bot. 47:1241-1247.
Kant, S, Bi, Y-M and Rothstein, SJ 2011. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J Exp Bot. 62:1499-1509.


Khan, KA, Shoaib, A, Arshad Awan, Z, Basit, A and Hussain, M 2018. Macrophomina phaseolina alters the biochemical pathway in Vigna radiata chastened by Zn2+ and FYM to improve plant growth. J Plant Interact. 13:131-140.

Khattak, SG, Dominy, PJ and Ahmad, W 2016. Effect of Zn as soil addition and foliar application on yield and protein content of wheat in alkaline soil. J Natl Sci Found Sri Lanka. 43:303-312.

Khokon, AR, Okuma, E, Hossain, MA, Munemasa, S, Uraji, M, Nakamura, Y, Mori, IC and Murata, Y 2011. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34:434-443.


Kim, DS and Hwang, BK 2014. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J Exp Bot. 65:2295-2306.



Kurusu, T, Yagala, T, Miyao, A, Hirochika, H and Kuchitsu, K 2005. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 42:798-809.


Liljeroth, E, Lankinen, Ć
, Wiik, L, Burra, DD, Alexandersson, E and Andreasson, E 2016. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Prot. 86:42-55.

Machado, PP, Steiner, F, Zuffo, AM and Machado, RA 2018. Could the supply of boron and zinc improve resistance of potato to early blight? Potato Res. 61:169-182.

Martos, S, Gallego, B, Cabot, C, Llugany, M, BarcelĆ³, J and Poschenrieder, C 2016. Zinc triggers signaling mechanisms and defense responses promoting resistance to Alternaria brassicicola in Arabidopsis thaliana. Plant Sci. 249:13-24.


Mathpal, B, Srivastava, PC, Shankhdhar, D and Shankhdhar, SC 2015. Improving key enzyme activities and quality of rice under various methods of zinc application. Physiol Mol Biol Plants. 21:567-572.



Minocha, R, Martinez, G, Lyons, B and Long, S 2009. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can J For Res. 39:849-861.

Mona, EE, Ibrahim, SA and Manal, FM 2012. Combined effect of NPK levels and foliar nutritional compounds on growth and yield parameters of potato plants (Solanum tuberosum L.). Afr J Microbiol Res. 6:5100-5109.
Nashwa, SMA and Abo-elyousr, KAM 2012. Evaluation of various plant extracts against the early blight disease of tomato plants under greenhouse and field conditions. Plant Protect Sci. 48:74-79.

Noulas, C, Tziouvalekas, M and Karyotis, T 2018. Zinc in soils, water and food crops. J Trace Elem Med Biol. 49:252-260.


Pandey, KK, Pandey, PK, Kalloo, G and Banerjee, MK 2003. Resistance to early blight of tomato with respect to various parameters of disease epidemics. J Gen Plant Pathol. 69:364-371.

Potarzycki, J 2011. Effect of magnesium or zinc supplementation at the background of nitrogen rate on nitrogen management by maize canopy cultivated in monoculture. Plant Soil Environ. 57:19-25.

Reguera, M, Bonilla, I and BolaƱos, L 2010. Boron deficiency results in induction of pathogenesis-related proteins from the PR-10 family during the legume-rhizobia interaction. J Plant Physiol. 167:625-632.


Rehman, A, Farooq, M, Naveed, M, Nawaz, A and Shahzad, B 2018. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur J Agron. 94:98-107.

Sarkar, D, Maji, RK, Dey, S, Sarkar, A, Ghosh, Z and Kundu, P 2017. Integrated miRNA and mRNA expression profiling reveals the response regulators of a susceptible tomato cultivar to early blight disease. DNA Res. 24:235-250.



Senbayram, M, Gransee, A, Wahle, V and Thiel, H 2015. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci. 66:1219-1229.

Sharma, P, Jha, AB, Dubey, RS and Pessarakli, M 2012 Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012:217037.

Sharma, SB, Sayyed, RZ, Trivedi, MH and Gobi, TA 2013. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2:587



Shen, Z, Chen, Z, Hou, Z, Li, T and Lu, X 2015. Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front Environ Sci Eng. 9:912-918.

Shi, R, Shuford, CM, Wang, JP, Sun, YH, Yang, Z, Chen, HC, Tunlaya-Anukit, S, Li, Q, Liu, J, Muddiman, DC, Sederoff, RR and Chiang, VL 2013. Regulation of phenylalanine ammonia-lyase (PAL) gene family in wood forming tissue of Populus trichocarpa. Planta. 238:487-497.


Sinsabaugh, RL 2010. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem. 42:391-404.

Song, Y, Wang, R, Wei, X, Lu, Y, Tang, Z, Wu, G, Su, Y and Zeng, R 2011 Mechanism of tomato plants enhanced disease resistance against early blight primed by arbuscular mycorrhizal fungus Glomus versiforme. J Appl Ecol. 22:2316-2324 (in Chinese).
Sten, J, Mahapatra, S and Das, S 2017. Effect of different levels of NPK on foliar diseases of potato under different fertility gradient soil in field Effect of different levels of NPK on foliar diseases of potato under different fertility gradient soil in field. J Crop Weed. 13:151-156.
Sugawara, E and Nikaido, H 2014. Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli. Antimicrob Agents Chemother. 58:7250-7257.



Tripathy, BC and OelmĆ¼ller, R 2012. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 7:1621-1633.



Vongsak, B, Sithisarn, P, Mangmool, S, Thongpraditchote, S, Wongkrajang, Y and Gritsanapan, W 2013. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod. 44:566-571.

- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




