Inhibitory Effect of Chitosan and Phosphate Cross-linked Chitosan against Cucumber Mosaic Virus and Pepper Mild Mottle Virus
Article information
Abstract
Cucumber mosaic virus (CMV) and Pepper mild mottle virus (PMMoV) causes severe economic loss in crop productivity of both agriculture and horticulture crops in Korea. The previous surveys showed that naturally available biopolymer material – chitosan (CS), which is from shrimp cells, reduced CMV accumulation on pepper. To improve the antiviral activity of CS, it was synthesized to form phosphate cross-linked chitosan (PCS) and compared with the original CS. Initially, the activity of CS and PCS (0.01%, 0.05%, and 0.1% concentration) compound against PMMoV infection and replication was tested using a half-leaf assay on Nicotiana glutinosa leaves. The total number of local lesions represented on a leaf of N. glutinosa were counted and analyzed with phosphate buffer treated leaves as a negative control. The leaves treated with a 0.1% concentration of CS or PCS compounds exhibited an inhibition effect by 40–75% compared with the control leaves. The same treatment significantly reduced about 40% CMV accumulation measured by double antibody sandwich enzyme-linked immunosorbent assay and increased the relative expression levels of the NPR1, PR-1, cysteine protease inhibitor gene, LOX, PAL, SRC2, CRF3 and ERF4 genes analyzed by quantitative reverse transcriptase-polymerase chain reaction, in chili pepper plants.
Chili pepper is the most important vegetable and spice crop because of its unique characteristics of color, taste, pungency, flavor and aroma in worldwide (Rohini and Lakshmanan, 2017). In 2020, chili pepper was produced 60,076 tons in South Korea (Statistics Korea, 2020). Chili productivity was decreased by 18,361 tons (~23.4%) with the value of 78, 347 tons of pepper as per the 2019 data. Among the pepper infecting viruses, the cucumber mosaic virus (CMV) is devastating in both agriculture and horticulture crops in the world (Elsharkawy et al., 2012). CMV is a type species of the genus Cucumovirus, in the family Bromoviridae and it has a wide host range of more than 1,071 species belonging to 521 genera from 100 families and is transmitted by 80 species of aphids (Palukaitis and García-Arenal, 2003; Yoon et al., 2019). CMV in chili pepper is the most wide-spread virus in South Korea (Cho et al., 2007; Choi et al., 2005; Kwon et al., 2018; Lee et al., 2015), severe symptoms were attributed to CMV infection such as mosaic, stunting growth, distortion and a characteristic shoestring like leaf appearance (Sudhakar et al., 2007). Pepper mild mottle virus (PMMoV) is also one of the major viruses in pepper species (Capsicum spp.) in the world (Adams et al., 2009). PMMoV is a single-stranded positive-sense RNA virus classified in the genus Tobamovirus, which includes viruses extremely resistant to physical and chemical agents (Anonymous, 2006; Wetter et al., 1984). PMMoV infection initially causes mild foliar mosaic symptoms followed by mottling and malformation of leaves and fruits, resulting in significant losses of pepper yield and cash values (Kim et al., 2012). To reduce the viral disease incidence, agrochemicals such as pesticides are indispensable tools to a boon in agriculture production (Qin et al., 2011; Voss-Fels and Snowdon, 2016). But these agrochemicals are a serious threat to the biogeochemical cycling of the entire biosphere (Damalas and Koutroubas 2016). At the point of a healthy biosphere environment, other alternative methods need to be explored.
Chitin is the second most abundant natural biopolymer after cellulose and it is a semi-crystalline homopolymer of β-(1→4)-linked N-acetyl-D-glucosamine units. Chitosan (CS) is extracted from the chitin by the deacetylation process, it is the major component of the fungal cell wall and the arthropod exoskeleton (Iriti and Varoni, 2015). CS is a heteropolysaccharide composed of N-acetyl D-glucosamine and D-glucosamine by β-(1→4) glycosidic bonds (Iriti and Varoni, 2015). CS is a biodegradable, non-toxic compound, with antimicrobial nature (Kumaraswamy et al., 2018) by electrostatic interactions and also induced plant defense mechanism to pathogens by systemic acquired resistance (SAR) (Xing et al., 2015). CS has been reported in various applications in the agriculture and horticulture commodities, including plant growth promotion, protecting food products from different pathogens, and inducing tolerance in biotic and abiotic stress and post-harvest technology (Lustriane et al., 2018; Malerba and Cerana, 2016; Pratiwi et al., 2015; Shahrajabian et al., 2021; Yin and Du, 2010).
There are some reports were reported on the CS application promotes plant growth promotion (Kumaraswamy et al., 2018; Sharif et al., 2018); and also act as delivery of agrochemicals, fertilizers, micronutrients as well as genetic materials in the form of CS nanoparticles (Malerba and Cerana, 2016). Liu et al. (2004) reported that CS has shown antimicrobial activity for bacteria, yeast, and molds. CS is also used as a conventional fungicide in both preharvest (Feliziani et al., 2015) and post-harvest applications (Sivakumar et al., 2016; Zhang et al., 2015). CS application-induced response against potato virus X and tomato mosaic virus was reported by (Chirkov et al., 2001; Jia et al., 2016; Nagorskaya et al., 2014). CS perception in the plant’s variation in the ion fluxes and membrane depolarization (Iriti and Varoni, 2015) and also recognized by the plant as a pathogen-mimicking stimulus, but the identification of a CS receptor is still mysterious (Malerba and Cerana, 2016; Povero et al., 2011). Although Petutschnig et al. (2010) found that the chitin elicitor receptor kinase 1 (CERK1) also bound weakly to CS, later Povero et al. (2011) demonstrated that the perception of CS was independent of CERK1. Recently, Liu et al. (2018) suggested wheat W5G2U8, W5HY42, and W5I0R4 as potential CS oligosaccharides receptors. However, the detailed mechanism of action of CS in reducing plant diseases has not been completely revealed (Hassan and Chang, 2017).
There is a piece of scanty information that has been available on the mechanism of controlling the viral diseases in chili pepper with not only CS, but also phosphate cross-linked chitosan (PCS). Therefore, the study has been aimed at understanding the resistant mechanism by CMV in chili pepper treated with CS or PCS.
Materials and Methods
Virus source
CMV-GTN was maintained in Nicotiana tabacum leaves and confirmed virus viability by the immunostrip method (Choi et al., 2015). Infected leaves were collected aseptically and ground with 0.01 M phosphate buffer (pH 7.0) using a sterilized plastic pouch in the ratio of 1 g leaf tissue per 10 ml of the buffer. The suspension is used as the source of inoculum for chili pepper infection. The first two true leaves of each chili pepper plant were lightly dusted with carborundum (Thermo Fisher Scientific, Waltham, MA, USA) and then rubbed with inoculum from leaf base to tip, contains CMV-infected tobacco leaf suspension. All the plants including the controls were inoculated with CMV.
Preparation of CS and PCS solution
CS flakes (MW ~ 600,000; viscosity 50–800 mPa, degree of deacetylation 80–95%) was purchased from Kwang Jin Chemical Co. Ltd., Siheung, Korea. To prepare a 2% CS solution, the required amount of CS flakes was dispersed in pre-prepared 2% acetic acid, with stirring for 12 h at a uniform temperature of 60°C. The obtained CS solution was cooled to room temperature. The structure of CS is represented in Supplementary Fig. 1A. For the synthesis of PCS, each CS and ammonium polyphosphate solution were placed over a heat bath with stirring at 600 rpm, and the temperature was gradually increased from room temperature to 80°C. Under these conditions, the reaction was allowed to continue for 12 h. After completion of the reaction, the contents of the flask were cooled to room temperature, and then vacuum filtered. The obtained pale yellow product was thoroughly washed with deionized water, dried in an oven at 70°C for 24 h, and ground into powder. Finally, a distinct pale-yellow powder of a novel compound based on CS and ammonium polyphosphate was obtained. Hereafter, the compound is referred to as PCS. This synthesis mechanism was depicted in Supplementary Fig. 1B. To prepare a 2% stock solution of CS or PCS, the required amount of CS or PCS powder was dispersed in pre-prepared 2% acetic acid, with stirring. Stirring was continued for 12 h at a uniform temperature of 60°C to obtain a homogeneous solution of CS or PCS and cooled to room temperature. The stock solution of CS or PCS was diluted to 0.01%, 0.05%, and 0.1%.
Local lesion count assay
Infectious PMMoV was quantified by a local lesion count assay with N. glutinosa. The seed and the seedling of N. glutinosa were grown and cultured in the soil pot. After cultivation, three leaves of each plant were painted with at the concentrations of 0.01%, 0.05%, and 0.1% of CS, and PCS materials once a day for three times, then covered with 600 mesh carborundum, and then PMMoV inoculums in 0.01 M phosphate-buffered saline was rubbed into each leaf with a gloved finger. After washing, the plant was incubated in the growth chamber at 25°C under a long-day photoperiod (16-h light, 8-h dark) for 4–5 days. At the end of the incubation, local lesions on each leaf were counted, and the average lesion count prepared from a single sample was considered as the infectious PMMoV concentration for that sample. The number of local lesions was compared with the control local lesions.
Pot experiment
The efficacy of antiviral agents in controlling CMV in chili pepper was assessed under greenhouse conditions. Chili pepper plants at a four-leaf stage were selected for spraying of CS and PCS at the concentrations of 0.01%, 0.05%, and 0.1% once a day for three days, then followed by CMV-GTN inoculation. Foliar spray with distilled water was carried out as the control treatment.
Double antibody sandwich enzyme-linked immunosorbent assay
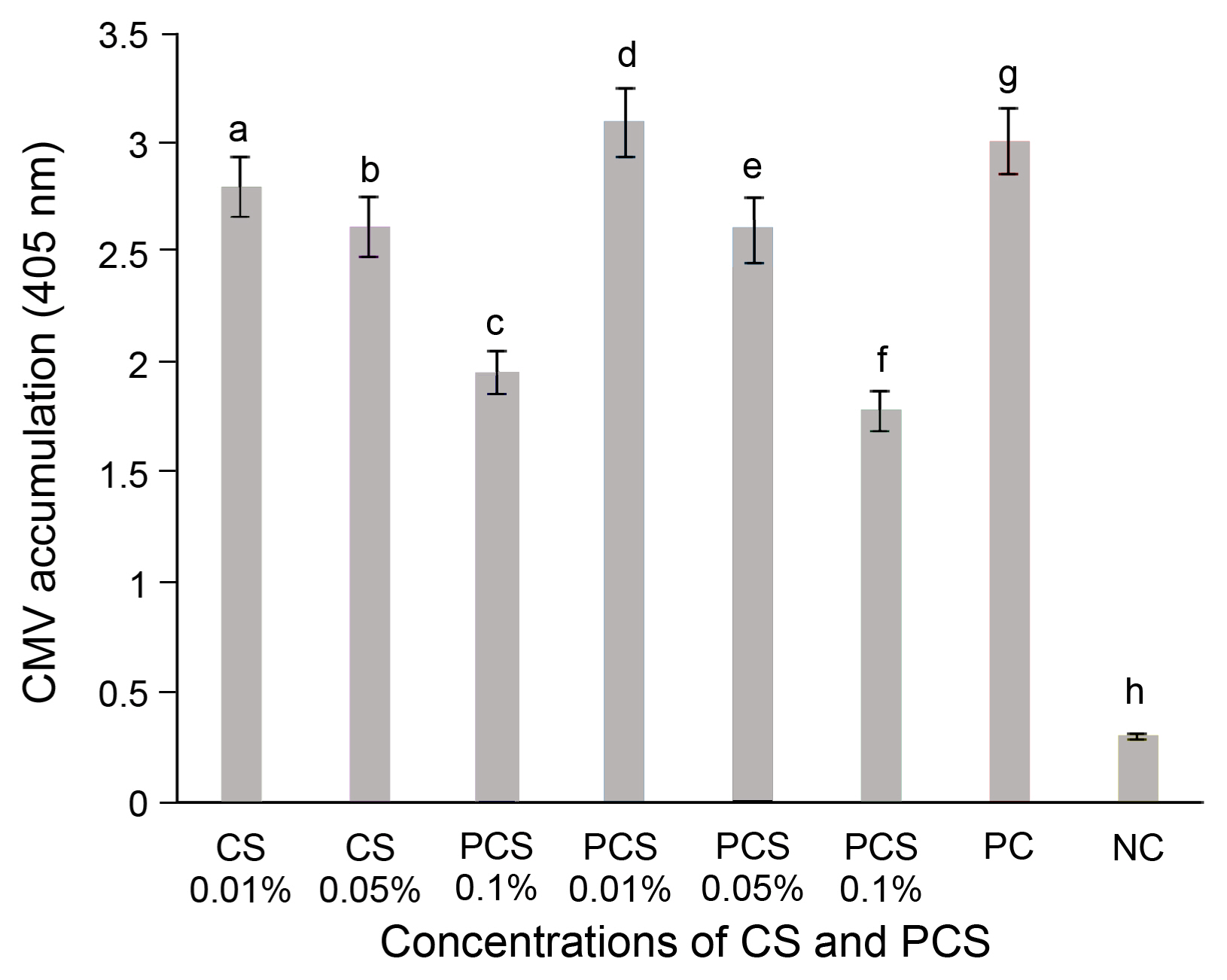
Double antibody sandwich enzyme-linked immunosorbent assay (Vitti et al., 2016) was used for the rapid detection of the virus as per the manufacturer’s protocol (Agdia, Elkhart, IN, USA). The experimental setup chili pepper seedlings upper leaf sample was collected at 2 weeks of post-inoculation (wpi) along with control samples and extracts the CMV antigen by using a general extraction buffer. The extracted samples of (100 μl) were analyzed after performing several steps in the protocol. The absorbance value of each sample was measured at 405 nm by an ELISA reader (Titertek, Huntsville, AI, USA) thirty minutes after the addition of 100 μl of the substrate (p-nitrophenyl phosphate at 1 mg/ml in 10% of diethanolamine pH 9.8). A sample was noted as positive if the OD exceeds 3 times the mean of the negative controls (Yoon et al., 2021).
Quantitative real-time PCR analysis
Quantitative real-time PCR (RT-qPCR) analysis was performed on chili pepper plants, which were sprayed with CS, PCS of 0.1%, and water (i.e., control). Leaf tissues (consisting of three biological replicates) were collected, immediately frozen in liquid nitrogen, and stored at −80ºC until RNA extraction. Total RNA was isolated using the Plant RNA Prep Kit (BCS Plan RNA Prep kit, Biocube, Gwacheon, Korea). RNA quantity and quality were checked by using a nanodrop (Bio-Rad, Hercules, CA, USA). Only RNA with an A260/ A280 ratio ≥ 1.95 was retained for subsequent analysis. The cDNA was synthesized using the SuperScript IVVILO cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RT-qPCR reactions were made to a final volume of 20 μl contained 2 μl of cDNA. The PCR cycling program was set as follows: initial denaturation at 95°C for 3 min, then 40 cycles of denaturation at 95°C for 15 s, and extension at 59°C for the 30 s. The program was completed with melt curve determination from 60°C to 95°C with incremental steps of 0.5°C for 5 s (Bio-Rad). CS and PCS treated chili pepper plants defense genes – such as CaNPR-1, CaPR-1, CaPR-4, Cysteine, defensin, lignin, CaLOX, CaPAL, CaSRC2, CaCRF3, and CaERF4; were studied and quantified relative to the expression level of CaUBZ. All primer pairs were designed using Primer Select of the Primer Quest tool (Integrated DNA Technologies, Inc., Coralville, IW, USA). Primers’ specificity in recognizing target genes in the chili genome was validated by sequencing the amplified fragments (170–200 bp). The primers used in this study are listed in Table 1.
Statistical analysis
The data were compared using a one-way ANOVA analysis. All were tested at P ≤ 0.05 significance level and the Duncan multiple range tests were used for separation between treatments. Statistica v.10, Statsoft was used for all statistical analyses.
Results
Antiviral efficacy
The application of CS and PCS at three different concentrations of local lesions count in N. glutinosa were analyzed (Fig. 1, Supplementary Fig. 2). The local lesions count on the leaves of N. glutinosa treated with phosphate buffer are more number compared with treatments of CS and PCS. The number of local lesions was inversely proposed to the concentrations of CS and PCS. The painting of CS and PCS at 0.1% decreased the PM-MoV local lesions by 40–75% related to the control local lesion count. Overall the CS at 0.1% is best suitable for the control of PMMoV in N. glutinosa. Based on this hypersensitive response of CS and PCS materials concentrations were further tested by spraying at the concentrations of 0.01, 0.05, and 0.1% in chili pepper infected with CMV.
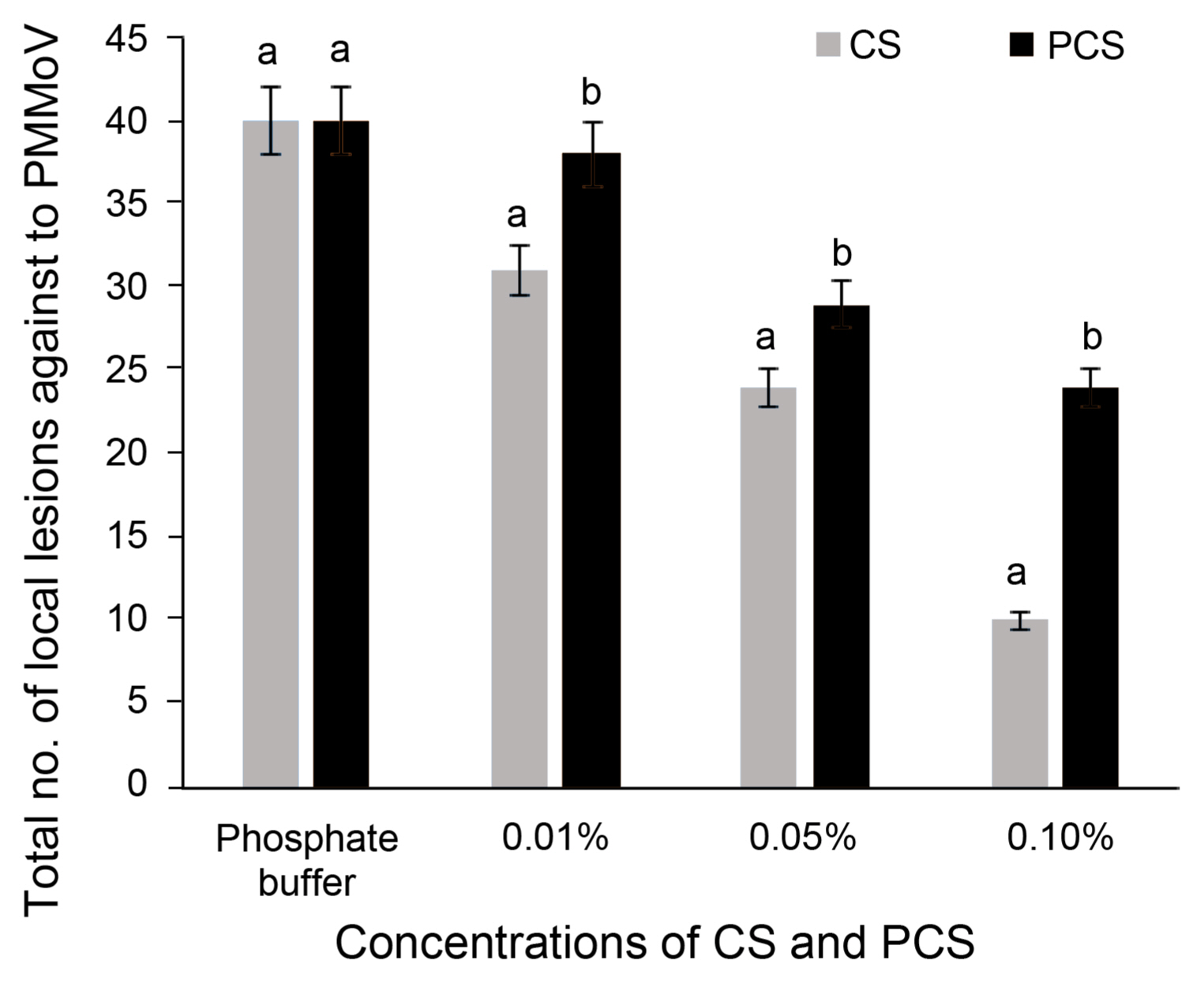
Antiviral activity chitosan (CS) and phosphate cross-linked chitosan (PCS) by hypersensitive response. PMMoV, pepper mild mottle virus.
For untreated chili pepper plants, symptoms of the disease appeared seven days after the infection and ranged from mild symptoms on new leaves to severe mosaic with stunting. However, chili pepper plants treated with CS and PCS by foliar spray showed an incredible reduction in CMV severity compared to the non-treated plants after 14 days of inoculation. Moreover, the reduction in the severity of CMV in chili plants by foliar spray treatment was higher than that of control-treated samples, ELISA test confirmed that CMV titer as an indicator for CMV accumulation was markedly reduced by 35–40% in chili pepper plants treated with CS and PCS at the concentrations of 0.05% and 0.1% in comparison to untreated plants after 2 weeks of virus inoculation (Supplementary Fig. 3). The results were expressed in the graphical representation form (Fig. 2). CMV accumulation was much lower in chili pepper plants treated with 0.1% of CS and PCS as foliar spray relative to plants treated with distilled water as a control.
Induction of defense genes in chili pepper by CS or PCS
The induction of defense genes in chili pepper by foliar spraying of CS and PCS at 0.1% level was evaluated by RT-qPCR and the expression of CaNPR1, CaPR1, CaPR4, Cysteine, defensin, lignin, CaLOX, CaPAL, CaSRC2, CaCRF3, and CaERF4 genes, were analyzed and shown (Fig. 3A). CS and PCS at 0.1% level the non-pathogenesis related proteins relative normalized expression is 3.8-fold and a 5-fold increase in the chili pepper in respective of CS and PCS compared with the control plants. In the same way, PR-1 expression is about 8-fold and 10-fold level increased in respective of CS and PCS. The chili pepper plants are relatively expressed a 2-fold level of cysteine, for both CS and PCS treatment. Whilst in the case of CaPR-4, lignin and defensin were more or less equal with the control plant’s expression levels.

(A) Genes expression in chitosan (CS) and phosphate cross-linked chitosan (PCS) in chili pepper plants: NPR1, PR-1, PR-4, cysteine protease inhibitor, lignin forming anion peroxidase, pepper defensin1. (B) PAL, LOX, SRC2, CRF3, ERF4 (ethylene response factor 4). Means of three replicates and standard errors are represented by bars. Significant differences (P ≤ 0.05) among treatments are indicated by different letters according to parameter of ANOVA One-way analysis.
In addition to these genes, other genes were further assessed, analyzed, and presented (Fig. 3B). In the phenylalanine propanoid pathway phenylalanine ammonialyase (PAL) is a key enzyme to synthesize defense-related enzymes to express. The foliar spraying of CS and PCS at 0.1% level in chili pepper induced the expression of two oxidative genes - CaPAL, CaLOX was normalized expression levels were 4.52, 4.01 and 3.62, 4.46 fold up-regulated in respective of CS and PCS correlated to the control chili pepper plants. To this gene product, we were further looked by foliar spraying of CS and PCS at 0.1% level in chili pepper, the genes such as - CaSRC-2, CaCRF-3 and CaERF-4 and the results were set side by side with the buffer treated control and shown (Fig. 3B), the gene products CaSRC-2, CaCRF-3, and CaERF-4 were 72.93, 19.06, 3.63 and 41.56, 16.40, 1.66 folds up-regulated to the CS and PCS treatments, respectively.
Based on the RT-qPCR study, we demonstrated that CS or PCS-treated chili pepper plants are stimulated in the expression of CaNPR-1, CaPR-1, CaPR-4, cysteine, defensin, lignin, CaLOX, CaPAL, CaSRC2, CaCRF3, and CaERF4 gene products. Overall the CS or PCS at 0.1% foliar spraying induced the expression of PR-1 proteins and other defense genes.
Discussion
CS and PCS were applied to chili plants as a foliar spray to induce defense-related genes. In the plant system, SAR is a long-lasting priming mechanism to many pathogenic infections (Mou et al., 2003), and also the plant responds to the production of reactive oxygen species (ROS), PR proteins, lignification and other cell wall structural proteins crosslinking (Pandey et al., 2017). Xing et al. (2015) reported that chitosan induced and enhanced the expression of defense-related enzymes, such as peroxidase, PAL, polyphenol oxidase, superoxide dismutase, and catalase.
In phenylpropanoid metabolism PAL is the key enzyme in the conversion of L-phenylalanine to ammonia and transcinnamic acid. Lee et al. (1994) demonstrated that multiple initiation sites in the PAL5 allow the tomato plant to respond to different environmental stimuli in a tissue-specific fashion. A recent study reveals a significant increase in the expression of PAL5 enzyme in tomato plants treated with CS alone and CS with CMV, compared to tomato plants treated with CMV only. These results are in agreement with the study of Mejía-Teniente et al. (2013), who treated Capsicum annum L., with CS. On the other hand, tobacco mosaic virus (TMV)-infected tobacco leaves increased PAL A and PAL B transcripts as well as PAL activity indicated that the phenylalanine pathway is the main route of salicylic acid (SA) synthesis. Compared to CMV-TP, the significantly higher PAL5 expression detected in CHT-CMV-TP suggests the involvement of phenylpropanoid-derived products as lignin and SA (Rendina et al., 2019). In our study also indicated that, increased the expression of PAL enzyme as to the control chili pepper plants. Not only PAL, and other defense-related genes are up-regulated in chili pepper by foliar application of both CS and PCS to the control (Fig. 3A and B).
CS and CS nanoparticle-treated tomato plants were enhanced in the expression of PR1, β-1,3 glucanase, chitinase, PR-1 gene, and PR-10 defense-related genes were markedly higher than the control. The same materials were also increased in the expression of superoxide dismutase (SOD) and catalase (CAT) in tomato plants along with the coinoculation of a fungal pathogen (Chun and Chandrasekaran, 2019). CS application to plants aggregate around the pathogen penetration sites creating a physical barrier and avoids pathogen entry and colonization, stimulation of ROS such as H2O2, and accumulation of PR proteins such as chitinase, that induce the formation and accumulation of phenolic compounds such as phytoalexins, which in turn promotes lignification, inhibits the action of proteinase and activates peroxidase, SOD and CAT enzymes (Ali et al., 2014). These results indicated that SOD and CAT potentially protect plants against different oxidative stresses.
The induced systemic resistance is triggered by some bacteria and fungi and requires jasmonic acid (JA) and ethylene (ET). Differently, the SAR requires SA, exogenously applied or endogenously produced. The non-expression of pathogenesis-related genes 1 (NPR1) in the cytosol regulates the cross-talk pathways of salicylate and jasmonate (Spoel et al., 2003). Bacillus cereus AR156 triggered the induction of the JA/ET-signaling pathway with the expression of NPR1 in Arabidopsis to Botrytis cinerea (Nie et al., 2017). Interestingly, another study reported that SAR induction by AR156 required NPR1 and SA-signaling pathway (Niu et al., 2016). For this, Wu et al. (2012) suggested that Arabidopsis NPR1 binds SA through cysteines 521/529 via copper. Mou et al. (2003) reported that SAR is activated via NPR1-PR1 and SAR activating mechanism. In our study, the results in Fig. 3A, CS and PCS have induced the expression of NPR1 over the control plants. However, NPR1 expression was up-regulated in CS and PCS treated chili pepper, compared to untreated ones. This could indicate the CS and PCS efficacy against CMV by triggering SAR-related defense responses in chili pepper plants. On the contrary at a low-level concentration (50 mg/l) of CS oligosaccharide treatment induced the SA-signaling pathway in Arabidopsis to TMV infection was reported by Jia et al. (2016). Interestingly, these results are correlated and also JA levels were increased in the tomato plants treated with the CS oligosaccharide reported by Doares et al. (1995).
The effect of foliar spray with CS on the level of jasmonates and abscisic acid (ABA) and their responsive genes was determined in roots of mycorrhizal and non-mycorrhizal plants at a late stage of the symbiosis (9 weeks after inoculation). Quantification of ABA showed that treatment with CS did not affect significantly the level of ABA in mycorrhizal and non-mycorrhizal roots. However, the expression of the 9-cis-epoxycarotenoid dioxygenase gene (NCED1), a gene encoding one of the ABA-biosynthetic enzymes, showed different results. The expression of NCED1 increased with increasing concentrations of CS. The transcript accumulation of NCED1 was significantly increased in roots of non-mycorrhizal plants treated with 1 mg/ml of CS. In this study the results are in the way with the study of other researchers, foliar spraying of CS and PCS materials inhibited CMV, PMMoV accumulation and induced the expression of defense genes in the chili pepper plants (Colson et al., 2010; Rendina et al., 2019).
Acknowledgments
This work was supported by a grant from the Basic Research Program (PJ01431803) of the National Institute of Horticultural and Herbal Science, Rural Development Administration, Republic of Korea. The authors are thankful to Dr. M.N. Prabhakar, Dept. of Mechanical Engineering, Changwon National University, Changwon, South Korea, for providing the CS and PCS materials.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).