Theobroxide Treatment Inhibits Wild Fire Disease Occurrence in Nicotiana benthamiana by the Overexpression of Defense-related Genes
Article information
Abstract
Theobroxide, a novel compound isolated from a fungus Lasiodiplodia theobromae, stimulates potato tuber formation and induces flowering of morning glory by initiating the jasmonic acid synthesis pathway. To elucidate the effect of theobroxide on pathogen resistance in plants, Nicotiana benthamiana plants treated with theobroxide were immediately infiltrated with Pseudomonas syringae pv. tabaci. Exogenous application of theobroxide inhibited development of lesion symptoms, and growth of the bacterial cells was significantly retarded. Semi-quantitative RT-PCRs using the primers of 18 defense-related genes were performed to investigate the molecular mechanisms of resistance. Among the genes, the theobroxide treatment increased the expression of pathogenesis-related protein 1a (PR1a), pathogenesis-related protein 1b (PR1b), glutathione S-transferase (GST), allen oxide cyclase (AOC), and lipoxyganase (LOX). All these data strongly indicate that theobroxide treatment inhibits disease development by faster induction of defense responses, which can be possible by the induction of defense-related genes including PR1a, PR1b, and GST triggered by the elevated jasmonic acid.
Theobroxide is an epoxy cyclohexene natural compound, which was isolated from the culture filtrates of the fungus Lasiodiplodia theobromae (Pat.) Griffon & Maubl (Strain OCS71) (Nakamori et al., 1994). As a putative plant growth regulator, theobroxide stimulates tuber formation in potato (Solanum tuberosum L.) under in vitro, in vivo, and non-inductive condition, and induces flower bud formation of morning glory (Pharbitis nil C.) under long day conditions (Gao et al., 2005; Yoshihara et al., 2000). Theobroxide treatment increased the activity of lipoxygenase (LOX) and allene oxide cyclase (AOC), and eventually increased the endogenous level of jasmonic acid (JA) and tuberonic acid (TA) in potato and morning glory (Gao et al., 2003; Kong et al., 2005; Yang et al., 2004).
In defense mechanisms of plants against pathogen attacks, JA plays an important role as a signaling molecule. JA is produced in response to pathogen infection, most probably through an increase of the activity of lipoxygenase in plants (Hammond-Kosack et al., 1996; May et al., 1996; Thomma et al., 1998). JA is one of the most important signals in the plant defense response against pathogens in addition to salicylic acid (SA). Through signal transduction using these molecules, plants respond to pathogen attack or external stresses by rapid changes in gene expression, resulting in the induction of genes involved in the defense response, such as the pathogenesis-related (PR) proteins. Therefore, the genes of PR proteins are induced and accumulated in host plants as a result of pathogen infection or abiotic stresses (Kim and Hwang, 2000; Yang et al., 1997). All defense signaling molecules including SA, JA, MeJA, and ethylene induce the production of antimicrobial compounds such as phytoalexins and PR proteins (Lamb and Dixon, 1997).
Theobroxide has been known to induce LOX activity in plants (Kong et al., 2009; Yang et al., 2004), which suggests that exogenous treatment can be assumed to elicit the pathway for JA synthesis and the newly evolved JA can inhibit disease infection in tobacco plants. Therefore, theobroxide treatment is supposed to induce the defense response against external stresses and pathogen attack in plants. In this study, we were able to verify the effect of theobroxide on inhibiting disease development by inducing defense responses in Nicotiana benthamiana plants.
N. benthamiana plants were grown at a growth room maintained at 23°C with a 16/8 h light/dark condition. Leaves of 5 to 6 week-old plants were sprayed with 2 or 5 mM theobroxide solution dissolved in distilled water without any organic solvent and then immediately infiltrated with Pseudomonas syringae pv. tabaci (Ps. tabaci), a pathogenic bacterium of N. benthamiana. Leaves were harvested at the indicated time points (0, 1, 3, 6, 12, 24, and 48 h) after infiltration, immediately frozen in liquid nitrogen, and then stored at −80°C for future use. Theobroxide was kindly provided by Dr. Yoshihara in Asahikawa University, Japan. Ps. tabaci was grown at 28°C in King’s B agar media (Peptone 20 g, potassium sulphate 1.5 g, magnesium chloride 1.5 g, glycerol 10 ml, and agar 15 g/ml). Bacterial cell suspensions (106 cfu/ml) were infiltrated into leaf mesophyll tissues of intact plants using a hypodermic syringe without a needle. The diameter of lesions on the pathogen-infected leaves was measured 3 and 5 days after infiltration. To monitor bacterial cell growth in the leaf tissues infiltrated with the pathogen, the tissue infiltrated with Ps. tabaci for 24 h was ground in 10 mM MgCl2 and spread on selective King’s B agar media. Numbers of bacterial populations were determined based on the number of colonies formed on the selective medium 48 h after incubation.
Total RNAs were extracted using the easy-BLUE kit (iNtRON Biotechnology, USA), according to the manufacturer’s instructions. From the total RNA (1 μg), first-strand cDNA was synthesized using the PrimeScript™ 1st strand cDNA synthesis kit (TaKaRa Bio Inc., Japan) and subsequently used as the template for PCR. The nucleotide sequences of housekeeping actin gene and gene-specific primers used in this study are shown in Table 1. The conditions for the PCR were as follows: an initial 5 min of denaturation at 94°C; 35 cycles at 94°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min; and final 7 min incubation at 72°C. The various-sized PCR products were identified by 1% (W/V) agarose gel electrophoresis with 0.5x TBE running buffer. After taking the gel picture by benchtop variable transilluminator (UVP, CA, USA), the images were analyzed the amount of expression by using a public domain image analysis system (NIH ImageJ, NIH Image, Bethesda, USA).
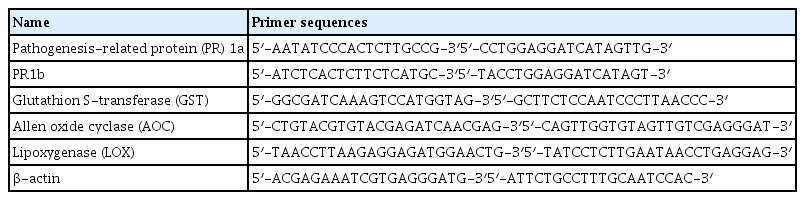
Development of wild fire disease lesion in the leaves of N. benthamiana plants was evaluated 3 and 5 days after inoculation of Ps. tabaci. The lesion sizes on the N. benthamiana leaves are shown in Fig. 1. Bacterial wilt symptoms were observed on the N. benthamiana leaves infiltrated with Ps. tabaci (Fig. 1A). The infiltrated areas of N. benthamiana leaves for the Ps. tabaci inoculation, 2 mM theobroxide treatment + Ps. tabaci inoculation and 5 mM theobroxide treatment + Ps. tabaci inoculation were 0.6 ± 0.03 mm, 0.6 ± 0.05 mm, and 0.6 ± 0.03 mm in diameter, respectively. Symptom development was inhibited in N. benthamiana plants sprayed with 2 and 5 mM theobroxide compared to control plants at 3 and 5 days after inoculation (Fig. 1B), indicating that the treatment of theobroxide enhanced the resistance to Ps. tabaci.
Disease symptoms (A) and disease development (B) on the leaves of N. benthamiana with P. syringae pv. tabaci. (A) P. syringae pv. tabaci inoculated at 106 cfu/ml and picture was taken 5 days after inoculation. (B) lesions on N. bethamiana leaves were measured 3 and 5 days after the bacterial infiltration. Data show the means ± standard deviation from three independent experiments. ■; Inoculation with P. syringae pv. tabaci,
 ; 2 mM theobroxide treatment and inoculation with P. syringae pv. tabaci (2 mM theo. + Ps. tabaci), □; 5 mM theobroxide treatment and inoculation with P. syringae pv. tabaci (5 mM theo. + Ps. tabaci). Mean separation within columns by the Duncan’s multiple range test, P < 0.05.
; 2 mM theobroxide treatment and inoculation with P. syringae pv. tabaci (2 mM theo. + Ps. tabaci), □; 5 mM theobroxide treatment and inoculation with P. syringae pv. tabaci (5 mM theo. + Ps. tabaci). Mean separation within columns by the Duncan’s multiple range test, P < 0.05.
The growth of bacteria in the controls and in the leaves treated with theobroxide was further measured by the cell counting method at 24 h after infiltration. The numbers of bacteria recovered from the lesions of theobroxide-treated leaves were significantly lower than those recovered from the lesions of controls, and a reduction of 81–95% of bacterial cell growth by the theobroxide treatment was observed (Fig. 2). The pattern of the bacterial growth was positively correlated with the symptom development, showing that the bacterial numbers in the pathogen-inoculated control exceeded 3.4 or 19 times only one day after inoculation compared with those in the 2 mM theobroxide + Ps. tabaci, and 5 mM theobroxide + Ps. tabaci treatment, respectively. Five mM theobroxide-treated plants showed higher resistance against Ps. tabaci than 2 mM theobroxide treated and control plants.
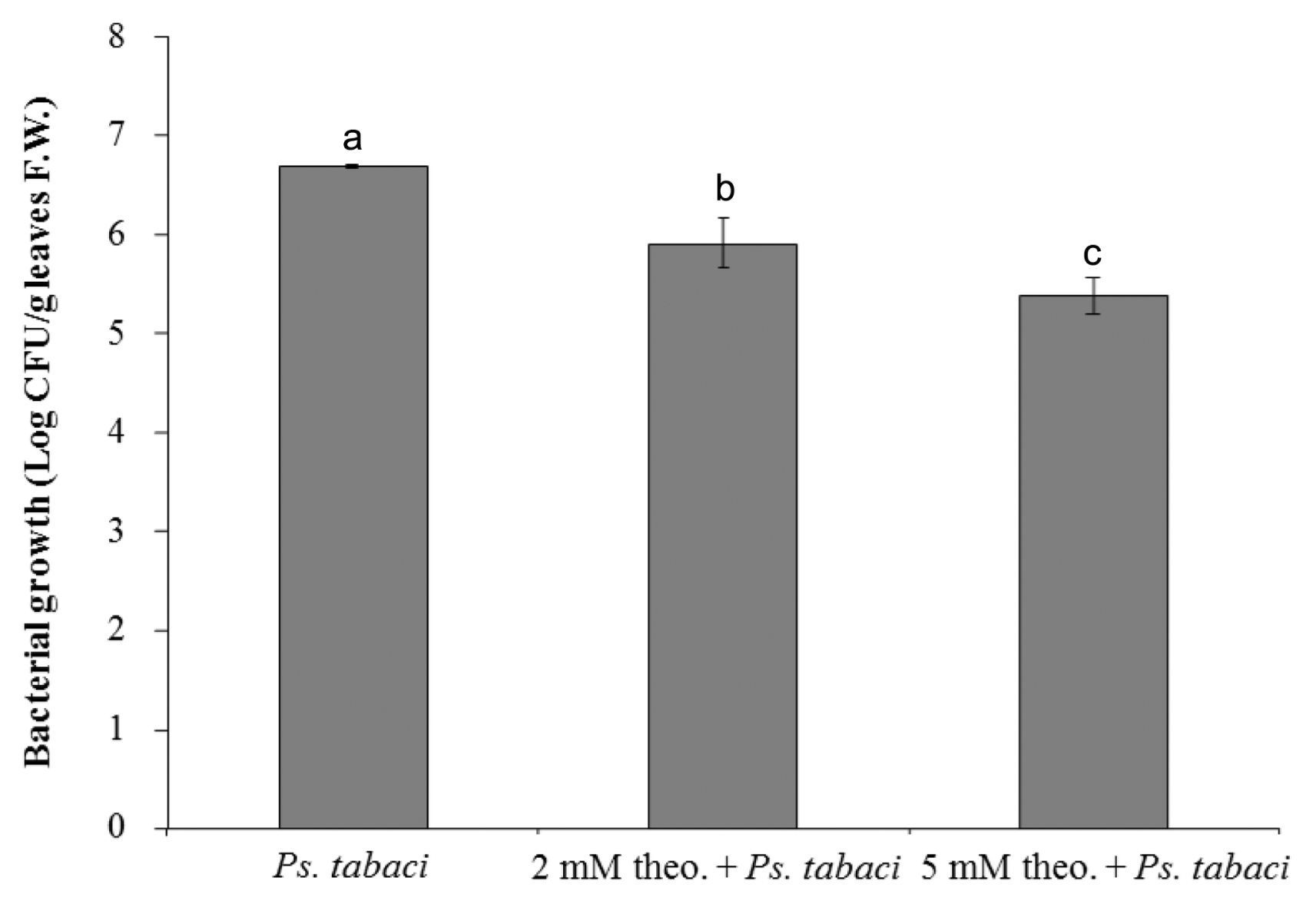
Numbers of colony forming unit (cfu) from the control, 2 mM theobroxide (2 mM theo.) + Ps. tabaci, and 5 mM theobroxide (5 mM theo.) + Ps. tabaci-treated leaves which was infiltrated with P. syringae pv. tabaci for one day. Data show the means ± standard deviation from three independent experiments. Mean separation within columns by the Duncan’s multiple range, P < 0.05.
RT-PCR analysis was carried out using the primer pairs listed in Table 1. Primers for β-actin were used as an internal control for the RNA quality and quantity. Among 18 primer sets for detecting the expression levels of defense-related genes, three genes, PR1a, PR1b, and GST were highly induced by the treatments of 5 mM theobroxide, 5 mM theobroxide + Ps. tabaci (5 mM theobroxide treatment and inoculation with Ps. tabaci) and the infection of Ps. tabaci (Fig. 3).

RT-PCR analysis (A) and the relative gene expression quantitation (B) for detecting the expression levels of three defense-related genes (PR1a, PR1b, and glutathione S-transferase), allen oxide cyclase (AOC) gene, and lipoxygenase (LOX) gene in N. benthamiana leaves treated with 5 mM theobroxide, 5 mM theobroxide + Ps. tabaci and infiltrated with P. syringae pv. tabaci. cDNA was constructed using the total RNAs extracted at the indicated time points and used for conducting semi-quantitative RT-PCRs. PCR profile was composed of an initial 5 min of denaturation at 94°C; 35 cycles at 94°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min; and final 7 min incubation at 72°C. For detecting LOX, only 28 cycles were performed with same profile. Equal usage of total RNA was identified by comparing the expression levels of the actin gene. ■; PR1a, ◆; PR1b, ▲; GST, ●; AOC, *; LOX, ×; actin.
In plants, JA is biosynthetically produced by the octadecanoid pathway (Schaller and Stinzi, 2009). In this pathway, AOC is crucially important to facilitate the enantiomeric structure of the cyclopentenone ring. RT-PCR data showed that the AOC and LOX genes increased the expression levels by all the treatments tested (Fig. 3). These results indirectly indicated that theobroxide treatment induced JA accumulation in inoculated tobacco leaves.
PR1a and PR1b genes were highly induced in N. benthamiana leaves treated with 5 mM theobroxide, 5 mM theobroxide + Ps. tabaci treatment and the infection of Ps. tabaci (Fig. 3). These treatments induced higher expression of the genes, even only after 1 h. The expression levels of PR1a were highly induced following the treatment of theobroxide and lasted for 48 h, but those of PR1b had a shorter treatment effect. PR1b decreased the expression levels to the original expression levels after 48 h of the treatment.
It has been reported that there were at least 16 PR-1-type genes in tobacco (Cornelissen et al., 1987). The PR-1 type proteins are very similar in their structures, and classified into four groups: three acidic (1a, 1b, and 1c) and one basic (1g) proteins (Van Loon and Van Strien, 1999). The PR-1 type proteins are often used as markers of the enhanced defensive state conferred by pathogen-induced systemic acquired resistance (SAR), and both PR1a and PR1b proteins were accumulated through JA synthesis pathway (Van Loon and Van Strien, 1999; Van Loon et al., 2006). Pathogen-induced SAR has been known to be related to PR-1 type protein accumulation through the action of the signal molecules such as SA and JA in plants (Mei et al., 2006; Van Loon et al., 2006). In the mechanisms of defense responses in plants, JA signal transduction is required for induction of the expression of genes encoding PR-1 type proteins, especially PR1a and PR1b (Niki et al., 1998; Pieterse et al., 1998; Santamaria et al., 2001). Therefore, the overexpression of PR1a and PR1b in our study can be explained by the newly induced JA, which was triggered by the theobroxide treatment.
GST gene expression was induced 1 h after pathogen inoculation in all plants treated with Ps. tabaci, 5 mM theobroxide and 5 mM theobroxide + Ps. tabaci. However, after 6 h, the expression in the leaves of the N. benthamiana treated with 5 mM theobroxide was higher than those treated with Ps. tabaci (Fig. 3). These higher expressions of GST in the leaves treated with 5 mM theobroxide and 5 mM theobroxide + Ps. tabaci were observed in all time points after 6 h of treatment. Plant GSTs were reported to be induced in response to pathogen attack and heavy metals (Moons, 2003; Ulmasov et al., 1995) and also to oxidative stress to protect cellular components from damage (Levine et al., 1994; Marrs, 1996). Twelve Arabidopsis GST genes exhibited a diverse range of responses to jasmonate, SA, ethylene, pathogen infection, and oxidative stress (Wagner et al., 2002). GSTs with peroxidase (POX) activity can catalyze the reduction of lipid hydroperoxides and alleviate oxidative stress during various stresses (Roxas et al., 1997). Therefore, the higher resistance level induced by the theobroxide treatment might be due to the higher expression of GST gene, and the high expression of GST might delay the development of disease symptoms.
The expression levels of other genes, e.g., SAR, PinI, PinII, PR-Q, and thaumatin genes were high even without infection of Ps. tabaci or theobroxide treatment (data not shown). These genes were constitutively expressed in normal growth conditions, and furthermore, the expression levels were not highly induced by the infiltration of Ps. tabaci or the treatment of theobroxide. In particular, SAR gene, which is the key factor for SA signal transduction, was not highly induced by theobroxide, indicating this gene is not regulated by JA signaling pathway. SAR, PinI, PinII, PR-Q, and thaumatin genes, which expressed at the same levels following infiltration of Ps. tabaci or the treatment of theobroxide, might be regulated by other signaling transduction mechanisms or may express their maximum amounts even under the normal growth conditions.
Plants resist biotic attacks by employing a complex array of physical and chemical defense mechanisms, including production of various signal compounds, production of so-called PR proteins, and the buildup of histological barriers (Ortíz-Castro et al., 2009). The induced resistance in plants could be initiated by the exogenous treatment of response-eliciting-chemicals such as SA, 2,6-dichloroisonicotinic acid, acibenzolar-S-methyl, tiadinil, copper, NN-acetylglucosamin, silicon, BTH, and beta-aminobutyric acid, and by application of microbes like plant growth promoting rhizobacterim (Hammerschmidt, 2009; Rudrappa et al., 2010; Schneider et al., 1996).
JA and its methyl derivative MeJA play important roles in the defense responses of plants against both phytophagous insects and necrotrophic pathogens. For the operation of defense responses in plants, JA signal transduction is required for induction of systemic resistance and mounts defense responses against infection of bacterial pathogens (Pieterse et al., 1998; Veena et al., 2003; Zipfel et al., 2006). The accumulation of JA is required for the expression of the antimicrobial peptides (Epple et al., 1995) and MeJA application induces resistance to Alternaria brassicicola and Botrytis cinerea in Arabidopsis (Thomma et al., 1998; Thomma et al., 2000). Especially, PR genes in tobacco (Niki et al., 1998) and AtGSTF6 in Arabidopsis (Wagner et al., 2002) have been reported to be induced by treatment of JA. In this study, exogenous application of theobroxide to N. benthamiana resulted in the increased expression of PR1a, PR1b, GST, AOC, and LOX, which genes have been reported to be induced by the JA signal transduction in the defense response in the plants. Therefore, the newly evolved JA by the treatment of theobroxide may induce the systemic resistance and mount defense responses through the up-regulation of PR1a, PR1b, GST, AOC, and LOX genes. Exogenous application of theobroxide has been reported to promote the activity of LOX, which enzyme is involved in the linolenic acid synthesis cascade (Gao et al., 2003; Kong et al., 2005). The treatment of theobroxide may activate LOX gene first, and increase the expression of AOC gene, which is related with the JA synthesis in N. benthamiana.
In this study, we examined the effect of theobroxide on controlling disease development by Ps. tabaci, and subsequently, detected the induction of defense-related genes. Theobroxide treatment significantly reduced lesion development of wild fire disease and the numbers of infected bacteria, and these delayed symptoms could be induced by the higher expression of the PR1a, PR1b, and GST genes. These data suggest that theobroxide, which has been known to induce JA-mediated pathways, can be utilized as an agent for controlling wild fire disease and possibly other diseases by inducing the responses related defenses in plants.
Acknowledgments
This work was supported by the 2011 Post-Doctoral Course Program of Yeungnam University, and by the Technology Innovation Program (Industrial Strategic Technology Development Program, 10033630) funded by the Ministry of Knowledge Economy (MKE, Korea).