Hot pepper (Capsicum annum L.) is cultivated on more than 55,000 ha in Korea and is one of the country’s most important cash vegetable crops (Kim, 2010). Pests of hot pepper can cause severe damage to yields (Kang et al., 2010; Myung et al., 2009). Its diseases include anthracnose (Colletotrichum acutatum J. H. Simmonds), Phytophthora blight (Phytophthora capsici Leonian), bacterial wilt (Ralstonia solanacearum (Smith) Yabuuchi et al.), and bacterial spot (Xanthomonas campestris pv. vesicatoria (Doidge) Dye); and its insect pests include tobacco budworm (Helicoverpa assulta Guene), beet armyworm (Spodoptera exigua Hbner), thrips, and aphids (Kim, 2010; Kwon and Lee, 2002). Controls of these pests are mainly depending on pesticides, but alternatives have been tried such as developing resistant varieties for Phytophthora blights. Since anthracnose is a devastating disease for hot pepper and an anthracnose-resistant cultivar has not yet been developed, its control is a priority over that of other hot pepper diseases (Kim, 2010; Manandhar et al., 1995). Tobacco budworm and beet armyworm belong to the family Noctuidae, and are controlled by some of the same insecticides (Jung, 2010). Therefore, the chemical control of both insects can be achieved simultaneously. Aphids and thrips are not only harmful in themselves, but can also act as vectors for Cucumber mosaic virus (CMV) and Pepper mottle virus (PepMoV). Natural enemies or chemical sprays are needed for their control (Kim, 2010). Hot pepper growers, highly dependent on pesticides to prevent pest infestations, protectively spray pesticides at 7- to 10-day intervals, leading to 10 to 19 treatments in a growing season (Kim et al., 2010; Kwon and Lee, 2002; Moral et al., 2012).
Environment-friendly pest management aims to manage the density of a pest and keep it below an economic threshold (Estrada et al., 1996; Funt et al., 1990). This can be accomplished by 1) increasing the resistance of a host, 2) providing a habitat or ecological niche for antagonistic microbes and natural enemies, and 3) using eco-friendly pesticides or natural enemies with proper timing and application rates when the pest population is over the economic threshold (Ji, 2010). The short-term goals of integrated pest management (IPM) are to rally cultural, physical, and biological controls to decrease the frequency of pesticide spraying and prevent the damage caused by crop pests (Bounds and Hausbeck, 2007; Gleason et al., 1997). Pest outbreak forecasts can predict the distribution, timing, and amount of the pest (Bounds et al., 2006; Byrne et al., 1997; Gugino et al., 2007). In order to decide the timing of curative pesticide spraying, one needs to establish a forecasting system that can monitor the current pest population as well as predict pest behavior at least few days into the future in order to prepare for IPM (Jung, 2010).
We have developed an IPM pest forecast system for pests of hot pepper, including anthracnose, tobacco budworm, and Phytophthora blight programs (Kim et al., 2010). Each program has a critical model for advising the proper time to treat hot peppers with pesticides. The anthracnose infection model calculates a cumulative infection risk (IR) value every hour based on the hourly temperature and moisture characteristics (Ahn et al., 2008; Huber and Gillespie, 1992; Kang et al., 2010; Wilson et al., 1990). The tobacco budworm program has a population model which calculates the cumulative eclosion rates for the first and second generations based on the degree-day model to control eggs and the younger stages of larvae, respectively (Jung, 2010). In the Gyeongbuk area, insecticides are normally sprayed in late June, late July, and mid- or late August. The Phytophthora model predicts the accumulated amounts of zoospores and germinated zoosporangia based on the daily soil temperature and water content (Do et al., 2012). For control of a soil-borne disease, it is important to be able to predict pest behavior prior to the occurrence of primary infection. The Phytophthora model estimates the date of the first outbreak, and recommends that fungicide be applied 14 or 7 days prior to this date. We also developed a bacterial wilt model (Kim et al., 2012), which estimates the bacterial population density in a root after successful infection. The program mechanistically predicts the primary infection date, at which point the bacterial population in a root first surpasses a threshold level.
Well-designed IPM strategies simplify the application of pesticides while controlling for multiple pests of a host plant (Gugino et al., 2007; Kim et al., 2006). We studied an IPM for hot pepper by considering anthracnose, tobacco budworm, and Phytophthora blight (Kim et al., 2010). We tested whether the pesticide spraying strategy should differ depending on the host growth stage. From the time of transplanting to the end of the vegetative stage, which is around late June in the central district, including Gyeonggi, Chungchung, and Gangwon provinces, we considered the chemical control of Phytophthora. When the Phytophthora blight model advised treatment, we applied not only a fungicide against Phytophthora blight but also a fungicide against anthracnose and an insecticide targeting tobacco budworm. From early July, when hot pepper enters the reproductive stage, the main concerns were the control of anthracnose and tobacco budworm. When the anthracnose model warned of disease potential, we sprayed a curative fungicide within 2 days, but tried to spray the fungicide less than three or four times in a season. We also sprayed a mix of a fungicide against anthracnose and an insecticide against tobacco budworm when the tobacco budworm model advised an application to control the first and second generations in late July to late August. However, validation of the IPM strategy was not always possible, depending on the disease pressure. The coefficient of variance was larger than that obtained in former studies (Kim et al., 2010). It was believed that the field plots were too small to be independent; the inocula could have been easily transferred from no-spray control plots to IPM or periodic plots. When the anthracnose pressure in a field was already severe, a curative fungicide spray was not as effective as a conventional periodic spray with a protective fungicide would have been.
The main goal of this study was to develop an improved hot pepper IPM system that can be used in the field. We studied and validated our modified IPM system in the field for three years, from 2010 to 2012. Different insecticide spray schedules for tobacco budworm were also compared, and two recently developed forecast models for bacterial wilt and bacterial spot were added (Kim et al., 2012). From 2005 to 2012, we conducted field studies to validate the anthracnose forecast program as well as other forecast programs for hot pepper and tried to build an IPM strategy for growers. This study also includes a review of eight years of results from farmers and experimental trials of hot pepper pest management.
Materials and Methods
Field preparation.
A field study was conducted at the Gyeonggi-do Agricultural Research & Extension Services (ARES) test field (37°13′17″ N, 127°2′18″ E) in Hwasung, Gyeonggi-do, Korea, from 2010 to 2012. Hot pepper seedlings of the cultivar Supermanitta were transplanted 0.4 m apart in rows spaced 1.0 m apart on 8 May 2010, 10 May 2011, and 12 May 2012. Each row was covered with vinyl mulch, and felt was laid between the rows to control weeds. Water was supplied by an irrigation system when needed. Each treatment plot consisted of seven rows of 30 m and contained at least 500 plants. Each treatment was tested in only one plot. The three treatments consisted of (1) a control that was not treated except for aphid control, (2) periodic spraying at intervals of 7 to 10 days, and (3) IPM spraying whenever the pest models advised pesticide treatment. Each of an attraction trap for tobacco budworm and beet armyworm were set up in the field to monitor these insects.
Forecasting programs for IPM.
Based on the pesticide spray advisories of the hot pepper pest models, curative fungicides or insecticides were sprayed at the IPM treatment field. The IPM fungicide spray for anthracnose was used from early June until harvest. A forecasting program for anthracnose (Kang et al., 2010) calculated the cumulative IR every hour based on hourly weather data, such as the temperature, wetness period, and relative humidity, which were obtained from an automated weather station in a paddy field about 2 km from the test field. The model provided an advisory decision to spray a curative fungicide just after the fungal infection was predicted to occur. The hourly weather data were transmitted to a PC server in the Epidemiology Laboratory at Seoul National University. Hourly IRs were summed and accumulated; when a threshold IR of 2.7 was reached, the fungicide spray was applied. 2.7 was successful threshold in the former studies (Ahn et al., 2008; Kim et al., 2010). A forecasting program for Phytophthora blight (Do et al., 2012) estimated the infection risk level, quantified as the accumulated amount of active inocula during the prior three days. Fungicides to control Phytophthora blight were applied to the soil 7 and 14 days ahead of the estimated first date of Phytophthora occurrence after overwintering. To estimate the infection risk level, data on the daily air temperature, relative humidity, and rainfall were used. A forecasting program for tobacco budworm (Jung, 2010) estimated the cumulative eclosion rate based on the daily temperature. When the first and second generations were predicted to have reached 100% eclosion, insecticides were applied at that time and again at 7 days following the first spray for eggs, or to the first and second stages of larvae, which are the most sensitive to the pesticide. Since the model predicted two generations, the insecticide was sprayed four times per growing season. To monitor the population of adult male insects, a corn trap was set up in the center of the field with a sex pheromone to lure adult males. In addition, a bacterial wilt model was developed and added to the IPM treatment in the 2012 season. The soil fumigant dazomet was used on 6 April 2012 in both the periodic and IPM treatment plots. The forecasting program for bacterial wilt (Kim et al., 2012) estimated the bacterial populations on the roots.
2010 field study.
An IPM treatment for anthracnose, tobacco budworm, and Phytophthora blight was conducted by operating the three forecast models and spraying with curative fungicides based on the model advisories within 2 days of the prediction. The periodic spray for anthracnose, applied at 7-to 10-day intervals, also contained the protective fungicides and the treatment for tobacco budworm, and Phytophthora blight was also treated by spraying on the same day as the IPM treatments.
The fungicides used for anthracnose in the IPM treatment were azoxystrobin and tebuconazole, and those used in the periodic treatment were azoxystrobin, chlorothalonil, dithianon, and tebuconazole. The insecticides used for tobacco budworm in both the IPM and periodic treatments were chlorpyrifos, methomyl, deltamethrin, and indoxacarb. The fungicides used against Phytophthora blight in both the IPM and periodic treatments were fluopicolide and dimethomorph. Diseases were assessed on 50 pepper plants in the center of the middle three or four rows of each plot on 28 July, 4 August, 11 August, 25 August, and 8 September 2010. The disease incidences were calculated based on the number of fruits that were damaged by anthracnose fungus or tobacco budworm larvae according to the formula:
A disease progress curve was generated from the incidence data for each treatment, and the area under the disease progress curve in each treatment was assessed as:
Yield calculation and disease assessment were performed on 11 August, 25 August, and 8 September 2010. Only ripened red fruits were harvested and assessed on 11 and 25 August from the same 50 plants that were used for the disease assessment. Both green and red fruits were harvested and assessed on 8 September from the same 50 plants. The fresh non-damaged fruits were weighed together to determine the yield of each plot.
2011 field study.
In 2011, the pesticide treatments used in the field were the same as in 2010. The only exception was in the periodic treatment for tobacco budworm, in which insecticides were sprayed along with fungicides against anthracnose at 7- to 10-day intervals. The fungicides used against Phytophthora blight in the IPM treatment were sprayed twice at 14 and 7 days ahead of the predicted first occurrence day according to the prediction of the model. The pesticides used to control anthracnose and tobacco budworm were sprayed together whenever the two models recommended spraying. The fungicides used against anthracnose in the IPM and periodic treatments were same as those used in 2010. The insecticides used against tobacco budworm in the IPM treatments were deltamethrin, indoxacarb, chlorpyrifos, and alpha-cypemethrin. Those used in the periodic treatments were deltamethrin, indoxacarb, chlorpyrifos, alpha-cypemethrin, and diflubenzuron. The fungicide used against Phytophthora blight in both the IPM and periodic treatments was fluopicolide. Diseases were assessed on 45 pepper plants growing in the center of the middle three or four rows of each plot in mid-July and on 3 August, 12 August, 26 August, and 9 September 2011. Yield and disease assessment were conducted on 12 August, 26 August, and 9 September. The methods used to assess disease and yield were same as in 2010.
2012 field study.
The pesticide treatments used in the field in 2012 were same as those used in 2011. Phytophthora blight was not controlled in either the IPM or the periodic treatment. Instead, the control of bacterial wilt and spot were added. Dazomet fumigant was applied to the soil of the IPM and periodic treatment plots on 6 April, one month ahead of planting. The fungicides used against anthracnose in the IPM treatment were azoxystrobin and tebuconazole, whereas those used in the periodic treatment were carbendazim and tebuconazole. The insecticides against tobacco budworm in the IPM treatment were chlorpyrifos and carbamate, and those in the periodic treatment were permethrin, resmethrin, carbamate, bifenthrin, and chlorfenapyr. Dazomet was the fumigant used for bacterial wilt. Diseases were assessed on 50 pepper plants in the center of the middle three or four rows of each plot on 21 June, 28 June, 9 July, 16 July, 26 July, 9 August, and 23 August. Yield and disease assessment were conducted on 26 July, 9 August, and 23 August. The methods of disease and yield assessment used were same as in 2010.
Results
Control in 2010.
IPM sprays for anthracnose were performed eight times in 2010, including four sprays that were done based on the recommendations from the anthracnose forecasting model when the cumulated IRs were over 2.7 on 3 July, 19 July, 18 August, and 26 August (Fig. 1A). The IPM sprays for anthracnose performed on 16 and 25 June were applied due to advisories from the tobacco budworm model regarding the timing of spraying for the first generation. The treatments for anthracnose on 24 July and 4 August were done due to warnings from the second generation tobacco budworm model. Therefore, a fungicide for anthracnose and an insecticide for tobacco budworm were mixed and sprayed in the IPM treatment plot on 16 June, 25 June, 24 July, and 4 August 2010. However, an anthracnose model warned of infection on 28 August, but no treatment was performed then because a fungicide was applied on 26 August and the fungicide should still have been effective until at least that day. In addition, the anthracnose model warned of infection on 6 September, but no spraying was done then due to the timing of the third harvest. A total of 11 periodic sprays for anthracnose were performed in 2010, from 16 June to 2 September (Fig. 1C). Four and two treatments for tobacco budworm and Phytophthora blight were performed, respectively. These were done at the same times as the IPM treatments.
Control in 2011.
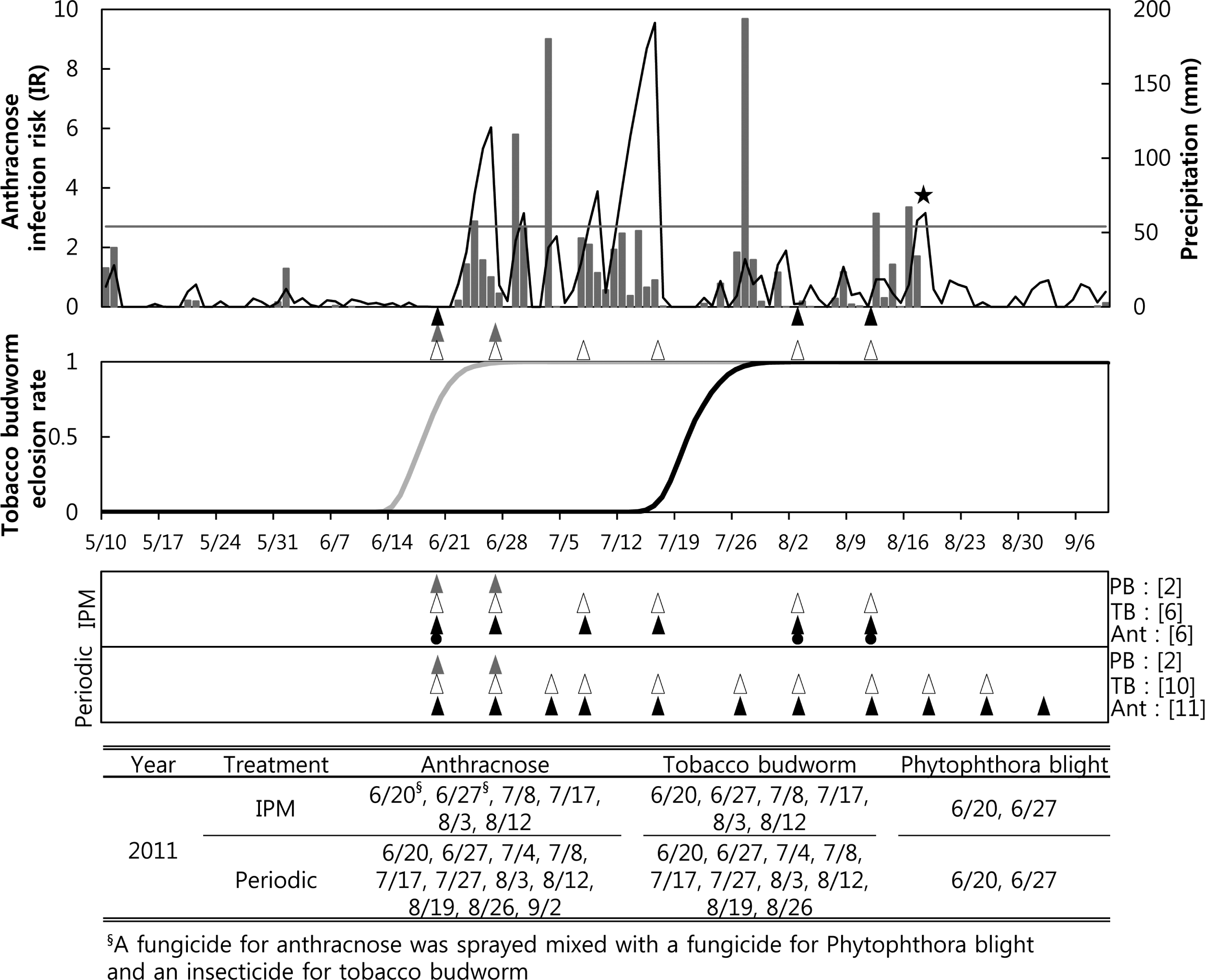
IPM sprays for anthracnose were performed six times in 2011, including two sprays when the anthracnose forecast model recommended application, when the cumulated IRs were over 2.7 on 8 July and 17 July (Fig. 2A). The IPM sprayings for anthracnose, on 20 June and 25 June, were instigated by the Phytophthora model warning of the date of the first infection. At those times, a fungicide against anthracnose and a fungicide against Phytophthora blight were mixed and sprayed. Since the IR threshold for anthracnose was greater than 2.7 from 22 through 24 June, we were able to control anthracnose according to the fungicide advisory of the anthracnose forecaster. The IPM sprays for anthracnose on 3 and 12 August were performed due to the tobacco budworm model advising the timing of control for the second generation. Therefore, a fungicide for anthracnose and an insecticide for tobacco budworm were mixed and sprayed at the IPM treatment plot on 3 and 12 August. The total six IPM sprays for tobacco budworm in 2011 were performed on the same dates as the IPM spray for anthracnose. The periodic sprays for anthracnose in 2011 totaled eleven, applied from 20 June to 2 September (Fig. 2C) at 7- to 10-day intervals, and those for tobacco budworm totaled ten applications, which were done on the same dates except for 2 September. The periodic treatments for Phytophthora blight were done on the same dates (20 and 27 June) as the IPM treatments.
Control in 2012.
Six IPM sprays for anthracnose were performed in 2012, including two applications based on recommendations from the anthracnose forecasting model when the cumulated IRs were over 2.7 on 9 and 16 July (Fig. 3A). The IPM sprays for anthracnose that were done on 21 and 28 June were due to warnings from the tobacco budworm model to control of the first generation of the pest. The treatments for anthracnose done on 2 and 9 August were due to warnings from the model of the tobacco budworm second generation. Therefore, a fungicide against anthracnose and an insecticide against tobacco budworm were mixed and sprayed at the IPM treatment plot on 9 and 16 July and 2 and 9 August. Two insecticide treatments each were performed for the first and second generations, for a total of four. The forecast models developed for bacterial wilt and bacterial spot were tested in the Hwasung field in 2012. The first symptoms of bacterial wilt were estimated to occur on 24 July, but the bacterial spot forecast model did not warn of this occurrence. On that day, no bacterial wilt or symptoms of spot were observed in the field. Twelve periodic sprays for anthracnose were performed in 2012, from 31 May to 22 August (Fig. 3C) at 7-to 10-day intervals, and five sprays were performed for tobacco budworm.
Anthracnose occurrence and incidence.
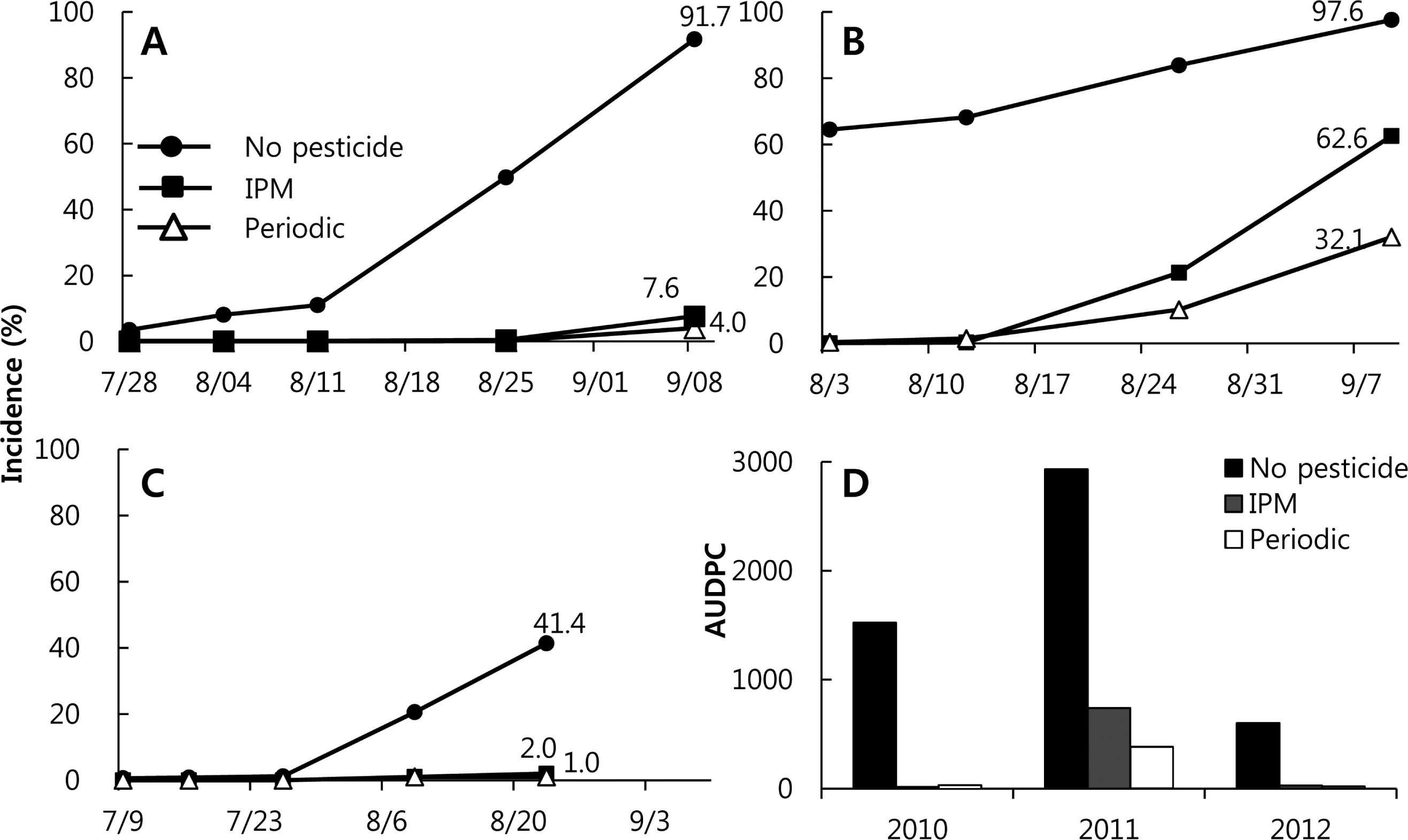
In 2010, the first anthracnose symptom found in the Hwasung field was on 28 July in all three treatment plots. The incidence of anthracnose with the control treatment was 3.46%, whereas the incidences in the IPM and the periodic treatment plots were less than 0.1%. The incidence in the control plot increased steadily until the last day of the harvest, increasing particularly steeply from 11 to 25 August, up to 49.8%. The incidence on the last harvest day in the control treatment was 91.7%, whereas those in the periodic and IPM treatment plots were 7.6 and 4.0%, respectively (Fig. 4A). The first date that anthracnose symptoms were observed in 2011 was in mid-July for the control treatment, and on 3 August for both the periodic and IPM treatments. In 2011, the anthracnose disease pressure was higher than in the other years. In that year, the incidence in the control plot increased steeply in July, whereas those in the IPM and the periodic treatment plots ascended steeply from 12 August to mid-September. The incidence on 9 September (the last harvest day) in the control plot was 97.6%, whereas those in the IPM and periodic treatment plots were 62.6 and 32.1%, respectively (Fig. 4B). In 2012, the first anthracnose symptom was found in the Hwasung field on 9 July. The incidence at the control treatment site ascended steeply from late July to 23 August, up to 41.4%. Symptoms were first observed at the IPM and periodic treatment plots on 9 August, and the incidences on the last day of the harvest were 2.0 and 1.1%, respectively (Fig. 4C).
Yield.
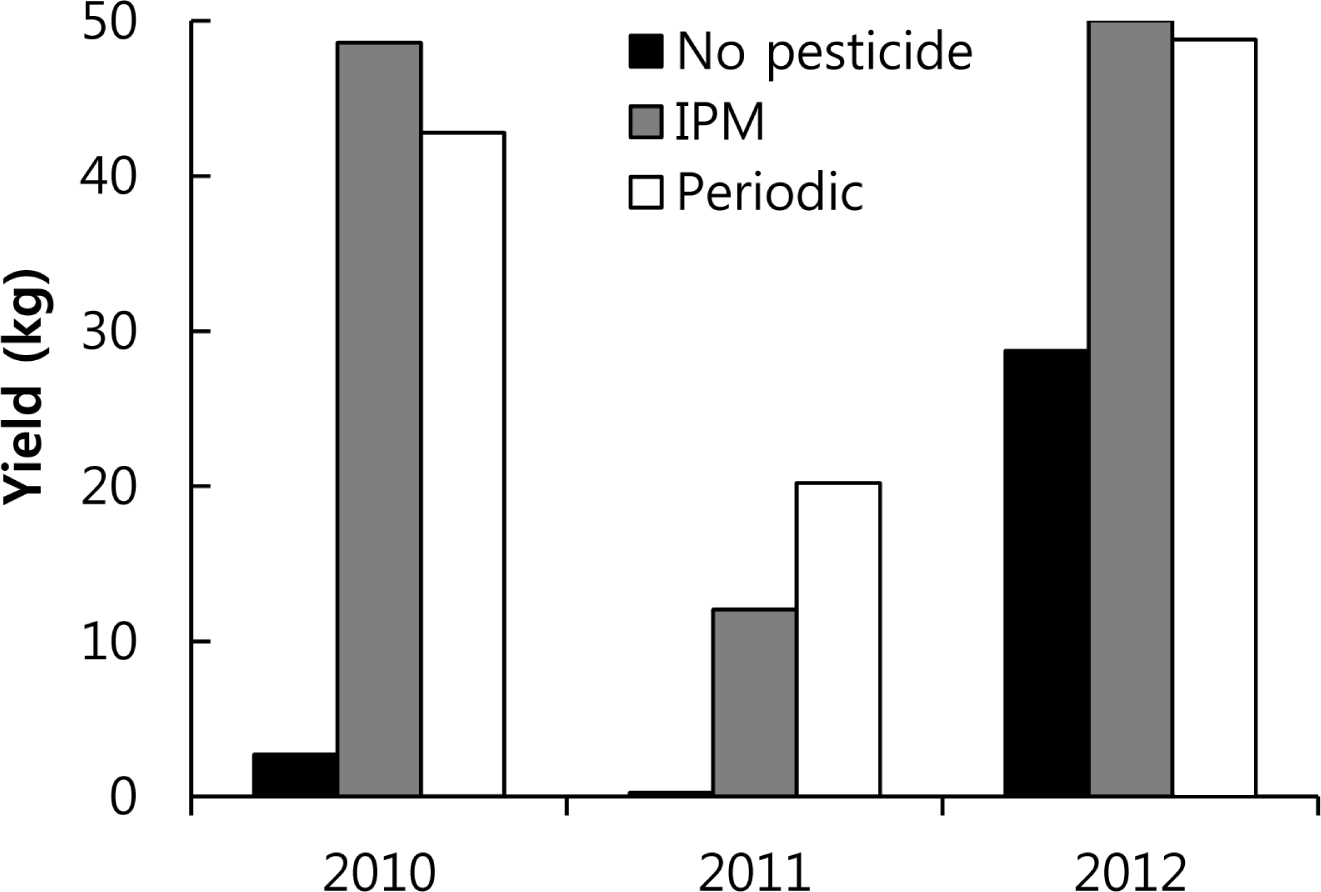
In 2010, fresh peppers randomly selected from 50 plants receiving each treatment weighed 48.6 kg for the IPM treatment, 42.8 kg for the periodic treatment, and 2.7 kg for control treatment (Fig. 5). The fresh pepper weights in 2011 from a randomly selected 45 plants were 20.2 kg for the IPM treatment, 12.1 kg for periodic treatment, and 0.25 kg for the control treatment. In 2012, the fresh pepper weights from a randomly selected 50 plants were 50.0 kg for the IPM treatment, 48.8 kg for the periodic treatment, and 28.7 kg for the control treatment (Fig. 5).
Discussion
IPM sprays were applied eight times in 2010, three times fewer than the periodic spray treatments. Since the yields from the IPM treatment were on average 5.8 kg higher than those of the periodic treatment, which received three more sprays, this IPM strategy is a successful means of pest control. The anthracnose pressure was greatest in 2011 when the yields were lower than those of the other years for all three treatments. Six IPM sprays were applied in 2011, five times fewer than in the periodic treatment; however, the yield of the IPM plot was 8 kg lower than the yield from the periodic treatment plot. IPM was not successful in 2011. Six IPM sprays were applied in 2012, six fewer than for the periodic treatment. Even though fewer sprays were done for the IPM treatment, the yield at the IPM site in 2012 was 1.2 kg higher than that at the periodic treatment site. The IPM strategy, therefore, successfully controlled anthracnose in 2012 (Fig. 5), compared with other studies (Byrne et al., 1997; Dillard et al., 1997; Estrada et al., 1996; Freeman et al., 1997)
The strategy for controlling tobacco budworm in 2010 was mainly to limit insecticide applications to four to attain lower pesticide residues. Then, we directly compared the IPM strategy of few insecticide sprays with periodic sprays at 7- to 10-day intervals. Although it was difficult to precisely assess the number of damaged fruits that had already fallen from the plants, some fruits that were obviously damaged by tobacco budworm were observed between the rows with felt. However, there were no significant differences in the damaged fruits at the IPM and periodic plots. The strategy for the control of Phytophthora blight in 2010 and 2011 was to obtain the estimated first occurrence date from the forecast models. Because it was difficult to find plants that were damaged by Phytophthora blight at the test field from 2010 through 2012, we did not consider controlling Phytophthora blight in 2012 as part of the IPM program. Rather, we chose the cultivar Supermanitta, which is resistant to Phytophthora blight, since a sensitive cultivar was not readily available at the market. In addition, almost no bacterial spot or wilt was found at our test field during the three years of the study.
The incidences of pests in treatments without pesticides (control) for the years 2010 through 2012 were 91, 97.6, and 41.4%, respectively. This means that anthracnose disease pressure was very high in this region throughout the study period. It is difficult to control anthracnose without chemical spraying, and yield losses due to anthracnose are tremendous: the yields at the control plot were less than 10% of those of the IPM or periodic treatments in 2010 and 2011. In 2012, when the disease pressure was lower than in the other years, the yield of the control plot was 60% that of either the IPM or the periodic treatments. The control values (%), the relative incidence between a control method and no treatment, for the periodic treatments were 67-97% higher than those for the IPM treatment, of 36-95% (Table 1).
In 2005 through 2008, the control values at the periodic treatment plot were between 42 and 85%, except for those in 2006 and 2007, when the disease pressures were high (Ahn et al., 2008; Ahn and Yun, 2008). At the same time, the values at the IPM plot were between 2.4 and 60.8% (Table 1); we were attempting to spray fewer than four times throughout the season. When the number of fungicide treatments at the IPM site increased to five to eight from 2009 through 2012, the control values of the IPM treatment were 32.3-95%. In the same years, the control values of the periodic treatment were 79-97%, with ten to twelve sprays. The incidences of pest outbreak at the control site were as high as 58.5, 80.4, and 97.6% at Taean of the two cultivars in 2009 and Hwasung in 2011. There were large differences between the IPM and periodic treatments, such as high disease pressure. If the growers had chosen to spray periodically rather than use IPM, they would have had higher yields and a lower incidence of diseased fruit, but with three to six more fungicide sprayings. Obviously, an IPM strategy would be successful at a relatively lower disease pressure. If the incidence is greater than 20% during the growing season, periodic spraying with a protective fungicide is much better than the IPM strategy of a curative spray. In an earlier study we conducted from 2005 to 2008, we attempted to spray one to four times in a growing season, but realized that it is more reasonable to spray four to six times as part of an IPM strategy to prevent severe yield losses (Yun, 2010). This sort of strategy would also be more acceptable to the agricultural industry.
According to De Wolf (2007) in a review paper, mechanistic modeling approaches are used to develop predictive models for plant diseases. To characterize multiple substages of the disease cycles, models use a series of submodels based on data from controlled experiments on pathogenic biology. The four general stages are dormancy, reproduction, dispersal, and pathogenesis. Our anthracnose model was considered at the infection substage of pathogenesis. The bacterial wilt model was considered at the colonization of reproduction and infection of pathogenesis substages. The Phytophthora blight model was based on the dormancy and reproduction of zoospores. It is acceptable to consider these substages for our three disease models, because these submodels are commonly used in other published models and works.
The most important disease for hot pepper growers is anthracnose, which must be controlled by means of a pesticide (Manandhar et al., 1995; Moral et al., 2012; Yun, 2010). Other pesticides can be mixed with a fungicide against anthracnose when they are needed for control. It is common for farmers to spray fungicides against anthracnose 11 to 19 times in a single growing season. That means that growers are attempting to suppress anthracnose in the host plant throughout the entire growing season. The greater the dependence on chemical control, the more that the environment and human health are threatened by chemical toxicity. In order to generate an alternative pest management technique that has the same effectiveness but is less dependent on pesticides (Funt et al., 1990; Gleason et al., 1995), it is best to maintain a monitoring field to act as a no-spray control, in addition to ideal IPM plots. These plots can tell hot pepper growers how important chemical control is, while IPM may represent an alternative and effective way to control anthracnose.
To achieve environmentally friendly pest management of hot pepper, alternative means to control pests are needed. There are tremendous efforts to develop hot peppers that are resistant against Phytophthora blights; most of the varieties used in this study are resistant to Phytophthora, leading to few Phytophthora symptoms at the test plots. Another soil-borne disease, bacterial wilt, is now becoming resistant to treatment. Although we fumigated the field with dazomet, this method is not recommended. For the purpose of controlling harmful insects on hot pepper, such as tobacco budworm and beet armyworm, it is necessary to not only forecast their populations in a field but also monitor for them using attractive traps. In addition, aphid control needs to occur to block both virus transmission and mechanical damage to hot pepper plants. Moreover, climate change is a potential concern in hot pepper cultivation areas, since viral and bacterial diseases may have a greater impact than fungal pathogens in warmer situations (Shin and Yun, 2010).
To develop a successful IPM strategy for controlling hot pepper pests, growers need to understand several aspects of pests. First, growers need to decide which chemical controls to order, prioritizing the most severe pest in a field. Anthracnose generally has the greatest impact, and some additional but minor diseases can be controlled when a fungicide for anthracnose is sprayed (Freeman et al., 1998). It is generally unknown when in a year the initial anthracnose infection occurs in a field; infection may occur early in a season (Ahn and Yun, 2009; Freeman et al., 1997). Vinyl canopies can also be used to limit precipitation penetration and prevent infection from spreading during the growth period, but growers need to be aware of existing infections throughout the season. Our field studies of eight years have shown that it is more desirable to spray after infection to reduce unnecessary spraying. If growers want to follow an IPM program, they need to spray fungicides against anthracnose at least four or five times in a season. In addition, we also recommend changing the strategy to conventional spraying when anthracnose-infected fruits in the field surpass 20%.
Growers also need to be aware of emerging pests, such as root-knot nematode and several bacterial diseases that will increase in severity if climate change accelerates (Shin and Yun, 2010). Hot pepper is usually grown without crop rotation. Soil fertility is declining, and the incidences of soil-borne diseases may increase. Crop rotation or growing in paddy plots are considered alternative control strategies. However, without efforts to reduce excessive dependence on chemical control, it will be difficult to achieve environmentally friendly pest management in hot pepper cultivation.



 ). The arrows for the periodic treatment in the lower panel were the timing of the periodic sprayings. Fungicides against anthracnose were not only sprayed based on an anthracnose model forecast (▴) but also mixed with other pesticides (
). The arrows for the periodic treatment in the lower panel were the timing of the periodic sprayings. Fungicides against anthracnose were not only sprayed based on an anthracnose model forecast (▴) but also mixed with other pesticides (
 ) based on the forecasts of the other two models. The brackets after each pest indicate the number of sprays for the pest in the 2010 growing season. The lowest table shows the date of spraying for each pest.
) based on the forecasts of the other two models. The brackets after each pest indicate the number of sprays for the pest in the 2010 growing season. The lowest table shows the date of spraying for each pest.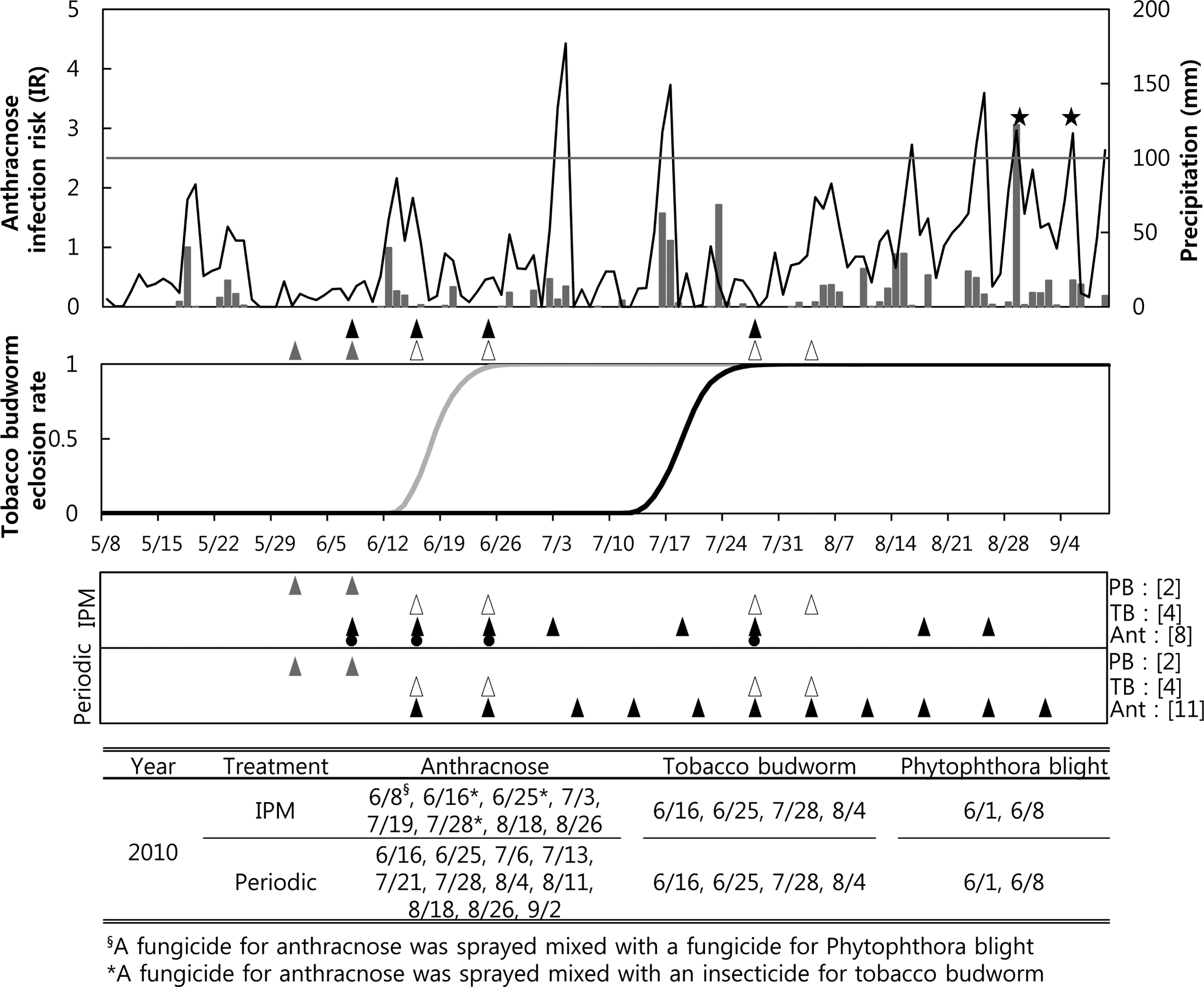

 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






