Inhibitory Effects of Pepper Mild Mottle Virus Infection by Supernatants of Five Bacterial Cultures in Capsicum annuum L.
Article information
Abstract
Pepper mild mottle virus (PMMoV), one of the most prevalent viruses in chili pepper (Capsicum annuum L.) is a non-enveloped, rod-shaped, single-stranded positive-sense RNA virus classified in the genus Tobamovirus. The supernatants of five bacterial cultures (Pseudomonas putida [PP], Bacillus licheniformis [BLI], P. fluorescens [PF], Serratia marcescens [SER], and B. amyloliquifaciens [BA]) were analyzed to find novel antiviral agents to PMMoV in chili pepper. Foliar spraying with supernatants (1:1, v/v) obtained from Luria-Bertani broth cultures of PP, BLI, PF, SER, and BA inhibited PMMoV infection of chili pepper if applied before the PMMoV inoculation. Double-antibody sandwich enzyme-linked immunosorbent assay showed that treatments of five supernatants resulted in 51–66% reductions in PMMoV accumulation in the treated chili pepper. To identify key compounds in supernatants of PP, BLI, PF, SER, and BA, the supernatants were subjected to gas chromatography-mass spectrometry. The 24 different types of compounds were identified from the supernatants of PP, BLI, PF, SER, and BA. The compounds vary from supernatants of one bacterial culture to another which includes simple compounds—alkanes, ketones, alcohols, and an aromatic ring containing compounds. The compounds triggered the inhibitory effect on PMMoV propagation in chili pepper plants. In conclusion, the cultures could be used to further conduct tissue culture and field trial experiments as potential bio-control agents.
The latest projections by the United Nations Development Program (UNDP) suggest that the global population grows to around 8.5 billion in 2030, 9.7 billion in 2050, and 10.4 billion in 2100 (United Nations, 2022). According to the predicted data of the UN Food and Agriculture Organization (FAO), farmers will have to generate 70% food to meet the oversized population on earth by 2050 (Searchinger et al., 2019). Plant diseases are one of the major obstacles to achieving the goal of food security for the growing population throughout the world. A wide range of methods, such as crop rotation, selection of resistant plant varieties, and so on other methods have been adopted to control plant diseases. However, agrochemicals are still a warranted approach to the prevalence of many plant pathogens. The excessive use of agrochemicals poses a major threat to other living organisms and it is environmentally hazardous too (Sponsler et al., 2019). Therefore, other alternative eco-friendly agents must be explored to control diseases or to enhance crop productivity. Microbes are omnipresent in the environment. By their omnipresence, microbes impact the entire biosphere. Soil microbes produce a broad spectrum of secondary metabolites that enable soil microbes to compete with neighboring microorganisms, which they have likely evolved to compete for the same resources in the soil (Brakhage and Schroeckh, 2011; Garbeva and Weisskopf, 2020). Amongst the microbes, there are microbial biological control agents that are applied to crops for the biological control of plant pathogens (Köhl et al., 2019). In some cases, some microbes have been selected which secrete already efficient secondary metabolites into the growth media during mass production that are applied together with or without living cells of antagonists in the biological control of crops. Production of secondary antimicrobial metabolite with inhibition effects against pathogens is a promising mode of action (Raaijmakers and Mazzola, 2012). In the last decade have had increased attention on the production of microbial secondary metabolites, and their mode of action on crops/plants is an alternative way to use pesticides and fertilizers in the agriculture sector (Thomas et al., 2020). The signals of microbial volatile organic compounds (mVOC) from some microbes suppress neighboring pathogens and also induce plant immunity, exploited as substitutes for chemical fertilizers and pesticides. The mVOC signals could provide a more sustainable solution, and also negligible or zero hazardous effects on animals and the environment (Tilocca et al., 2020).
Pepper mild mottle virus (PMMoV) has been found to infects a wide range of pepper varieties (Wetter et al., 1984) and is one of the major pathogens in pepper species in the world (Jarret et al., 2008; Roberts and Adkins, 2001). Recently PMMoV was found to be the most abundant RNA virus in human feces and also a potential viral indicator for human fecal pollution in aquatic environments (Ferraro et al., 2021). PMMoV is a non-enveloped, rigid, rod-shaped, single-stranded positive-sense RNA, which belongs to the genus Tobamovirus. On pepper fields, PMMoV also remains viable for long periods in soils after infected crops have been removed or harvested. Composting and drying have been shown to only slightly reduce the PMMoV infection, but the impact of the virus infectivity remains available (Aguilar et al., 2010; Petrov, 2014). Chili pepper infected with PMMoV exhibits symptoms like mottling and yellow/green mosaic in leaves, and small malformed, mottled fruit, resulting in significant loss of pepper yield (Jarret et al., 2008; Kim et al., 2012; Rialch et al., 2015; Roberts and Adkins, 2001).
Plant-beneficial bacteria and fungi, living in the soil as free organisms or as endophytes, trigger plant growth and they also protect plants from phyto-pathogens. The influence of abiotic factors on crop yield has been reported by several researchers (Radhakrishnan et al., 2014; Tonelli et al., 2010). The application of plant-beneficial microorganisms is an alternative to chemical fungicides, bactericides, and nematicides. The foliar application of microbes/its active compound/s is the feasibility of an effective, environmentally friendly approach to improving plant growth, and controlling many plant diseases (Adam et al., 2014; Choudhary and Johri, 2009; Egamberdieva et al., 2014; Lee et al., 2020; Radhakrishnan et al., 2013). Among several species of plant growth-promoting bacteria/rhizobacteria (PGPB/PGPR), Pseudomonas spp. and Bacillus spp. have been identified as the predominant communities (Kang et al., 2015), and a few of the PGPB have been commercialized due to their survival within a diverse range of biotic and abiotic environments. PGPRs can produce and induce a wide diversity of useful bioactive metabolites (Beneduzi et al., 2012). Specifically, Pseudomonas spp. and Bacillus spp. were important members of the protective microbiome (Wei et al., 2019). Several research results have shown the control of bacterial and fungal pathogens in many crops (Castaldi et al., 2022; Kim et al., 2017; Vitti et al., 2016), whereas the controlling of plant viruses with microbes/microbial secondary metabolites was limited reports (Elsharkawy et al., 2022; Kong et al., 2018; Tan et al., 2015, 2017). Thus, this study aimed to access supernatants of five bacterial cultures on PMMoV in chili pepper and identification of its active metabolites in the supernatant.
Materials and Methods
Virus source
The single isolated PMMoV-P1,2 strain used in this study was maintained in Nicotiana tabacum cv. Samsun (Choi et al., 2014). PMMoV-infected tobacco leaves were collected aseptically and homogenized with 10 mM phosphate buffer (pH 7.0) using a sterilized plastic pouch. The sap is used as the source of inoculum for chili pepper infection. The inoculum was confirmed by the immunostrip kit specific to PMMoV (Agdia, Elkhart, IN, USA).
Bacterial cultures and foliar spray
The five bacterial species were obtained from the Korean Agricultural Culture Collection (KACC), Rural Development Administration (RDA), South Korea. Pseudomonas putida (KACC no. 12538; PP), Bacillus licheniformis (KACC no. 10307; BLI), Pseudomonas fluorescens (KACC no. 12553; PF), Serratia marcescens (KACC no. 11743; SER), Bacillus amyloliquifaciens (KACC no. 10116; BA). The Luria-Bertani (LB) broth contained (g/l)—Tryptone 10, yeast extract 5, sodium chloride 5, and pH 7.0. LB broth from BD Difco, Thermo Fisher Scientific Inc. (Sparks, MD, USA) was used for the growth of bacteria.
To grow PP, BLI, PF, SER, and BA cultures, the autoclaved LB broth was inoculated separately with the individual bacterial cultures. The culture flasks were incubated in an orbital shaker at 175 rpm at 37°C for 48 h. The 48 h grown culture broth was centrifuged at 8,000 ×g, 15 min, and 4°C to separate the bacterial culture from the supernatant. The supernatants of bacterial cultures were diluted with distilled water in the ratio of (1:1), and 10 ml for each plant (run-off the plant) was foliar sprayed separately in chili pepper plants at a 4-leaf stage (cv. Cheongyang) once a day for three days at 24 h time intervals (Chung et al., 2020). Foliar spray with distilled water was carried out as the positive control treatment. The whole setup of experimental chili pepper plants was lightly dusted with carborundum and then rubbed with PMMoV inoculum from the leaf base to the tip with a gloved finger. After washing, the plants were placed in controlled conditions maintained in a greenhouse chamber for the development of the symptoms.
Double-antibody sandwich enzyme-linked immunosorbent assay
Double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) was subjected to measure the quantity of the virus in samples according to the manufacturer’s protocol (Agdia). This method is a bit different from the detection of the PMMoV virus by rapid antigen test as mentioned in the section on virus source. The experimental setup of chili pepper seedling’s upper leaf sample was collected at 2 week-post-inoculation (wpi) along with control samples and extracts the PMMoV antigen by using a general extraction buffer. The extracted samples of one hundred microliters were placed in the capture-antibody coated enzyme-linked immunosorbent assay (ELISA) plate and incubate at room temperature for 4 h. The ELISA plate-coated wells were washed eight times with phosphate buffered saline (pH 7.5 containing 0.05% Tween-20). DAS-ELISA for PMMoV quantification was carried out as described previously (Yoon et al., 2021). DAS-ELISA data were analyzed with analysis of variance. Differences among the mean values of supernatants of five bacterial cultures were determined with Duncan’s multiple range test with significance set at P < 0.05.
Gas chromatography-mass spectrometry analysis of supernatants of bacterial cultures by solid-phase microextraction method
To identify the active components present in supernatants of bacterial cultures, a 48 h grown five bacterial cultures—PP, BLI, PF, SER, and BA in LB broth were centrifuged separately, then the supernatant was collected. Then the supernatants of bacterial cultures (10 ml) were transferred to a 20 ml capacity headspace vial containing 10 μl of acetonitrile and 0.3 g of NaCl. Acetonitrile was used as an internal standard to quantify secondary metabolites. The sample vial was incubated at 70°C with constant stirring for 1 h. Solid-phase microextraction method (SPME) fiber (50/30 μm DVB/CAR/PDMS; Supelco, Bellefonte, PA, USA) was introduced into the headspace for 20 min to adsorb secondary metabolites in the supernatants. There was a similar approach was also carried out to detect active compounds present in the supernatants of bacterial cultures by gas chromatography-mass spectrometry (GC-MS)-SPME (Chen et al., 2020; Syed-Ab-Rahman et al., 2019). The obtained chromatogram data were analyzed, and peak-to-peak curing was performed using the NIST v.11 (National Institute of Standards and Technology) Mass Spectrum Library. Statistical analysis was performed using the MetaboAnalyst 5.0 online tool in the auto-scaling process. Dendrograms (hierarchical clustering) heatmaps and Pearson’s correlation coefficient were performed visually to compare metabolites.
Results
DAS-ELISA
Chili pepper plants treated with supernatants of five bacterial cultures—PP, BLI, PF, SER, and BA by foliar spray showed lesser symptoms of PMMoV in chili pepper compared to the non-treated plants (control) after 2 weeks of inoculation (data not shown). Moreover, the reduction in the severity of PMMoV in chili plants by foliar spray treatment was higher than that of control-treated samples, DAS-ELISA test confirmed that PMMoV titer as an indicator for PMMoV accumulation was markedly reduced by 51–66% in chili pepper plants with supernatants of five bacterial cultures in comparison to non-treated plants (control) after 2 weeks of virus inoculation (Fig. 1). PMMoV accumulation was much lower in chili pepper plants treated with foliar spraying of supernatants of five bacterial cultures relative to control plants.
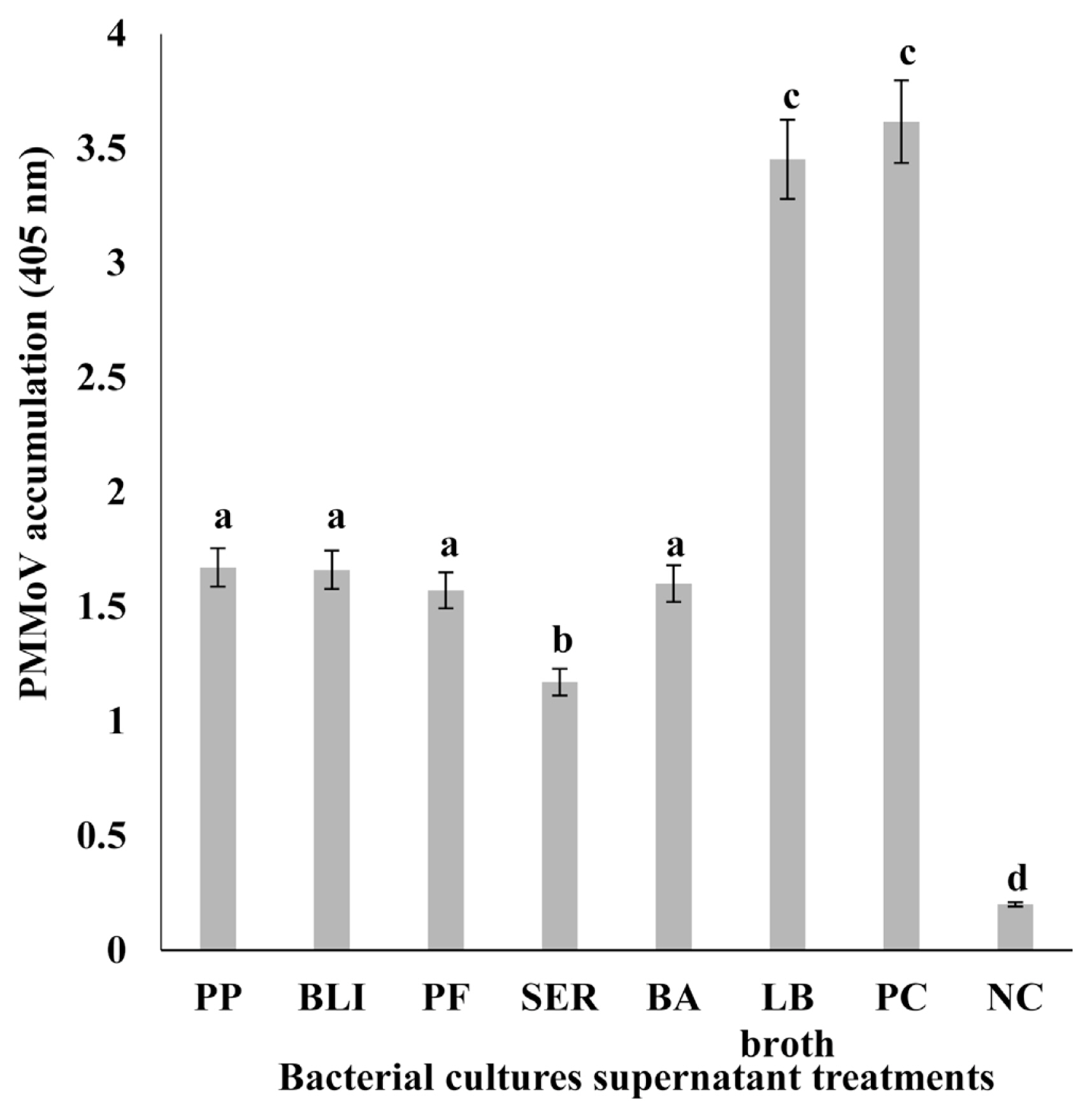
Comparison of pepper mild mottle virus (PMMoV) accumulation in chili pepper plants foliar sprayed with the supernatants of five bacterial cultures. PMMoV detection was performed with double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) kits (Agdia, Elkhart, IN, USA) according to the manufacturer’s protocol. A sample was considered positive if the optical density exceeded 3 times the mean of the negative controls. DAS-ELISA data were analyzed with analysis of variance and differences among the mean values were determined with Duncan’s multiple range test with significance set at P < 0.05. PP, Pseudomonas putida (KACC no. 12538); BLI, Bacillus licheniformis (KACC no. 10307); PF, Pseudomonas fluorescens (KACC no. 12553); SER, Serratia marcescens (KACC no. 11743); BA, Bacillus amyloliquifaciens (KACC no. 10116). Luria-Bertani (LB) broth was the control for foliar spraying. PMMoV-infected chili pepper and healthy pepper leaf were used as the positive control (PC) and negative control (NC), respectively.
GC-MS analysis
The active components present in the supernatants of PP, BLI, PF, SER, and BA culture were done by GC-MS analysis. The GC-MS chromatograms of supernatants of bacterial cultures—PP, BLI, PF, SER, and BA were carefully observed in the NIST library and identified 24-different types of compounds. Of the compounds, acetone, 2-heptanone, benzene, and phenyl ethyl alcohol compounds were commonly identified in supernatants of five bacterial cultures. 2-Butanone is identified in all, four cultures except in BA. Methyl isobutyl ketone is not identified in the PF culture filtrate. Nonanone is found in supernatants of all four bacterial cultures except in BLI. Benzyl alcohol is found in the supernatants of bacterial cultures—BLI, PF, SER, and BA and except in PP2. The compounds/metabolites identified in the supernatants of five bacterial cultures were presented in Table 1. These compound’s Kovats retention index values were calculated with the Kovats formula based on their retention time, and the values were validated with the standard polar values of the compounds in PubChem and presented in Table 1. The Kovats retention index values proved the confirmation of the metabolites in the supernatants of five bacterial cultures. The identified compounds in the supernatants of five bacterial cultures were analyzed with the help of MetaboAnalyst 5.0 and the heat map visualization, and the clustering of all identified compounds was expressed to the control and represented (Fig. 2). The compounds in the heat map are from simple aliphatic compounds to aromatic compounds. The correlation coefficient of all the metabolites of supernatants of five bacterial cultures was expressed (Fig. 3). The positive correlation coefficient was observed for the identified compounds in all supernatants of five bacterial cultures. These compounds are involved in the inhibitory effect on PMMoV titer in chili pepper. Recent reports evidenced that, a few of the compounds from different microbial cultures are involved in the controlling of various diseases caused by pathogenic microbes in the agriculture sector (Naamala and Smith, 2021).

List of metabolites identified in the supernatants of 48-h grown bacterial cultures: PP, BLI, PF, SER, BA, and control by GC-MS-SPME method
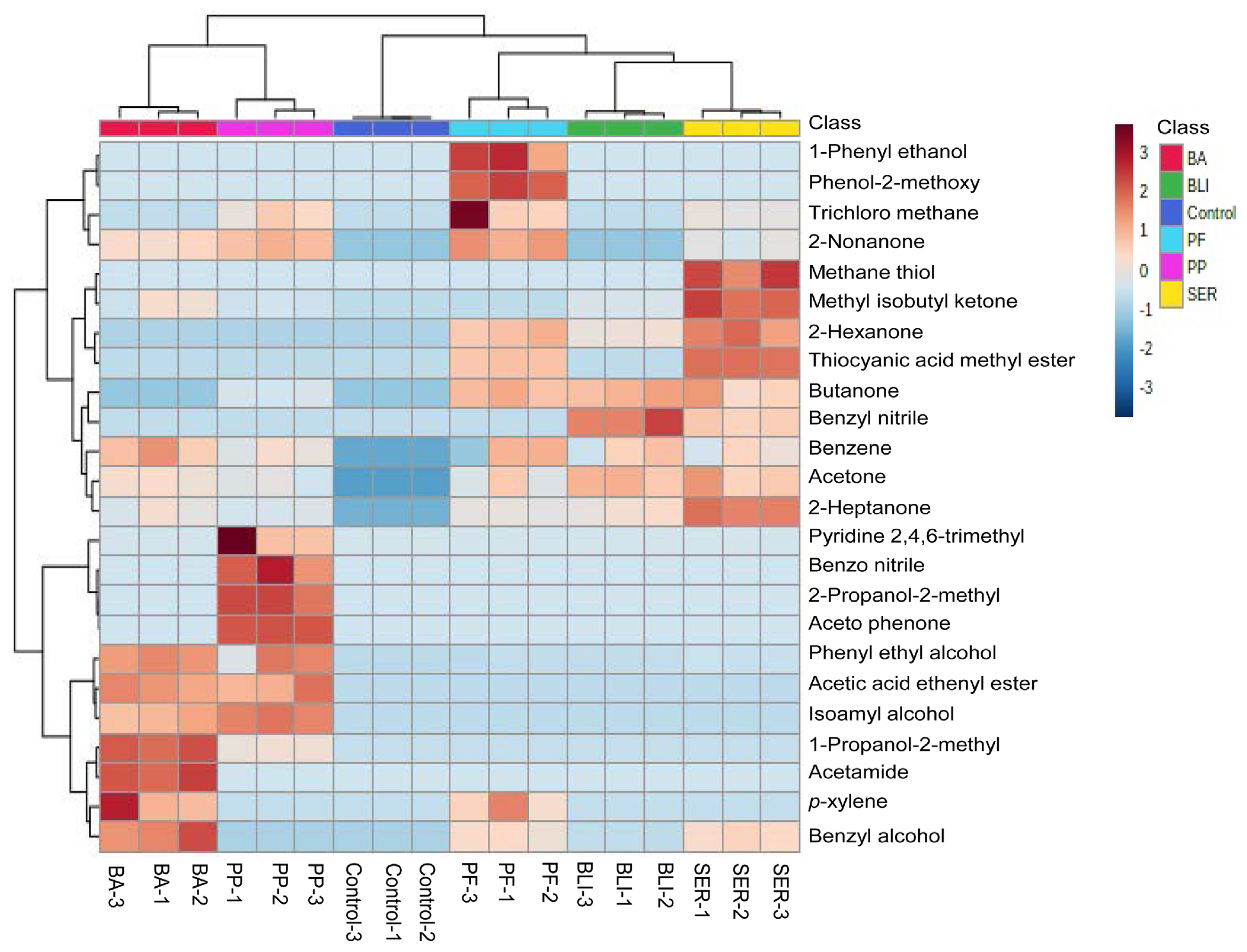
Heat map visualization and clustering of identified 24 compounds in the supernatants of five bacterial cultures—PP, BLI, PF, SER, BA, and control. The data obtained in the GC-MS runs were analyzed manually, and peak-to-peak curing was performed using the NIST v.11 Mass Spectrum Library. Statistical analysis was performed using the MetaboAnalyst 5.0 in the auto-scaling process. In the heat map, all the treatments were mentioned in three biological replicates including the control. BA, Bacillus amyloliquifaciens; BLI, Bacillus licheniformis; PF, Pseudomonas fluorescens; PP, Pseudomonas putida; SER, Serratia marcescens; GC-MS, gas chromatography-mass spectrometry.
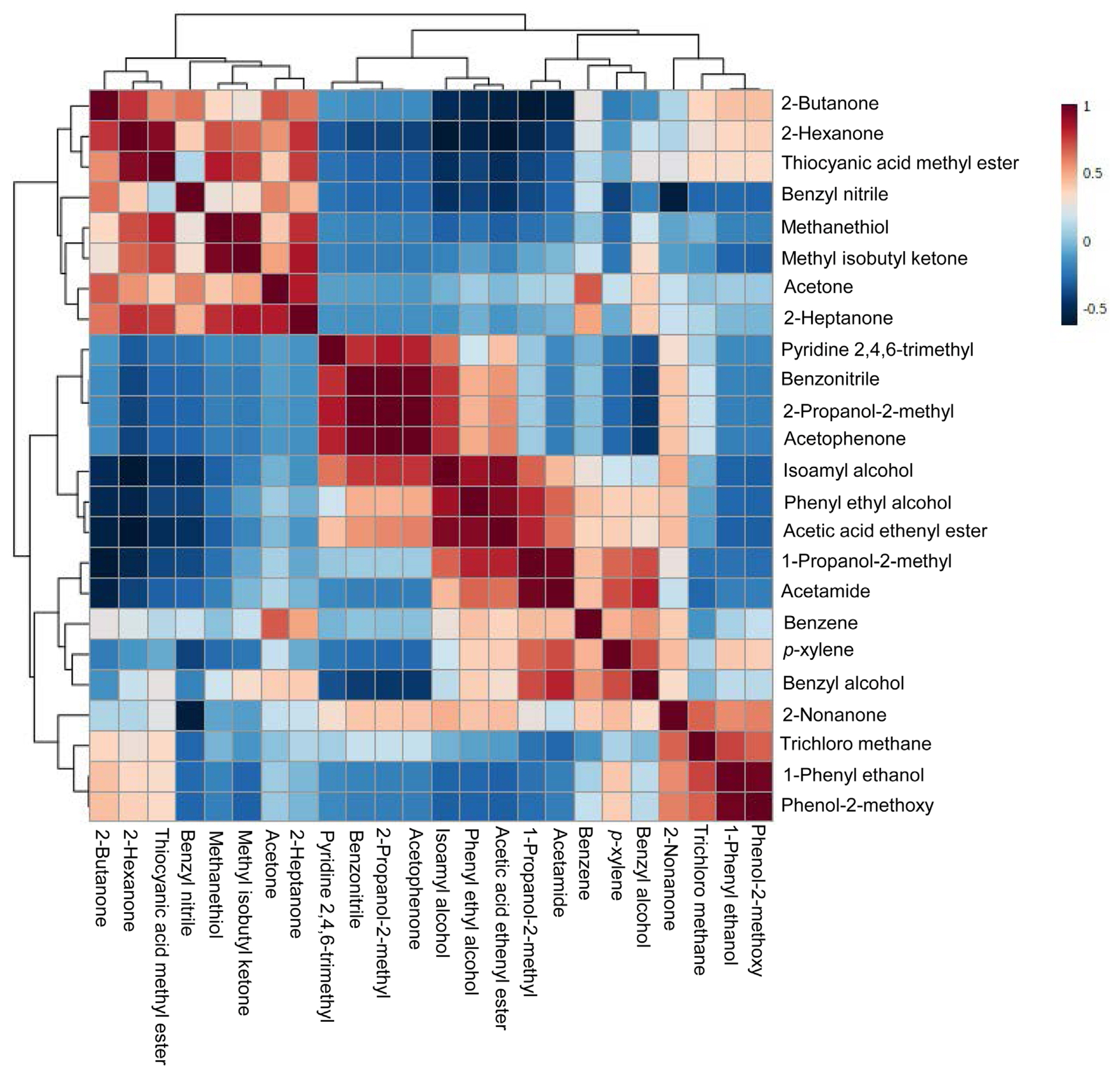
The Pearson’s correlation coefficient patterns of metabolites identified in the supernatants of five bacterial cultures—PP, BLI, PF, SER, and BA. Correlation coefficient were performed visually to compare metabolites (mean ± SE). Pearson’s correlation coefficient (r) measures a linear dependence between two variables (x and y). Positive correlations are indicated in red and negative correlations are in blue. PP, Pseudomonas putida; BLI, Bacillus licheniformis; PF, Pseudomonas fluorescens; SER, Serratia marcescens; BA, Bacillus amyloliquifaciens; SE, standard error.
Discussion
Microbes in general, and bacteria in particular, are well known for their biochemical versatility; using a wide array of inorganic and organic compounds to fuel their metabolism, they are also prolific producers of secondary metabolites of diverse biological activities. Many of these metabolites have antimicrobial properties and are used as antibiotic and antifungal drugs (Ait Barka et al., 2016; Brader et al., 2014; Caulier et al., 2019). Traditionally, the study of microbial metabolism and the search for bioactive molecules has focused on soluble compounds. However, mounting evidence suggests that microbes, and especially bacteria, emit diverse volatile compounds with significant biological activities on a wide range of target organisms, including plants and their pathogens (Groenhagen et al., 2013; Piechulla et al., 2017). When facing biotic stresses such as pest or pathogen attacks, plants defend themselves via two main resistance pathways, the salicylic acid (SA)-mediated systemic acquired resistance (SAR), and the jasmonic acid/ethylene-mediated induced systemic resistance (ISR) (Pieterse et al., 2011; Tsuda and Somssich, 2015). Whereas SAR is mainly induced by pathogenic microbes, plants have been shown to express ISR when their roots are colonized by beneficial Pseudomonas secreting specific metabolites (e.g., siderophores) or harboring molecular determinants such as flagellin or the O antigen of the outer membrane lipopolysaccharides (Meziane et al., 2005).
Some studies observed the effects of small organic compounds emitted by bacteria in modulating plant growth, development, defense, and inter-and intraspecific communication (Kanchiswamy et al., 2015; Schreiter et al., 2014; Wenke et al., 2018). For instance, B. amyloliquefaciens (UQ154), B. velezensis (UQ156), and Acinetobacter sp. (UQ202) cultures released isoamyl alcohol and act against P. capsici and inhibition of 41.1 and 66.9% at 5 and 10 μg/ml in I-plate in vitro assay compared with the control. In this study, supernatants of five bacterial cultures produced different types of compounds, those from simple alkanes, ketones, alcohols, and aromatic ring compounds. The compound 3-methyl butanol (isoamyl alcohol) was found in the supernatants of PP and BA. Moreover, phenyl ethyl alcohol and benzyl alcohol are aromatic compounds produced by B. velezensis (UQ156), and Acinetobacter sp. (UQ202) and inhibit the Phytophthora capsici infection in chili pepper plants (Syed-Ab-Rahman et al., 2019). The compound – phenyl ethyl alcohol was also one of the non-polar antifungal components released by Trichoderma virens 7b, which exhibited antifungal activity against Ganoderma boninense (Angel et al., 2016). Benzyl alcohol has also been shown to inhibit Colletorichum camelliae Massea, the causal agent of anthracnose in tea (Camellia sinensis) plants (Zhang et al., 2006). In agreement with this result, supernatants of BLI, PF, SER, and BA bacterial cultures also produced phenyl ethyl alcohol and benzyl alcohol. The aromatic compound 2-phenyl-ethanol emitted by two bacteria Serratia and Stenotrophomonoas, acts as a signal molecule, and negatively impacts wild A. thaliana and prominent player in the rearrangement of plants physiology, copes with the stress in the mutants of A. thaliana insertion of WRKY18 T-DNA (Wenke et al., 2018). In addition, 2-phenyl ethanol was isolated from Kloeckera apiculata and inhibited postharvest phytopathogenic fungi Liu et al. (2014). Furthermore, nonan-2-one, nonan-2-ol, and decanal were produced by B. amyloliquefaciens (UQ154) and B. velezensis (UQ156). All these compounds have been documented to antagonize fungi (Bruce et al., 2003; Kai et al., 2009; Liu et al., 2008). For example, nonan-2-one was associated with the inhibition of fungal growth by Serratia strains (Bruce et al., 2003). Similarly, nonan-2-ol which was produced by B. subtilis G8 also suppressed phytopathogenic fungi (Liu et al., 2008). In a similar fashion supernatants of PP, PF, SER, and BA bacterial cultures also produced a 2-nonanone compound. The volatile organic compound (VOC) 3-methyl butanal was emitted by the bacterium Staphylococcus pasteuri in the presence of the fungus Tuber borchii, whose mycelium was notably inhibited by VOCs (Barbieri et al., 2005). Another study that used GC-MS analysis to characterize the VOCs of Trichoderma viride revealed that 3-methyl butanal was one of the most abundant VOCs and showed that VOCs emitted by T. viride have growth-promoting effects on A. thaliana in the absence of direct physical contact (Hung et al., 2013), which supports the results observed in this study. Furthermore, 3-methylbutanal was reported to be antagonistic to Colletotrichum gloeosporioides, a fruit fungal pathogen (Gao et al., 2018). To parallel to this Acinetobacter sp. (UQ202) produced 3-methylbutanal, inhibits Phytophthora capsici in chili pepper, and promotes plant growth (Syed-Ab-Rahman et al., 2019).
The drench application of 1 mM 3-pentanol and 0.1 μM 2-butanone on cucumber seedlings consistently triggered plant systemic defense responses against Pseudomonas syringae pv. lachrymans. Drench application of 3-pentanol and 2-butanone resulted in a reduction in disease severity in cucumber in the open field at 28 days post-seeding (dps), i.e., 7 days after spray-challenge of P. syringae pv. lachrymans. The treatment of cucumber plants with 1 mM 3-pentanol, 0.1 μM 2-butanone, or 10 nM 2-butanone caused 24%, 26%, or 17% less symptom severity, respectively, than the water control. The disease severity of plants treated with 10 μM 3-pentanol was not statistically different from that of the control (P = 0.05). Plants treated with BTH, which was employed as a positive control, showed similar levels of disease severity to plants treated with 0.1 μM 2-butanone (Song and Ryu, 2013). For instance, 2-phenyl ethanol emitted from T. asperellum T76-14 was reported to control the postharvest fruit rot of muskmelon (Intana et al., 2021). Therefore, the VOCs of T. koningiopsis PSU3-2 containing azetidine, 2-phenyl ethanol, and ethyl hexadecanoate may be associated with the suppression of the mycelial growth of the C. gloeosporioides, suggesting the antibiosis mechanism of T. koningiopsis PSU3-2 (Ruangwong et al., 2021). In the plants, the protection was due to induced defenses, not to pathogen inhibition (Sharifi and Ryu, 2016). This relative importance of plant resistance induction vs direct pathogen inhibition is likely to be strain and compound dependent, although some volatile compounds were shown to display both types of effect: reduction of pathogen virulence and up-regulation of plant genes involved in the SA-mediated defense pathway (Tahir et al., 2017). NPR1 is one of three currently known SA receptors activating SA-mediated defenses via a function in the nucleus (Ding et al., 2018; Fu et al., 2012).
Based on the experimental results, our efforts will further continue on exposure of these bacterial cultures/compounds on Petri dishes (I shaped/separated), one side of the Petri dish containing nutrient medium/LB for growth of bacteria, another side of the Petri dish with Murashige & Skoog (MS) medium for the growth of chili seedlings/PMMoV-infected seedlings. We need to accentuate the virus titer/pathogenicity, plant defense mechanism pathways, and also field experiments.
Acknowledgments
This work was supported by a grant from the Basic Research Program (PJ01431803) of the National Institute of Horticultural and Herbal Science (NIHHS), Rural Development Administration (RDA), Republic of Korea (ROK). The authors are thankful to Dr. Kyeong-Ok Choi, Fruit Research Division, NIHHS, RDA of Korea for the analysis of GC-MS and data discussion.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
