Detection and Quantification of Fusarium oxysporum f. sp. niveum Race 1 in Plants and Soil by Real-time PCR
Article information
Abstract
Fusarium wilt caused by Fusarium oxysporum f. sp. niveum (Fon) is the most serious soil-borne disease in the world and has become the main limiting factor of watermelon production. Reliable and quick detection and quantification of Fon are essential in the early stages of infection for control of watermelon Fusarium wilt. Traditional detection and identification tests are laborious and cannot efficiently quantify Fon isolates. In this work, a real-time polymerase chain reaction (PCR) assay has been described to accurately identify and quantify Fon in watermelon plants and soil. The FONRT-18 specific primer set which was designed based on identified specific sequence amplified a specific 172 bp band from Fon and no amplification from the other formae speciales of Fusarium oxysporum tested. The detection limits with primers were 1.26 pg/μl genomic DNA of Fon, 0.2 pg/ng total plant DNA in inoculated plant, and 50 conidia/g soil. The PCR assay could also evaluate the relationships between the disease index and Fon DNA quantity in watermelon plants and soil. The assay was further used to estimate the Fon content in soil after disinfection with CaCN2. The real-time PCR method is rapid, accurate and reliable for monitoring and quantification analysis of Fon in watermelon plants and soil. It can be applied to the study of disease diagnosis, plant-pathogen interactions, and effective management.
Fusariums wilt of watermelon (Citrullus lanatus (Thunb.) Matsum.et Nakai) is the most serious soil-borne disease in the world and has become the main limiting factor of watermelon production (Martyn and Vakalounakis, 2012; Petkar and Ji, 2017). The disease was caused by Fusarium oxysporum f. sp. niveum (Fon), which can remain active in the soil for many years. So for watermelon production, continuous cropping should be avoided for at least 5 years (Costa et al., 2018). Even so, Fusarium wilt may occur at every stage of watermelon growth. Generally, Fusarium wilt can cause 20–30% yield loss in watermelon production and even up to 50–60% in severely affected areas (Chang et al., 2015; Raza et al., 2015). To date, three races (0, 1, and 2) of Fon have been detected based on pathogen specificity against various watermelon cultivars (Martyn, 2014). Fon race 3 was found and has highly virulent in Maryland, Florida, and Georgia in the United States (Amaradasa et al., 2018; Petkar et al., 2019; Zhou et al., 2010). Unfortunately, there are no varieties of watermelon with effective resistance to all races of Fon. Although some fungicides are available for Fusarium wilt on watermelon, it could be argued that these chemicals are not very effective because chlamydospores can survive in soil (Everts et al., 2014; Faheem et al., 2015; Zhang et al., 2017), leading to great difficulty in the prediction and management of Fusarium wilt in watermelon. Disease management practices need not only to identify pathogen in early stages, but also to understand the critical density of pathogen leading to economic losses. Timely and appropriate management measures can prevent the spread of the disease and minimize losses. Therefore, reliable, quick detection and quantification of Fon are essential at early stages of the infection process for control Fusarium wilt of watermelon.
Traditional methods for identification of Fon have been based on isolation and culture of pathogen and symptoms of infected host. These methods are laborious, time consuming and sometimes inaccurate, so that pathogens had enough time to spread rapidly and cause serious economic losses. Therefore, the development of an efficient, rapid and accurate detection assay for Fon in plants and soil is essential for taking appropriate measures to control plant diseases. A nested polymerase chain reaction (PCR) protocol has been developed using the primers ITS1/ITS4 and Fn-1/Fn-2 for the detection of Fon (Zhang et al., 2005). Moreover, Lin and associates developed a conventional PCR assay for the identification of Fon from other Fusarium species in Taiwan (Lin et al., 2010). However, none of these assays allows accurate quantification of the pathogen in soil and plants. Real-time PCR technology has been widely applied in detection and quantification of soil-borne pathogens, providing a basis for understanding the occurrence and development of soil-borne disease pathogens, and timely planning of prevention and control measures. Jiménez-Fernández and Daniel developed identification and detection techniques for F. oxysporum in hosts and soil (Jiménez-Fernández et al., 2010). In addition, real-time PCR technology has been applied for the detection of formae speciales of F. oxysporum in many plant species, such as maize (Atoui et al., 2012), banana (Lin et al. 2013), strawberry (Li et al., 2014), cucumber (Scarlett et al., 2013), tomato (Huang et al., 2016), bean (de Sousa et al., 2015), chickpea (Jiménez-Fernández et al., 2011), radish (Kim et al., 2017), Chinese water chestnut (Zhu et al., 2016), and celery (Epstein et al., 2017). Zhang also described a method for detecting Fon and Mycosphaerella melonis from soil by real-time PCR (Zhang et al., 2005), but the large product size (327 bp) led to low efficiency, sensitivity and reliability of the assay. A multiplex TaqMan and a real-time fluorescence loop-mediated isothermal amplification (RealAmp) assay was conducted for specific detection of Fon (Peng et al., 2013; van Dam et al., 2018). Compared with TaqMan and RealAmp assay, the real-time PCR based SYBR-green method has obvious advantages, the most prominent of which is simple and rapid as well as does not complex primer design and fluorescently tagged. However, there is no efficient real-time PCR assays based SYBR green to detect and quantify Fon in watermelon plants and soil to date.
Previous studies have shown that the disease index (DI) of watermelon was positively correlated with the quantity of Fon (Lü et al., 2014). The accurate identification and quantification assay of pathogen density in plants can assess risk and potential crop loss, and is essential for prevention and control of watermelon Fusarium wilt in the early stage of infection. Moreover, the quantity of pathogen in soil could be applied to predict the occurrence of disease in watermelon cultivation (Zhu et al., 2016). Quantification of pathogenic Fon in plant tissues or soil can help determine whether and when to take action. In this manuscript, we developed a real-time PCR assay to accurately identify and quantify Fon in watermelon plants and soil, evaluated the relationships between the DI and Fon DNA quantity of watermelon Fusarium wilt, and revealed the role of conidia density in soil for predicting the occurrence and development of Fusarium wilt. The results provide an important reference for guiding the early risk prediction for Fon and formulating comprehensive control measures.
Materials and Methods
Fungal isolate culture and genomic DNA extraction
Twenty isolates of Fusarium oxysporum, Rhizoctonia solani and Verticillium dahliae were used (Table 1). All isolates were collected and stored on potato dextrose agar at the College of Horticulture, Hebei Agricultural University, PR China. Isolates were grown on the culture medium for 5 days at 25°C. The mycelia were collected and ground in liquid nitrogen. Genomic DNA was extracted with the Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech, Shanghai, China) and stored at −40°C.
Primer design and specificity
Based on the sequence from the F. oxysporum genome database (https://www.ncbi.nlm.nih.gov/genome/genomes/707), comparing the sequence information of the strains including Fon (GCA_001702505.1, GCA_001702785.1, GCA_ 001702775.1, GCA_001702715.1, GCA_001702805.1); F. oxysporum f. sp. lycopersici (GCA_001703175.1, GCA_001703185.1, GCF_000149955.1, GCA_001702875.1, GCA_001702905.1); F. oxysporum f. sp. melonis (GCA _001703355.1, GCA_001703215.1, GCA_001703205.1, GCA_001703255.1, GCA_001703265.1); F. oxysporum f. sp. cucumerinum (GCA_001702515.1, GCA _001703455.1, GCA_001702495.1, GCA_001702575.1, GCA_001702635.1); F. oxysporum f. sp. conglutinans (GCA_000260215.2); F. oxysporum f. sp. vasinfectum (GCA_000260175.2); F. oxysporum f. sp. raphani (GCA_000260235.2); F. oxysporum f. sp. cubense (GCA_000260195.2, GCA_001696625.1, GCA_000350345.1); F. oxysporum f. sp. nicotianae (GCA_002234055.1); F. oxysporum f. sp. gladioli (GCA_002234195.1); and F. oxysporum f. sp. narcissi (GCA_002233775.1), specific sites in the sequence of Fon were identified. Based on the specific sequence (GCA_001702505.1, Fon005blob3c_contig_463 (GenBank no. MAKY0100046 2.1), primers were designed using PRIMER PREMIER v. 5.0 (Premier Company, Palo Alto, CA, USA). Then the specificity of primers was evaluated with 20 isolates F. oxysporum, Rhizoctonia solani, and Verticillium dahliae (Table 1). The specific primers of Fon were preliminary screened by conventional PCR, and were further screened by the real-time PCR assay. The experiment was carried out three times.
Real-time PCR standard curve establishment
The 10-fold serial dilutions of genomic DNA of Fon were amplified as a template with specific FONRT-18 primers (FONRT-18F: 5-TAATGCTCGTAAGTCA GGTCAGGTCAGGTTCA-3; FONRT-18R: 5-AGTTGGAGTCAGCGATTCAT-3) in triplicate for real-time PCR. Standard regression curve was generated by the quantified target DNA and Ct values of the dilution series. The PCR reaction contained 2 μl of DNA template, 0.8 μl (10 μmol/l) of primer, 6.8 μl of RNase-free ddH2O and 10.4 μl of 2× qPCR SuperMix (Beijing Quanshijin Biotechnology Co., Ltd.). The thermal profile was as follows: 95°C for 30 s; 45 cycles of 95°C for 5 s, 58°C for 20 s, 72°C for 20 s; and 72°C for 1 min. Fluorescence was monitored during each PCR cycle at 72°C using a LightCycler 96 Eppendorf (Eppendorf Co., Ltd., Hamburg, Germany). Real-time PCR experiments were performed in three biological and technical replicates.
Plant material and pathogen inoculation
Seeds of the watermelon cultivars “Zao Jia 8424” (susceptible to Fon race 1) and “Xi Nong 8” (resistant to Fon race 1) were disinfected in 1.5% sodium hypochlorite for 20 min and then immersed in distilled water for 4 h. The seeds were germinated and sown in sterilized vermiculite and grown in a greenhouse at 20–25°C at 60–80% relative humidity. Fon race1 was inoculated into potato lactose medium and cultured on a rotating shaker (180 rpm, 25°C) for 5 days as previously described (Lü et al., 2014). The potato lactose medium was filtered through four layers of sterile filter paper. The density of the conidial suspension was adjusted to 5.0 × 106 spores/ml with sterile distilled water for inoculation. The roots of watermelon seedlings with both cotyledons open were dipped into the conidial suspension for 15 min, and all the seedlings were replanted in sterilized soil in a greenhouse. The experiments were performed in three biological replicates. As detailed by Lü et al. (2014), the disease response of the watermelon plants was scored using a disease grade from 0 to 4 and recorded daily for 19 days post inoculation (dpi). The DI of each inoculated cultivar was calculated according to the formula shown in (Lü et al., 2014): Disease index = ∑(nv)100/NV, where n = degree of infection rate according to the 5-grade scale, v = number of plants in a category, V = total number of plants screened, N = highest degree of infection rate. Roots of “Zao Jia 8424” and “Xi Nong 8” were harvested at 0, 1, 3, 5, 7, 9, 11, 13, 15, 17, and 19 dpi, quickly frozen in liquid nitrogen, and stored at −70°C. Genomic DNA of the infected plants was extracted according to the CTAB method and frozen at −20°C.
Soil materials and pathogen inoculation
To detect fungi in soil-based samples, Fon race 1 conidial suspensions (10 ml each of 5 × 106, 5 × 105, 5 × 104, 5 × 103, and 5 × 102 spores/ml suspensions) were added to 100 g of autoclaved soil (5 × 105, 5 × 104, 5 × 103, 5 × 102, and 5 × 101 cfu/g) collected from a watermelon field (sandy soil, Baoding, China) with no Fusarium wilt appearance. Control soil samples were mixed with 10 ml of distilled water. Seeds of “Zao Jia 8424” (susceptible to Fon race 1) and “Xi Nong 8” (resistant to Fon race 1) were grown in artificially inoculated soil. The soil of the watermelon rhizosphere was harvested at 0, 4, 8, 12, 16, and 20 dpi. The experiments were performed in three biological replicates. The disease was scored and recorded on a scale from 0 to 4 every day (Lü et al., 2014). All soil samples were dried at room temperature for 2 weeks, and sieved through a 50-mesh sieve. The Fast DNA SPIN Kit for Soil (MP Bio, Solon, OH, USA) was used to extract soil DNA essentially according to the instructions. The DNA was frozen at −20°C for using in the real-time PCR assay.
Plate dilution test
Fresh plant and soil samples inoculated with Fon were placed in a sterilized mortar, thoroughly ground, added to 1 ml of sterile water, and stirred evenly. Then, 200 μl of the solution was taken and evenly smeared on Bengal Red Medium. Five replicate plates were included in each treatment. The samples were cultured at 27°C for 24 h, and the number of colonies on the medium was recorded.
Statistical analysis
The SAS version 8.1 statistical program (SAS Institute Inc., Cary, NC, USA) was used to analyse the effects of the various treatments using Tukey’s tests, A P-value <0.05 was considered statistically significant.
Results
Specificity of the primers for Fon and standard curve analysis
The specificity of primers was evaluated with 11 isolates of Fon and 11 isolates of other soil-borne disease pathogens. The primers FONRT-18F (5-TAATGCTCGTAAGTCAGGTCAGGTCAGGTTCA-3) and FONRT-18R (5-AGTTGGAGTCAGCGATTCAT-3) amplified a unique 172-bp target fragment for 11 strains of Fon (Fon race 0, Fon race 1, Fon race 2), but the other fungi did not show any amplified target product (Table 1). It indicated the primer set was high specific. The FONRT-18 primer set was used for subsequent experiments for quantitative detection of Fon by the real-time PCR assay.
The sensitivity of the assay was tested by using 12.6 fg/μl-126 ng/μl Fon race 1 DNA as the template for real-time PCR. The optimum detection range of this quantitative assay ranged from 126 ng/μl-1.26 pg/μl (Supplementary Fig. 1). The real-time PCR results showed that the primer FONTR-18 had a single peak in a melting curve, indicating that the primers had strong specificity for Fon and showed no nonspecific amplification (Supplementary Fig. 2). Standard regression curves were generated using Ct values obtained for genomic DNA at concentrations ranging from 1.26 pg/μl to 126 ng/μl. The standard regression curve equation was y = −3.462 log(x) + 23.069 (R2 = 0.9993, E = 94.45%, P < 0.0001), with a detection limit of 1.26 pg/μl (Fig. 1). The maximum coefficient of variation within the group was 0.66%, and the maximum coefficient of variation between groups was 1.13%. The data obtained by this method had good intergroup repeatability (Supplementary Table 1).

Standard curve analysis of real-time polymerase chain reaction (PCR) assay by the FONRT-18 primers. Real-time PCR assay standard curve of Fusarium oxysporum f. sp. niveum (Fon). Various concentrations of Fon DNA, ranging from 1.26 pg to 126 ng/μl, were used to develop the standard curve. Each point represents the average of in three biological and technical replicates.
Detection and quantification of Fon in inoculated watermelon plants
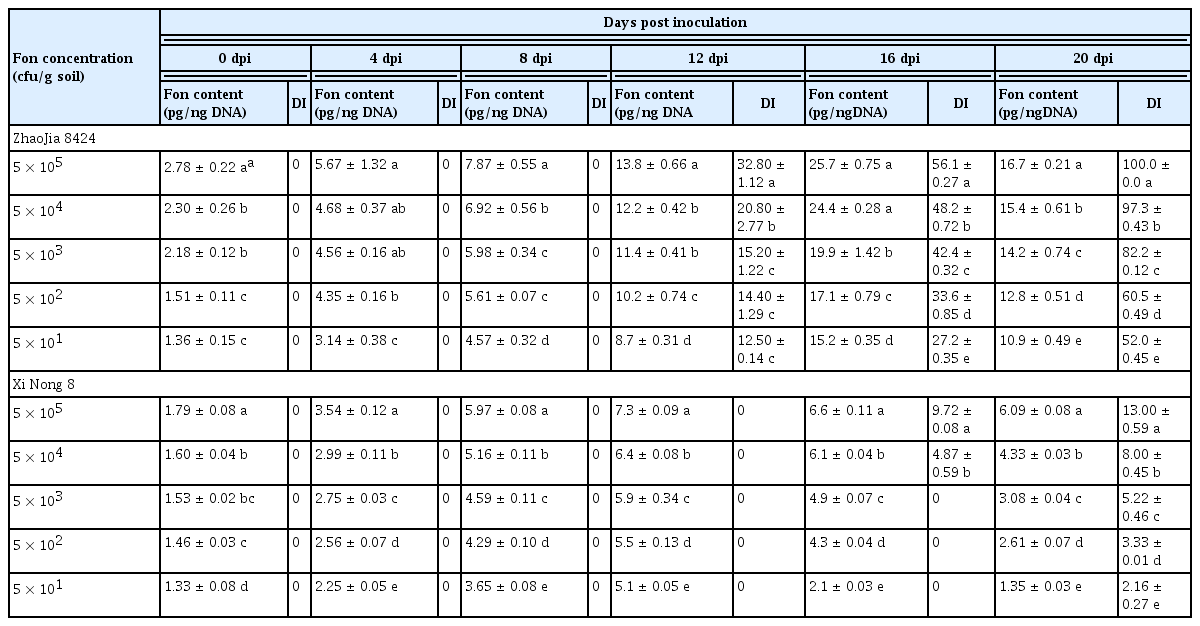
The results showed that there were significant differences in the number of pathogens between resistant and susceptible varieties after inoculation (Table 2). The pathogen quantity in ‘Zhao Jia 8424’ was consistently higher than that in ‘Xi Nong 8’. The pathogen quantity in ‘Zhao Jia 8424’ increased slowly from 0–5 dpi, increased rapidly from 7–15 dpi, reached a peak of 14.16 pg/ng of total DNA at 15 dpi, and decreased slowly from 17–19 dpi; the final pathogen amount was 11.47 pg/ng of total DNA, which may be attributed to the complete death of the plant at the final sampling. The ‘Zhao Jia 8424’ plants had no symptoms, and the DI was 0 from 1–4 dpi; at 5 dpi, the plants began to show mild wilting symptoms, and the DI was 1.77; the DI continued to increase from 5–15 dpi, reaching 96.67 at 15 dpi; from 17–19 dpi, the watermelon plants had almost all withered and died.

Detection and quantification of Fon DNA from the watermelon plants ‘Zao Jia 8424’ and ‘Xi Nong 8’ in different Fusarium wilt disease index groupings by real-time PCR assay
‘Xi Nong 8’ showed an increase in the pathogen amount from 1 to 5 dpi and the amount increased rapidly from approximately 5–7 dpi, with no significant change at 7–9 dpi, and the maximum pathogen amount reached 2.06 pg/ng of total DNA. At 9 dpi, the pathogen amount decreased slowly, indicating that the resistant varieties had strong resilience after being stressed by the pathogens and could inhibit the reproduction of pathogens, the final pathogen amount was 0.47 pg/ng of plant genomic DNA. ‘Xi Nong 8’ showed no symptoms from 0–13 dpi, and the symptoms began to become apparent at 15 dpi, when the DI was 2.67; the highest DI reached was 12.72 in this study (Table 2, Supplementary Fig. 3). The DNA quantity of Fon in the roots of the susceptible cultivar was 36.31-fold greater than that in the roots of the resistant cultivar at 15 dpi (Table 2). The detection limits of the assay were 0.2 pg/ng total DNA in plants.
Using the plate dilution method, the number of colony-forming units on ‘Zao Jia 8424’ was found to continue to increase from 1 to 15 dpi, and the rate was relatively stable. The number of colony-forming units on ‘Zao Jia 8424’ reached a peak of 271.67 cfu/g at 15 dpi and decreased slightly from 15–19 dpi. The number of colony-forming units on ‘Xi Nong 8’ from 0–9 dpi slowly increased, and at 9 dpi, the number peaked at 103 cfu/g, while the overall number from 9–19 dpi decreased gradually (Fig. 2). Regression analyses of the relations between plate dilution method and the real-time PCR assays are carried out, the relevant formulas of the regression relations are set up (y = 0.0665x – 2.0909, R2 = 0.8413, P = 0.05, y = Fon content (pg/ng total DNA), x = PDM (cfu/g)). R2 and P indicated that there was positive correlation between the plate dilution method and the real-time PCR assays, which could be used to detect and quantify Fon in the early stages of infection.
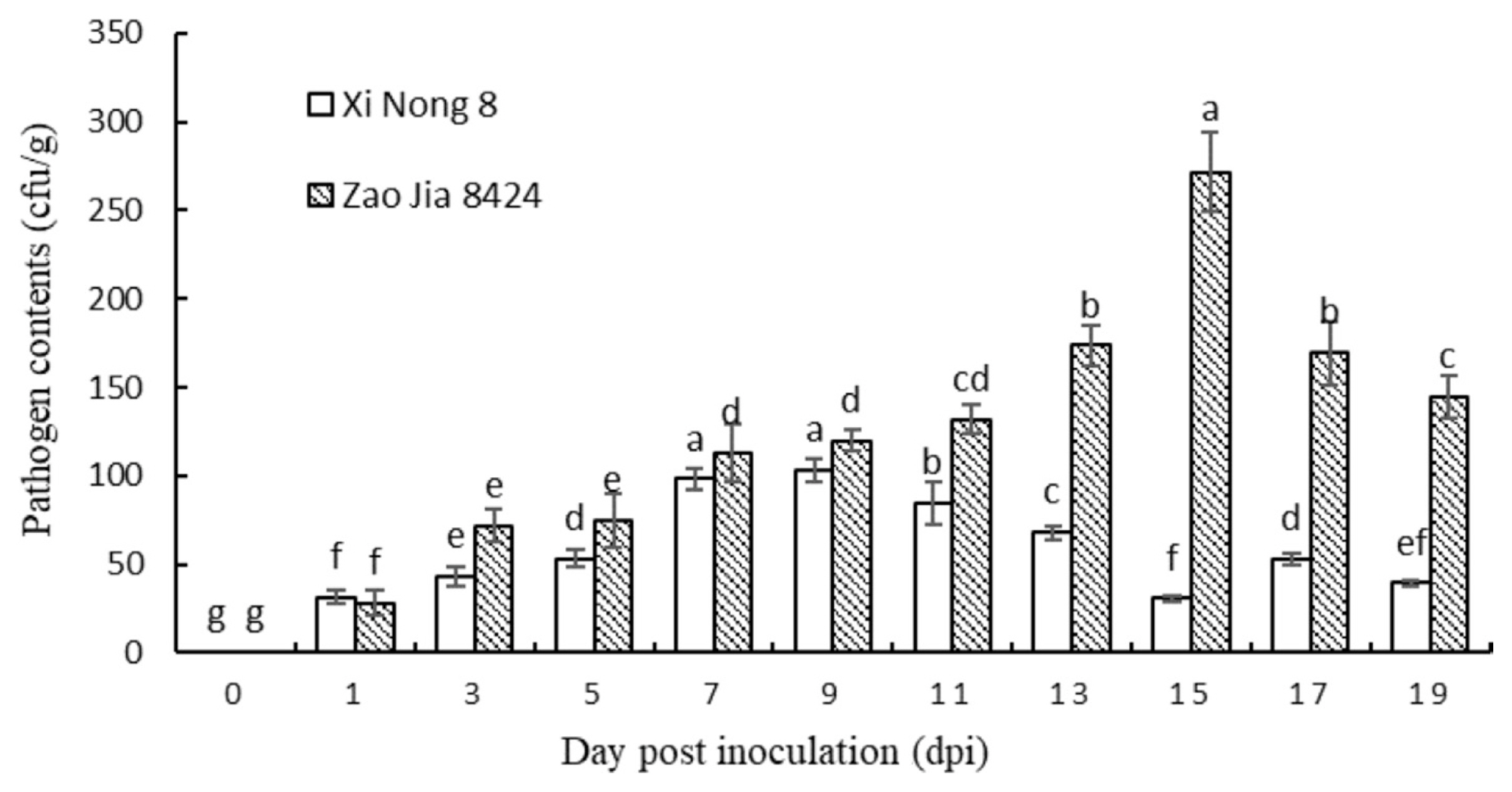
Colony-forming units (cfu) on ‘Zao Jia 8424’ (susceptible to Fusarium oxysporum f. sp. niveum [Fon] race 1) and ‘Xi Nong 8’ (resistant to Fon race 1) watermelon inoculated with Fon race 1 by the plate dilution method. Each value is the mean ± standard deviation of five replicates. Different lowercase letters in each column shape indicate significant difference at P < 0.05 by Tukey’s test.
Detection and quantification of Fon in inoculated soil
A real-time PCR assay was used for quantification of Fon DNA in soil to estimate the relationship between pathogen density and the DI of plants. From 0–16 dpi, the pathogen content of susceptible ‘Zao Jia 8424’ continued to increase at different soil inoculation concentrations. At 16 dpi, the amount of pathogen in soil inoculated with 5 × 105–5 × 101 cfu/g was 25.71–14.12 pg/ng of total DNA, which was significantly positively correlated with the change in the DI. At 20 dpi, the amount of pathogen decreased to 16.57–10.79 pg/ng of total DNA, which was likely caused by plant death (Table 3). For the resistant variety ‘Xi Nong 8’, from 0–12 dpi, the pathogen quantity continued to increase. The maximum pathogen quantity in the soil inoculated with a concentration of 5 × 105–5 × 101 cfu/g was 7.34–5.06 pg/ng of total DNA; at 12–20 dpi, the pathogen quantity decreased slowly, and at 20 dpi, the quantity decreased to 6.09–1.35 pg/ng of total DNA (Table 3). The DNA quantity of Fon in the roots of the susceptible cultivar was 2.70–7.99-fold greater than that in the roots of the resistant cultivar at 20 dpi, which may be because resistant varieties can inhibit the reproduction of pathogens. The change trend for the amount of pathogen in the resistant and susceptible varieties obtained by the plate dilution method was basically consistent with the change trend obtained by real-time PCR detection (Supplementary Fig. 4). The detection limit of the real-time PCR assay was 50 conidia/g of soil. Regression analyses of the relations between Fon DNA concentration and DI are carried out on 20 dpi, the relevant formulas of the regression relations are set up (y = 0.1417x + 2.743, R2 = 0.9675, P = 0.001, y = Fon content (pg/ng total DNA), x = DI). R2 and P indicated that the concentration of Fon DNA in soil was significantly positively correlation with the severity of Fusarium wilt.
Detection of Fon in soil after disinfection with CaCN2
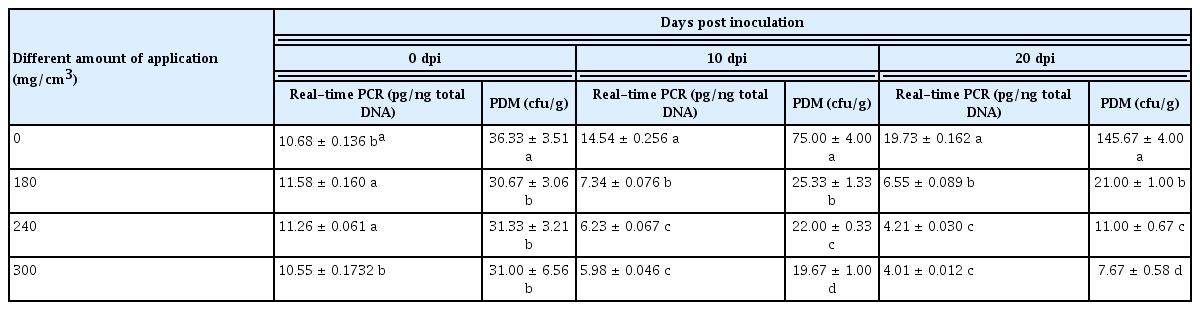
After disinfection with calcium cyanamide (CaCN2), the soil was examined by a real-time PCR assay (the Fon conidial concentration in the soil was 2 × 104 cfu/g) (Table 4). The amount of pathogen in the soil without disinfection increased continuously from 10.68 to 19.73 pg/ng total DNA. At CaCN2 concentrations of 180, 240, and 300 mg/cm3, the amount of pathogen in the soil decreased from 11.58 to 6.55 pg/ng of total DNA, 11.26 to 4.21 pg/ng of total DNA and 10.55 to 4.01 pg/ng of total DNA, respectively, indicating that 240–300 mg/cm3 CaCN2 had a better disinfection effect than 180 mg/cm3 CaCN2. The plate dilution method was used to verify that the number of colonies in the unsterilized soil reached a maximum of 145.67 cfu/g in 20 days; the number of colonies in the CaCN2-disinfected soil decreased from 31.33 and 31 cfu/g further to 11 and 7.67 cfu/g. Regression analyses of the relations between plate dilution method and the real-time PCR assays are carried out, the relevant formulas of the regression relations are set up (y = 7.489x – 32.258, R2 = 0.8319, P = 0.05, y = PDM (cfu/g), x = Real-time PCR (pg/ng total DNA)). R2 and P indicated that there was positive correlation between the plate dilution method and the real-time PCR assays.
Discussion
Rapid and quantitative real-time PCR assay
The rapid detection of Fon is critically important for the prevention of the damage to watermelon production caused by Fusarium wilt. Although many molecular methods have been developed to detect Fon isolates, there methods have some limitations. Lin used randomly amplified polymorphic DNA molecular markers to develop the specific primers Fon-1/Fon-2 and established a conventional PCR detection assay for Fon in Taiwan (Lin et al., 2010). Cao used two pairs of primers, the F. oxysporum-specific primers W106R/W106S and Fon-specific primers FOW-4F/FOW-4R, to establish a duplex PCR system to detect Fon rapidly (Cao et al., 2015). Zhang detected Fon in susceptible watermelon plants by duplex PCR using Fn-1/Fn-2, which are specific primers for Fon (Zhang et al., 2005). However, none of these protocols allow accurate quantitative detection of the pathogen in plant and soil. A multiplex TaqMan assay using primers and probes of Fon-specific amplicons can detect pathogens at template concentrations as low as 2.5 pg (van Dam et al., 2018). The RealAmp assay was developed for quantification and monitoring of Fon in only soil and the detection limit was 103 spores/g of artificially inoculated soil (Peng et al., 2013). Real-time PCR assay for Fon in this paper is as sensitive as TaqMan and RealAmp assay, but it is simpler and effective than them. The detection limits of real-time PCR assay were 1.26 pg/μl Fon genomic DNA, 50 conidia/g of soil, and 0.2 pg/ng total DNA in diseased plant tissue.The real-time PCR assay does not require complex primer design and fluorescently tagged, as the TaqMan and RealAmp assay does. In addition, the specificity and sensitivity of real-time PCR assay can accurately identify and quantify the target pathogen. As a reliable and efficient method for detection and quantitative of Fon in plants and soil, this assay is of great value to the monitoring and comprehensive management of Fusarium wilt.
Real-time PCR specificity
The primers for conventional PCR assays are useful as rapid molecular diagnostic tools for identifying Fon. However, these primers cannot be used in real-time PCR quantitative assays, which reduce the efficiency and reliability of the assay due to the large product size (Cao et al., 2015; Lin et al., 2010; Zhang et al., 2005). In this paper, novel Fon-specific primer sets were designed and screened based on genomic comparison of F. oxysporum f. sp. including melonis, cucumerinum, lycopersici, vesinfectum and conglutinans. The primer FONRT-18 that amplified a 172-bp DNA fragment was specific for Fon isolates, which had been confirmed by other F. oxysporum isolates. Since the product fragment generated by the FONRT-18 primers is shorter than that obtained with the Fon-specific primers in previous studies (Zhang et al., 2005), the FONRT-18 primer has higher sensitivity in quantitative PCR than the previously used primers and higher specificity in distinguishing Fon from closely related species of Fusarium oxysporum f. sp., such as lycopersici, melonis, cucumerinum, and conglutinans. These species often coexist in the same soil in vegetable fields for crop rotation in agricultural production in China.
Application of real-time PCR assays
Rapid and sensitive real-time PCR assays could provide an accurate survey of the occurrence and distribution of pathogens in plants and soil and could be applied to investigate in epidemiology, disease management practices and plant-pathogen interactions. The real-time PCR assay was used to detect and quantify F. oxysporum in compatible and incompatible interactions of grafted melon genotypes (Haegi et al., 2013). The FOR2-F/FOR2-R primer could detect F. oxysporum f. sp. raphani strain of radish plants in the early stage of disease development (Kim et al., 2017). Willsey used a multiplex qPCR assay to detect the dynamic changes in the rates of colonization of pea roots between Fusarium spp. and Aphanomyces euteiches (Willsey et al., 2018). In this experiment, dynamic changes in pathogens in watermelon plants were detected using real-time PCR. The result showed that the amount of pathogen in susceptible watermelon varieties was consistently higher than that in resistant varieties and increased quickly. The resistant variety showed a delay in the infection process and a lower in quantity. These results were consistent with the observation of the dynamic changes in pathogens using GFP (Lü et al., 2014). These indicates that there was a relationship between the resistance of varieties and the propagation rate of fungi in roots. The resistance of watermelon to Fusarium wilt can be evaluated by assaying pathogen density in the roots and stems of plant (Zhou and Everts, 2004). In addition, detection of the pathogen in plants could be a very valuable tool, because management strategies could be applied before the occurrence of symptoms, which will be more effective.
The detection and quantification of pathogens in soil by real-time PCR can be used for epidemiological research and evaluation of the effect of disease management measures. The TaqMan assay of Fusarium oxysporum f. sp. lycopersici revealed a significant quadratic regression between the DNA concentration of pathogens and disease severity, and the results could be used to establish a model for predicting the occurrence of tomato Fusarium wilt (Huang et al., 2016). Leplat evaluated the effect of the different types of crop residues on the colonization dynamics of Fusarium graminearum in soil by real-time PCR measurements to better understand how this organism survives in soil and residues (Leplat et al., 2016). A real-time fluorescence loop-mediated isothermal amplification (LAMP) assay was established for rapid and quantitative detection of Fon in only soil. The sensitivity of the LAMP assay was 103 conidia/g of artificially inoculated soil (Peng et al., 2013), whereas the detection limit of our real-time PCR was as low as 50 conidia/g of artificially inoculated soil. The conidia density of pathogenic in soil was significantly positive correlation with the DI in susceptible and resistant watermelon cultivars. The real-time PCR assay was also successfully used for quantifying F. oxysporum in fumigated soils to evaluate the control effect of the novel fumigant combination on F. oxysporum in strawberry greenhouses (Li et al., 2014). In our study, the real-time PCR assay also was used to estimate the Fon amount of soil after disinfection with CaCN2.
The real-time PCR protocol also was applied for current phytosanitary seed health testing. Sousa established a sensitive real-time PCR and TaqMan assay for the detection of F. oxysporum f. sp. phaseoli in bean seeds, which helped to prevent the pathogen from spreading over long distances through infected seeds (de Sousa et al., 2015). The FnSc-1/2 and Fon-1/2 primers were used to examine watermelon seeds on the market by real-time PCR assays; the results showed that “JingXinYiHao” watermelon seeds carried Fon (Li, 2013). The LAMP assay was tested F. oxysporum f. sp. lactucae with lettuce seeds, the detection limit was 0.004% infected seeds (Ortega et al., 2018).
Conclusion
In this paper, we developed a real-time PCR assay, which can quickly and accurately detect Fon from watermelon plant tissue and soil. The FONRT-18 primers had high specificity and could distinguish Fon from closely related species of F. oxysporum. The detection limits of the assay were 1.26 pg/μl Fon genomic DNA, 0.2 pg/ng total DNA in plant DNA, and 50 conidia/g of soil. The quantitative detection method can be applied for early prediction and disease management of watermelon Fusarium wilt.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Acknowledgments
This research was performed by the Collaborative Innovation Center of Vegetable Industry in Hebei, which was financially supported by the National Nature Science Foundation of China (No. 31872132), The Key Research Projects of Hebei (No. 21626901D), Hebei Facility Vegetables Innovation Team of Modern Agro-industry Technology (No. HBCT2021030213).
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.