Evaluation of Soil Streptomyces spp. for the Biological Control of Fusarium Wilt Disease and Growth Promotion in Tomato and Banana
Article information
Abstract
Fusarium oxysporum f. sp. lycopersici (Fol) and Fusarium oxysporum f. sp. cubense (Foc), are the causal agent of Fusarium wilt disease of tomato and banana, respectively, and cause significant yield losses worldwide. A cost-effective measure, such as biological control agents, was used as an alternative method to control these pathogens. Therefore, in this study, six isolates of the Streptomyces-like colony were isolated from soils and their antagonistic activity against phytopathogenic fungi and plant growth-promoting (PGP) activity were assessed. The results showed that these isolates could inhibit the mycelial growth of Fol and Foc. Among them, isolate STRM304 showed the highest percentage of mycelial growth reduction and broad-spectrum antagonistic activity against all tested fungi. In the pot experiment study, the culture filtrate of isolates STRM103 and STRM104 significantly decreased disease severity and symptoms in Fol inoculated plants. Similarly, the culture filtrate of the STRM304 isolate significantly reduced the severity of the disease and symptoms of the disease in Foc inoculated plants. The PGP activity test presents PGP activities, such as indole acetic acid production, phosphate solubilization, starch hydrolysis, lignin hydrolysis, and cellulase activity. Interestingly, the application of the culture filtrate from all isolates increased the percentage of tomato seed germination and stimulated the growth of tomato plants and banana seedlings, increasing the elongation of the shoot and the root and shoot and root weight compared to the control treatment. Therefore, the isolate STRM103 and STRM104, and STRM304 could be used as biocontrol and PGP agents for tomato and banana, respectively, in sustainable agriculture.
Fusarium wilt is one of the most economically significant plant diseases caused by many forms of the soil-inhabiting fungus Fusarium oxysporum. Several hundred species are susceptible, including tomatoes and bananas. The fungal pathogen Fusarium oxysporum f. sp. lycopersici (Fol) is the causal agent of tomato wilt disease and causes losses in tomato production and yields up to 100% in some production areas (Kirankumar et al., 2008; McGovern, 2015). F. oxysporum f. sp. cubense (Foc) is the causal agent of the Fusarium wilt disease of bananas (Shen et al., 2015; Wang et al., 2017). It is one of the most destructive diseases of bananas in tropical and subtropical areas and poses a significant economic loss to banana production worldwide (Mintoff et al., 2021; Moore et al., 2001). Currently, prevention and control methods for these diseases include chemical control (Amini and Sidovich, 2010; Bauer et al., 2016), disease resistant varieties (Ambrico and Trupo, 2017; Chen et al., 2019; Zuo et al., 2018), cultural control measures (Ben Abdallah et al., 2018), and biological control (Alijani et al., 2019). However, the first three methods are ineffective; fungicide applications risk human health and harm nontarget organisms and the environment (Arcury and Quandt, 2003). Furthermore, chemical treatments resulted in the emergence of fungicide-resistant pathogens (Kanini et al., 2013b; Reis et al., 2005). Therefore, the researchers focused on finding biocontrol agents and used them as an alternative and effective method. Recently, bacteria in the genus Streptomyces (Duan et al., 2020), Bacillus (Keikha et al., 2015), and Pseudomonas (Suresh et al., 2021; Weller 2007) have been used as biocontrol agents.
The bacteria in the genus Streptomyces are ubiquitous in soil and have been known as a potential impact biological control agent in vitro and planta for many years. The Streptomyces genus can grow in various environments, and they have been used as a biocontrol agent against various soilborne diseases due to their antagonistic activities by the production of many bioactive compounds and/or extracellular hydrolytic enzymes (Al-Askar et al., 2011, 2013; El-Tarabily and Sivasithamparam, 2006; Ghorbel et al., 2014; Kanini et al., 2013a; Sajitha and Florence, 2013; Singh et al., 2008; Srividya et al., 2012; Xio et al., 2002). Some Streptomyces species such as S. aureofaciens, S. avermitilis, S. humidus, S. hygroscopicus, S. lividans, S. lydicus, S. olivaceoviridis, S. plicatus, S. roseoflavus, S. scabies, and S. violaceusniger have successfully been applied to control the soilborne fungal pathogens (El-Tarabily and Sivasithamparam, 2006; Taechowisan et al., 2009; Xio et al., 2002).
Streptomycetes are also appointed as plant growth-promoting (PGP) bacteria due to their activities to produce various lytic enzymes during their metabolic processes such as enzymes can degrade insoluble organic polymers chitin and cellulose, resulting in breaking them and uptake (Bertram et al., 2004; Chater et al., 2010; Dias et al., 2017). Streptomycetes also enhance plant growth by direct stimulation, such as iron chelation, phosphate solubilization, nitrogen fixation, IAA production, and siderophore production (Basak and Biswas, 2009; Hao et al., 2011; Kawicha et al., 2020; Panhwar et al., 2012). Furthermore, they have indirect stimulation, such as induction of systemic resistance in host plants, resulting in the suppression of plant pathogens (Basak and Biswas, 2009; Hao et al., 2011; Panhwar et al., 2012).
Based on the advantage of antagonistic and PGP activities of Streptomyces spp., as mentioned above, the present study was to isolate Streptomyces bacteria from soils and to screen their activity against the highest pathogenicity strain of Fol and Foc. The taxonomic identity of the selected isolates was determined by morphological characteristics and analysis of the 16S rDNA sequence. The growth promoting characteristics and growth improvement traits of these strains in tomato and banana plants were also investigated.
Materials and Methods
Isolation of Streptomyces from soils
Streptomyces isolating from soils was performed using the method described by Sangdee et al. (2016). Briefly, soil samples were collected from tomato rhizospheres; approximately 50 g of each soil sample was collected and air dried at ambient temperature for 2–3 days. The soil samples were suspended and incubated at room temperature for 30 min before being diluted 10-fold serially. The diluted soil suspensions were spread on arginine-glycerol mineral salt agar (AGMA) and incubated at 37°C for 7 days. Streptomyces-like colonies were selected. The pure cultures were cultured on half potato dextrose agar (HPDA) before being kept on HPDA slants at 4°C for further studies.
Isolation of plant pathogenic Fusarium
Plant pathogenic fungi Fol race 1, including isolates TFPK101 and TFPK401, were isolated from tomatoes showing typical symptoms of Fusarium wilt disease, and plant pathogenic fungi Foc race 1 isolates KPS1-3 and PT4-3 were isolated from bananas showing typical symptoms of Fusarium wilt disease using tissue transplanting technique. The emerging colonies were subcultured on potato dextrose agar (PDA) and identified by morphological characteristics and analysis of the internal transcribed spacer sequencing. The pathogenicity of Fol and Foc were tested on the tomato and banana plants, respectively, in strict conformity with Koch’s postulates. For further studies, pure cultures were cultured and kept on PDA slants at 4°C.
Characterization of Streptomyces
Morphological characterizations
The six Streptomyces isolates were cultured on AGMA medium at 37°C and the colony characteristics of the strains were inspected daily for 14 days. The isolates’ spore-chain arrangement was prepared using the slide culture method (Cross, 1989) then the spore chains were examined under a light microscope.
Molecular identification by 16S rDNA sequence analysis
The six Streptomyces isolates were cultured in potato dextrose broth medium (PDB) at 37°C for 7 days before extracting the genomic DNA. Partial 16S rDNA was amplified by the polymerase chain reaction (PCR) technique with primers fD1 and rP2 (Weisburg et al., 1991). The thermal cycle profiles and PCR reaction followed the protocol of Sangdee et al. (2016). The 16S rDNA partial sequence was sequenced and then compared and aligned with the database of the sequences in the National Center for Biotechnology Information (NCBI). Next, the phylogenetic tree assessment was analyzed using Mega 6 (Tamura et al., 2013), and a neighbor-joining tree was constructed with 1,000 bootstrap replicates using the Kimura 2 parameter method. Bacillus subtilis was used as an outgroup.
In vitro antagonistic activity test
Six isolates of the soil Streptomyces-like colonies were isolated and screened for their antagonistic ability against Fol isolates TFPK101 and TFPK401, and Foc isolates KPS1-3 and PT4-3 using the dual culture method. The isolates of Streptomyces-like colony were streaked on PDA plates and kept at 37°C for 7 days before inoculating the 7 mm diameter hypha tips of the fungal pathogens at a 30 mm distance from the Streptomyces-like colony. The dual culture plates were incubated at 28°C for 7 days. Individual cultures of fungal isolates were used as a control plate. The experiment was carried out in three replicates. After 7 days, the plates were measured for antagonistic ability based on the reduction of the radius of the pathogen colony. The percentage of mycelial growth reduction (PGI) was calculated using the following formula.
, where KR is the colony radius of the fungal pathogen (mm) of the control treatment and R1 is the distance of the fungal colony radius (mm) toward the Streptomyces growth (Živković et al., 2010). The derived data were analyzed with variance analysis, and the means were compared using Tukey’s range test (P < 0.01).
Effect of Streptomyces spp. culture filtrate on Fusarium wilt control under pot experiment
Preparation of the culture filtrate
A 7-day-old disk of the selected antagonistic Streptomyces was cultured in AGMA broth for 14 days at 37°C in a shaker incubator at 125 rpm. The Streptomyces culture filtrate was collected and filtered through a 0.2 μm filter membrane before being used.
Biocontrol of Fusarium wilt disease in tomato
The two isolates of Streptomyces sp. isolates, STRM103 and STRM104, have the highest percentage of PGI against the Fol isolate TFPK401 and TFPK101, respectively and were evaluated for their ability to control Fusarium wilt in tomatoes. The tomato seeds, cv Seedathip 3, were seeded in peat moss for 20 days, then the seedlings were planted in sterilized loamy soil for 30 days. The plants were treated with 10 ml of culture filtrate of the Streptomyces isolates by drenching them on the ground near the root crown. Fol isolates were inoculated into the plants after application of the culture filtrate for 48 h. Ten milliliters of Fol spore suspension, 1 × 106 spores/ml, were drenched in the 4 sites of wounded tomato root created by stabbing a cutting blade, an 18 mm wide blade, 5 cm below the soil surface, and 5 cm from the tomato stem. Ten ml of the culture filtrates were applied every week after Fol inoculation for 4 weeks. The AGMA broth was used as a control. All tested tomato seedlings were grown in a greenhouse under natural sunlight and a temperature of 30–33°C/25–28°C (day/night). Seedlings were watered once a day. At 30 days after inoculation, the disease severity score, disease index, and disease control efficiency were evaluated. The severity of the disease was classified into five scores (0–4) described by Song et al. (2004), where 0 represents no infection, and 4 denotes complete infection. A completely randomized design (CRD) with 7 replications was used for statistical analysis.
Biocontrol of Fusarium wilt disease in banana
One isolate of Streptomyces sp. (STRM304), which has the highest percentage of PGI against Foc isolates KPS1-3 and PT4-3, respectively, was evaluated for their ability to control Fusarium wilt on the banana. Banana, Musa (ABB Group) ‘Pakchong 50’, propagated by micropropagation, was transferred to peach moss, grown in an evaporated greenhouse for 1 month, and then transferred to sterile loamy soil. The plants were drenched with 10 ml of the Streptomyces culture filtrate 1 week after the second transplantation. Forty-eight h after application of the culture filtrate, 20 ml of spore suspension of Foc isolates KPS1-3 and PT4-3 at 106 spores/ml were inoculated into banana plants through 2 sites of wound root created by stabbing a cutting blade, 18 mm wide blade, 5 cm below the soil surface and 2 cm from banana stem. Ten ml of the culture filtrates were continuously applied 2, 3, and 4 weeks after the second transplant. The AGMA broth was used as a control. All tested banana seedlings were grown in a greenhouse under natural sunlight and a temperature of 30–33°C/25–28°C (day/night). Seedlings were watered once a day. At 60 days after transplantation, the disease severity score, disease index, and disease control percentage were measured. The disease severity scores depended on external symptoms and ranged from 1 to 5, according to Pérez-Vicente et al. (2014), where 1 represents no infection and 5 denotes complete infection. The experiment design was CRD with 8 replications.
In vitro PGP traits investigation of Streptomyces spp
IAA production
The production of IAA of six isolates of Streptomyces, isolates STRM103, STRM104, STRM302, STRM304, STRM305, and STRM403, was determined according to the modified method of Bano and Musarrat (2003). The isolates were cultured in PDB supplemented with 0.2% w/v tryptophan and incubated at 37°C in a shaking incubator at 125 rpm for 14 days. After that, Streptomyces cells were discarded by centrifugation at 10,000 rpm for 10 min. Two ml of Salkowski reagent was mixed with 1 ml of the supernatant and incubated for 20 min at room temperature. The development of a pink color indicated the presence of IAA (μg/ml). The IAA produced was quantified by determining the optical density at 530 nm and compared with a standard curve of IAA.
Phosphate solubilization
The phosphate (P) solubilization of the six isolates of Streptomyces was screened in National Botanical Research Institute’s Phosphate growth medium (NBRIP). A loop of Streptomyces was streaked in the media and incubated for 5 days at 37°C. After that, a halo zone surrounding the streak lines was observed and measured.
Cellulase activity
The cellulase activity of the six isolates of Streptomyces was screened in PDA medium supplemented with carboxy methyl cellulose. A loop of Streptomyces was streaked in the media and incubated for 5 days at 37°C. After that, the plates were flooded with 2% Congo-red solution and incubated for 15 min, then washed with 1 M NaCl solution. Next, the clear zone was observed and measured around the streak lines.
Amylase activity
The amylase activity of the six Streptomyces isolates was screened in PDA medium supplemented with soluble starch. A loop of Streptomyces was streaked in the media and incubated for 5 days at 37°C. After that, the plates were flooded with Grams iodine and measured the clear zone around the streak lines.
Lignin hydrolysis activity
The lignin hydrolysis activity of the six isolates of Streptomyces was screened in tryptic soy broth supplement with methylene blue. A loop of Streptomyces was streaked in the media and incubated for 5 days at 37°C. After that, the clear zone around the streak lines was observed and measured.
Evaluation of Streptomyces culture filtrates on the growth of tomato and banana
In vitro tomato seed germination test
The six Streptomyces isolates, STRM103, STRM104, STRM302, STRM304, STRM305, and STRM403, were cultured in AGMA broth for 14 days at 37°C in a shaker incubator at 125 rpm. Tomato seeds were surface sterilized with 1% sodium hypochlorite and washed with sterile water. Sterilized tomato seeds cv. Seedathip 3 was soaked with the culture filtrate of Streptomyces overnight. The seeds were blotted in a sterile plate containing wet filter papers and kept at room temperature for 7 days. The percentages of seed germination, seedling lengths, shoot lengths, root lengths, and fresh weight and dry weight of shoot and root were measured and compared with the control treatments. Each treatment had three replicates (50 seeds per replication).
Effect of culture filtrate on tomato growth under pot experiment
The six isolates of Streptomyces were evaluated in a pot experiment for their PGP potential in tomatoes according to the method described by Kawicha et al. (2020). Tomato seedlings were planted in sterilized loamy soil for 30 days. The tomato seedlings were then drenched with 5 ml of the culture filtrate of Streptomyces at 1, 2, 3, and 4 weeks after planting. The AGMA broth was used as a control. All tested tomato seedlings were grown in a greenhouse under natural sunlight and a temperature of 30–33°C/25–28°C (day/night). Seedlings were watered once a day. The growth parameters, including plant height, root length, and fresh and dry weight of shoot and root, were measured 30 days after drenching the culture filtrate. The experiment was carried out with 3 replicates (3 plants per replication).
Effect of culture filtrate on banana growth under pot experiment
In a pot experiment, the six isolates of Streptomyces were evaluated for their PGP potential on a banana. Micro-propagated banana, Musa (ABB Group) ‘Pakchong 50’ was transferred to peach moss, grown in an evaporated greenhouse for 1 month, and then transferred to sterile loamy soil. Then, the plants were drenched with 5 ml of the culture filtrate of Streptomyces isolates at 1, 2, 3, and 4 weeks after planting. The AGMA broth was used as a control. All tested banana seedlings were grown in a greenhouse under natural sunlight and a temperature of 30–33°C/25–28°C (day/night). Seedlings were watered once a day. At 30 days after planting, the growth parameters, including plant height and root length and fresh and dry weight of shoot and root, were measured. The experiment was carried out with 3 replicates (3 plants per replication).
Results
Isolation and identification of the Streptomyces isolated from soils
Six isolates of the Streptomyces-like colony were isolated, including STRM103, STRM104, STRM302, STRM304, STRM305, and STRM403. The various colors of aerial mycelia, including white, brown, and grey, were observed. Two types of spore-bearing aerial hyphae, including rectiflexbiles spore-chain arrangement (STRM302, STRM305, and STRM403) and spira spore-chain arrangement (STRM103, STRM104, and STRM304), were observed under a light microscope (Fig. 1). The 16S rRNA gene sequence of all isolates has the highest homology with bacteria in the genus Streptomyces. A phylogenetic tree based on the neighbor-joining method was generated and indicated that the six isolates of the Streptomyces-like colony were located in the same clade as the Streptomyces species (Fig. 1). Based on the molecular data from the 16S rDNA gene sequence and the bacterial morphology, these isolates belonged to the genus Streptomyces, which should be referred to as Streptomyces sp. isolate STRM103, STRM104, STRM302, STRM304, STRM305, and STRM403.
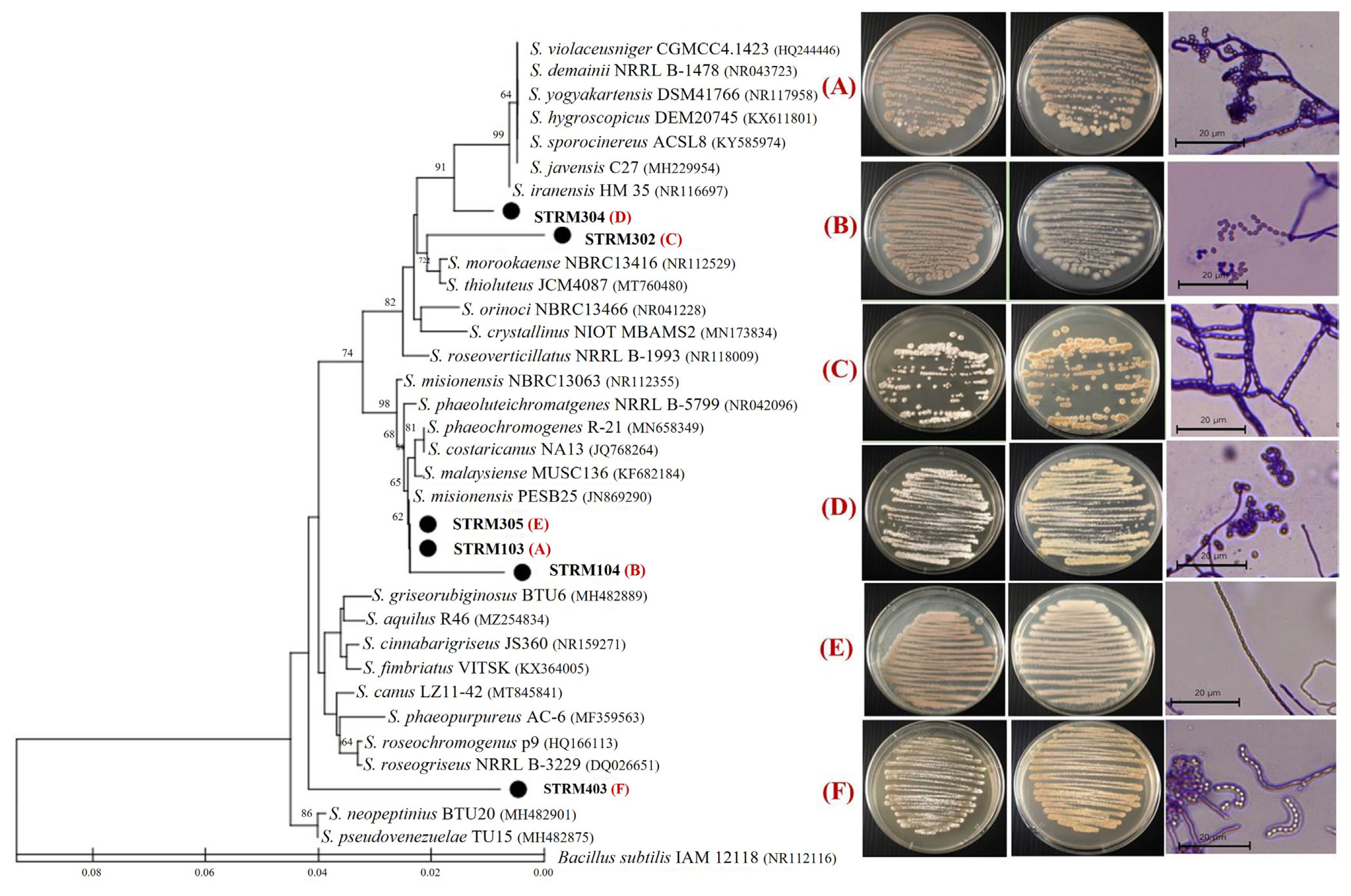
Phylogenetic relationship of the six strains of Streptomyces spp. based on partial 16S rDNA gene sequences. A neighbor-joining tree was constructed using Mega 6. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches (cut-off value of 50%). Pictures shown on the right side are a colony and spore-chain morphology.
In vitro antagonistic activity test
The dual culture assay assessed all isolates’ antagonistic ability against the two isolates of Fol, isolate TFPK101 and TFPK401. The results presented in Table 1 showed that Streptomyces isolate STRM103 showed the highest PGI against the isolate TFPK401 (78.1 ± 2.5%), followed by Streptomyces isolates STRM305 (75.3 ± 2.4%), STRM104 (70.5 ± 0.7%), STRM304 (54.1 ± 1.8%), and STRM403 (36.9 ± 2.5%), respectively. Streptomyces isolate STRM104 showed the highest PGI against the isolate TFPK101 (65.0 ± 0.6%), followed by Streptomyces isolates STRM103 (51.2 ± 1.9%), STRM305 (50.6 ± 1.6%), and STRM304 (41.9 ± 1.9%), respectively. Interestingly, the isolates STRM103, STRM104, and STRM305 showed a broad-spectrum activity against all isolates of the tested plant pathogenic fungi (Table 1). These results indicated that the antifungal activity depended on the isolates of Streptomyces and Fol.

In vitro antifungal activity of six isolates of Streptomyces spp. against Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. cubense
For the antagonistic ability against Foc, most Streptomyces isolates inhibited the growth of Foc, ranging from 41.0 ± 3.9% to 100.0 ± 0.0%, except isolate STRM103, and STRM104. The percentage of fungal mycelial growth reduction varied depending on the isolates of Streptomyces and Foc. Among them, the selected soil Streptomyces isolate SRTM304 showed the highest PGI in all the tested fungi. The results presented in Table 1 showed that Streptomyces isolate STRM304 showed the highest PGI against the isolate KPS1-3 (89.5 ± 0.8%), followed by Streptomyces isolates STRM302 (77.4 ± 2.1%) and STRM403 (52.4 ± 1.6%), respectively. Streptomyces isolate STRM304 showed the highest PGI against the isolate PT4-3 (100.0 ± 0.0%), followed by Streptomyces isolates STRM302 (76.1 ± 0.8%), STRM403 (76.1 ± 3.8%), and STRM305 (41.0 ± 3.9%), respectively. Interestingly, the isolates STRM304 showed a broad-spectrum activity against all isolates of the Fusarium oxysporum (Table 1).
Effect of Streptomyces spp. culture filtrate on Fusarium wilt control under pot experiment
Biocontrol of Fusarium wilt disease in tomato
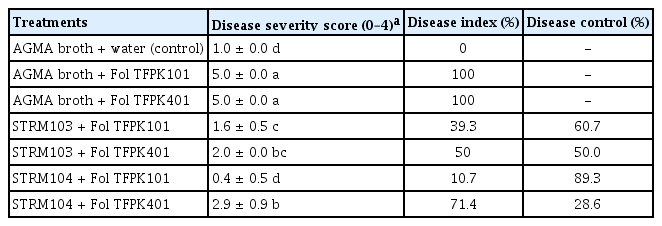
Based on the in vitro antifungal activity, Streptomyces isolate STRM103 and STRM104 were selected to be assessed their ability to control Fusarium wilt in tomatoes under greenhouse conditions. The results showed that the disease severity score and disease index were affected by the application of culture filtrates. The disease severity score of the culture filtrate STRM103 and STRM104 -treated tomatoes significantly decreased from 5.0 ± 0.0 to 1.6 ± 0.5, and 0.4 ± 0.5 on tomatoes inoculated with Fol isolate TFPK101, respectively. The disease severity score on tomatoes inoculated with Fol isolate TFPK401 was also decreased from 5.0 ± 0.0 to 2.0 ± 0.0 and 2.9 ± 0.9 when the culture filtrate of STRM103 and STRM104 were applied, respectively. Culture filtrate derived from Streptomyces sp. isolate STRM104 showed the lowest disease severity score when applied against Fol isolate TFPK101, followed by the culture filtrate from Streptomyces sp. isolate STRM103. The disease control efficiency of culture filtrates ranged from 28.6% to 89.3%, which depends on Fol isolates and Streptomyces isolates (Table 2, Fig. 2).
Effect of culture filtrate from Streptomyces sp. isolate STRM103 and STRM104 on disease severity, disease index, and disease control of Fusarium wilt of tomato under pot experiments

Fusarium wilt disease symptom in tomato after 4 weeks of Fusarium oxysporum f. sp. lycopersici (Fol), isolate TFPK101 and TFPK401 inoculation. Culture filtrate of Streptomyces sp. isolate STRM103 and STRM104 were drenched on the soil near the tomato’s root crown 2 days before Fol inoculation and every week after Fol inoculation.
Biocontrol of Fusarium wilt disease in banana
Based on the in vitro antifungal activity, Streptomyces isolate STRM304 was selected to be assessed its ability to control Fusarium wilt in bananas under greenhouse conditions. The culture filtrate of isolate STRM304 significantly decreased the disease severity score and disease index on bananas inoculated with Foc isolates (Table 3, Fig. 3). The disease severity score of the culture filtrate-treated banana significantly decreased from 5.0 ± 0.0 and 5.0 ± 0.0 to 2.1 ± 0.8 and 1.9 ± 0.6 on bananas inoculated with Foc isolate KPS1-3 and PT4-3, respectively. Also, the disease index of culture filtrate applied bananas were lower than without culture filtrate application. The percentage of disease control on all Foc isolates inoculated bananas ranged from 71.9% to 78.1%.
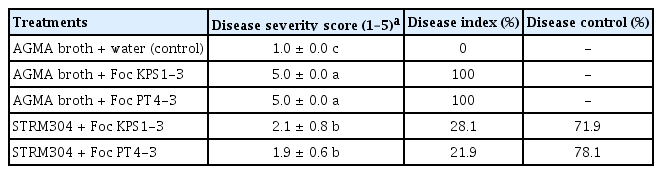
Effect of culture filtrate from Streptomyces sp. isolate STRM304 on disease severity, disease index, and disease control of Fusarium wilt of micro-propagated banana seedling under pot experiments
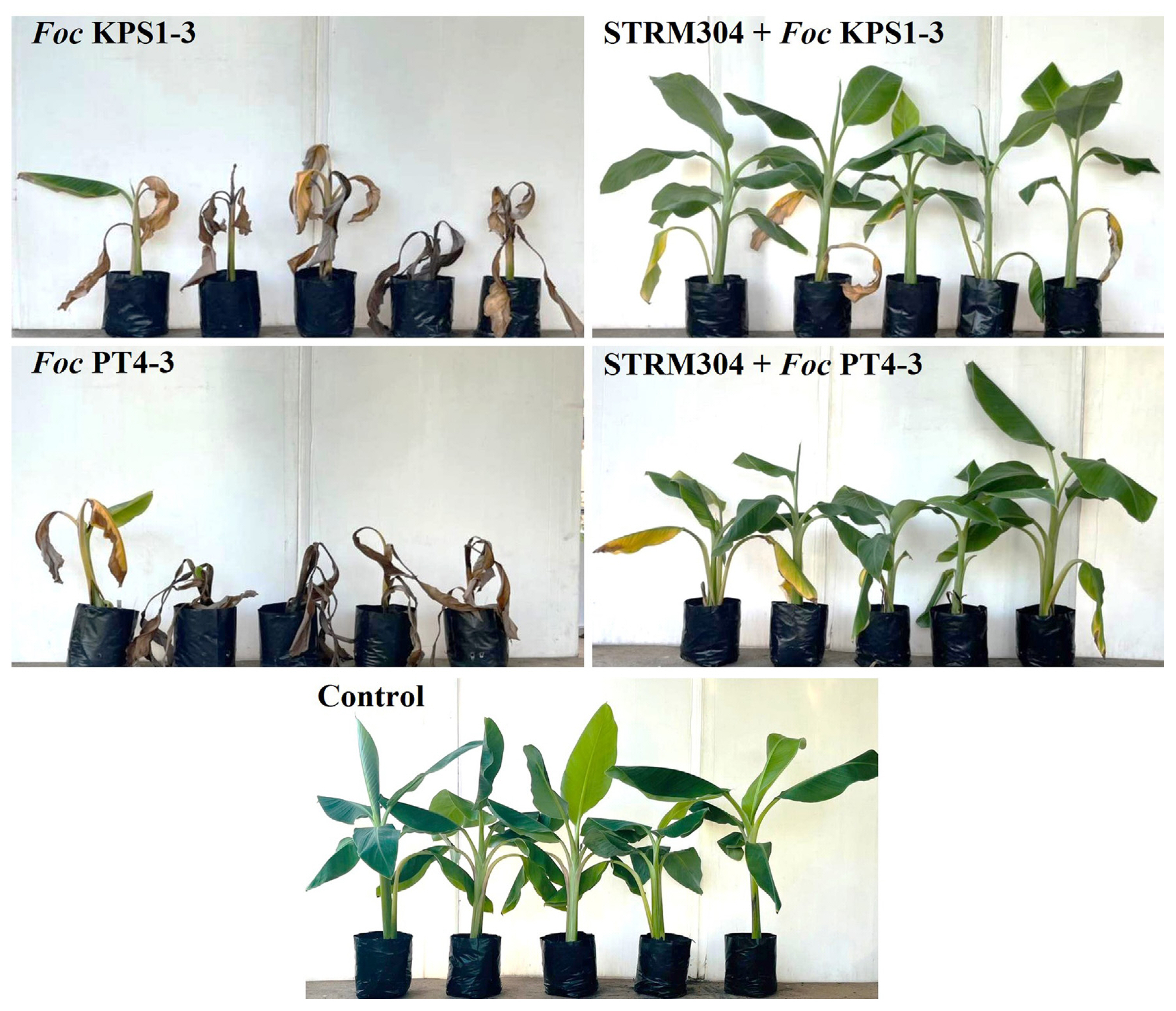
Fusarium wilt disease symptom in banana after 4 weeks of Fusarium oxysporum f. sp. cubense (Foc) isolate KPS1-3 and PT4-3 inoculation. Culture filtrate of Streptomyces sp. isolate STRM304 was drenched on the soil near the banana’s root crown 2 days before Foc inoculation and every week after Foc inoculation.
In vitro PGP traits investigation of Streptomyces spp
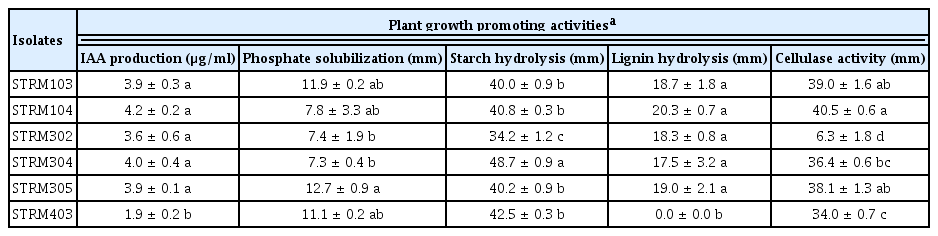
All the six strains of Streptomyces spp. produced IAA in the presence of L-tryptophan with the highest activity by the isolate STRM104 (4.2 ± 0.2 μg/ml) followed by isolate STRM304 (4.0 ± 0.4 μg/ml), whereas the remaining isolates produced IAA between (1.9 ± 0.2)-(3.9 ± 0.3) μg/ml. The six isolates were able to solubilize phosphate. The isolate STRM305 presented the highest activity with 12.7 ± 0.9 mm of a halo zone surrounding the streak lines, followed by isolate STRM103 (11.9 ± 0.2 mm) and STRM403 (11.1 ± 0.2 mm), whereas the halo zone of remaining isolates was between (7.3 ± 0.4)-(7.8 ± 3.3) mm. For the starch hydrolysis activity, isolates STRM304 showed the highest activity with the large zone (48.7 ± 0.9 mm), followed by the isolate STRM403 (42.5 ± 0.3 mm), whereas the clear zone of remaining isolates was between (34.2 ± 1.2)-(40.8 ± 0.3) mm. Five strains (STRM103, STRM104, STRM302, STRM304, and STRM305) of Streptomyces spp. could produce the enzyme to lysis of lignin substrate with the clear zone ranging from (17.5 ± 3.2)-(20.3 ± 0.7) mm, whereas the remaining isolates (STRM403) had no hydrolysis activity of lignin. The cellulase activity was observed in all the tested strains. The isolate STRM104 has the highest activity with 40.5 ± 0.6 mm of clear zone, whereas the clear zone of the remaining isolates was between (6.3 ± 1.8)-(39.0 ± 1.6) mm (Table 4).
Evaluation of Streptomyces culture filtrates on the growth of tomato and banana
In vitro tomato seed germination test
The blotter method investigated the effect of culture filtrate on seed germination of tomato plants. After treatment, we found that the isolates STRM103, STRM104, and STRM305 significantly enhanced seed germination percentage compared with the control treatment and other treatments (Table 5). The isolates STRM103, STRM104, and STRM305 also significantly enhanced PGP parameters, total seedling length, shoot, and root length. Among them, isolate STRM103 showed the highest total seedling length (10.6 ± 0.2 cm), followed by isolate STRM305 (9.3 ± 0.7 cm) and STRM104 (9.1 ± 0.6 cm). Moreover, STRM103 and STRM104 significantly enhanced total seedling fresh weight, shoot, and fresh weight compared with the control and other treatments (Table 5). The STRM104 and STRM103 showed the highest total seedling fresh weight (1.5 ± 0.1 g), followed by isolate STRM302 and STRM305. These indicated that the PGP activity depended on the Streptomyces isolates.
Effect of culture filtrate on tomato growth under pot experiment
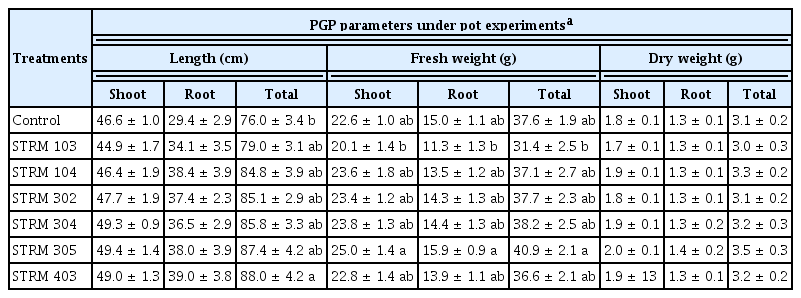
After observation, the PGP ability of six isolates of Streptomyces showed that the isolates STRM302 and STRM304 positively affect some tomato growth parameters such as shoot length, root length, and the total length measured 30 days after culture filtrate application. In contrast, the remaining isolates did not affect these parameters after being compared with the control treatment and other treatments except the isolates STRM305 and STRM403, which negatively affect tomato growth (Table 6). The shoot and root fresh weight and shoot and root dry weight of Streptomyces culture filtrate-treated tomatoes and untreated tomatoes (control) were significantly different. Streptomyces isolate STRM302 had the highest activity of PGP traits based on the values of shoot fresh weight (4.9 ± 0.0 g), root fresh weight (0.39 ± 0.0 g), and total fresh weight (5.3 ± 0.1 g) and shoot dry weight (0.37 ± 0.0 g), root dry weight (0.06 ± 0.0 g) and total dry weight (0.43 ± 0.0 g). The results showed that STRM305 and STRM403 reduced the growth of tested tomatoes based on some growth parameters, such as plant length and weight (Table 6).
Effect of culture filtrate on banana growth under pot experiment
After observation, the PGP ability of six isolates of Streptomyces showed that isolate STRM305 had exhibited a more significant effect on all the growth parameters of micro-propagated banana seedlings. In contrast, the remaining isolates affected some parameters, with some variation, compared with the control treatment (Table 7). The most extended shoot length (49.4 ± 1.4 cm) and longest total length (87.4 ± 4.2 cm) as well as heaviest shoot fresh weight (25.0 ± 1.4 g), root fresh weight (15.9 ± 0.9 g), and total fresh weight (40.9 ± 2.1 g), and heaviest shoot dry weight (2.0 ± 0.1 g), root dry weight (1.4 ± 0.2 g) and total dry weight (3.5 ± 0.3 g) were observed in the banana seedlings treated with the isolate STRM 305. The results showed that STRM 103 seems to reduce the growth of tested banana seedlings based on growth parameters such as shoot length, shoot and root fresh weight, and shoot and root dry weight (Table 7).
Discussion
Streptomyces spp. has been used as a natural source of new antibiotics. Around 7,600 biologically active secondary metabolites have been characterized and produced by the genus Streptomyces (Subramaniam et al., 2016; Watve et al., 2001). They also produce various bioactive secondary metabolites, such as antimicrobial, antitumor, and antihypertensive (de Lima Procópio et al., 2012; Liu et al., 2013). Moreover, several Streptomyces species have also been used as biocontrol agents against many plant pathogens because they have shown the potential to produce bioactive compounds, reducing or inhibiting the mycelial growth of several fungi (Bressan and Figueiredo, 2008; Ezziyyani et al., 2007; Mukherjee and Sen, 2006). Therefore, Streptomycetes can be a promising agent to replace some synthetic fungicides (Verma et al., 2012). In the present study, six isolates of Streptomyces-like colonies were isolated from soil and identified by the 16S rDNA; the region comprising about 1,500 bp with hypervariable and conserved regions is universal in all bacteria. Moreover, the hypervariable regions of the 16S rDNA sequences provide species-specific signature sequences widely used in bacterial identification (Ling et al., 2020). After the blast search alignment, the high homology sequence (more than 97% identity) between six isolates and the members of the genus Streptomyces was observed. The result indicated that six isolates were the bacteria in the genus Streptomyces based on the systematic identification of the prokaryote’s cut-off value at 97.0 (Stackebrandt and Ebers, 2006). Moreover, the neighbor-joining phylogenetic tree and their morphological characters also concluded that six isolates belong to the genus Streptomyces. However, further work is required to identify species.
After bacterial identification, the antifungal activity of the six isolates of Streptomyces was evaluated against 2 isolates of the phytopathogenic Fol and 2 isolates of the phytopathogenic Foc. The various in vitro antifungal abilities against the isolates of Fol and Foc were observed. The isolate STRM304 has shown the most potent broad-spectrum antagonistic activity. Among them, the isolate STRM103 and STRM104 showed the highest antagonistic activity against the phytopathogenic Fol, whereas the isolate STRM304 showed the highest antagonistic activity against the phytopathogenic Foc. These results indicated that the antifungal activity depended on the interaction between the isolate of antagonistic Streptomyces and the fungal pathogen. The variation in antifungal activity is due to the production of extracellular hydrolytic enzymes such as cellulase, amylase, and ligninase, which are well-known antifungal compounds that can directly or indirectly attack the fungal pathogen by degrading the cell wall. However, secondary metabolites and/or antibiotics must be produced at the right time and at high enough levels to attack the pathogens (Marois, 1990). In our study, high-level production of cellulase enzyme activities was detected and may provide these three isolates (STRM103, STRM104, and STRM304) with the potential to control phytopathogenic Fol and Foc. Cellulase is one of the lytic enzymes found in antagonistic microbes and plays a key role in cleaving cellulose bonds. Three types of cellulase have been reported and secreted by fungi: β-glucanase, cellobiohydrolase, and β-glucosidase (Panchapakesan and Shaker, 2016). This agreed with the previous research of Mukherjee and Sen (2006), who indicated that lytic enzymes such as β-glucanase from the strains of Streptomyces sp. had the activity to control plant pathogens. Similarly, Trejo-Estrada et al. (1998a, 1998b) demonstrated that the production of β-glucanase from S. violaceusniger YCED-9 could inhibit the mycelia of the fungal pathogen. Moreover, this was supported by the in vivo biocontrol experiment. The culture filtrate derived from the Streptomyces sp. isolate STRM104 and STRM103 showed a lower disease severity score and disease index in inoculated tomatoes than in the control treatment. Similarly, the culture filtrate derived from Streptomyces sp. isolate STRM304 showed a lower disease severity score and disease index in inoculated bananas than the control treatment. This indicated that the culture filtrate had the potential to protect and suppress the fungal pathogen in the inoculated tomato and banana plants treatments until 4 weeks after inoculation when compared with those of the control treatments (Tables 2 and 3, Figs. 2 and 3).
The PGP activity under in vitro and planta and lytic enzyme of the six isolates of Streptomyces spp. was investigated. Key indicators to clarify PGP ability, including IAA activity and phosphate solubilization, were detected, with some variation. In this study, the six strains could improve the percentage of tomato seed germination compared to the control treatment (Table 5). Furthermore, the culture filtrates of all strains also stimulate the growth of tomato seedlings compared to the total length and fresh weight with the control treatment (Table 6). In banana seedlings, the culture filtrates from all isolates of Streptomyces, except isolate STRM103, could stimulate the growth of banana seedlings. The shoot length of banana seedlings treated with the isolate STRM302, STRM304, STRM305, and STRM403 had longer than the remaining treatments and control treatment. Moreover, the shoot fresh weight, root fresh weight, total fresh weight, shoot dry weight, root dry weight, and total dry weight in the STRM305 treatment were also more remarkable than those of the other treatments (Table 7). These results reveal that Streptomyces spp. stimulated the significant efficient growth promotion due to its production of IAA. IAA is a common plant hormone belonging to the class of auxins that play an essential role in plant growth and development since it induces cell elongation and division (Vurukonda et al., 2018). These results agree with those of El-Tarabily et al. (2009) and Khamna et al. (2010), who reported that the various endophytic Streptomyces species, such as S. rimosus, S. viridis, and S. olivaceoviridis, have been produced IAA, which improves the growth of the host plants by increasing germination of seeds, root elongation, and root dry weight. Furthermore, the high growth rates of tomato and banana seedlings in the treatments applied with Streptomyces spp. may be due to the inorganic phosphate solubilization activity of Streptomyces spp., because it also plays an essential role in the enhancement of plant growth (Hamdali et al., 2008). The lytic enzyme, such as amylase from Streptomyces, plays a vital mechanism in competitive saprophytic ability (Bhai et al., 2016). The ability to produce cellulase of the Streptomyces strain may be the crucial antagonistic activity against some plant pathogenic fungi (Bhai et al., 2016).
Based on these results, the six strains of Streptomyces spp. showed antifungal activity against Fol and Foc by producing lytic enzymes including cellulase, amylase, and ligninase and PGP trait including IAA activity and phosphate solubilization under in vitro conditions. In the seed germination test and pot trial, applying culture filtrate to tomato and banana plants greatly enhanced the percentage of tomato seed germination and plant growth. Moreover, culture filtrate had the activity to suppress the fungal pathogens by delaying the disease symptom on inoculated tomato and banana plants. Thus, our results demonstrated the potential of soil Streptomyces isolate STRM103, STRM104, and STRM304 to be used as a biocontrol agent in tomatoes and bananas, respectively, without adverse effects on tested plant growth. However, a greater understanding of the environmental conditions, rhizosphere colonization, and the interaction between the antagonist and the pathogen are required for the long-term use of the biological control agent of Fusarium wilt disease.
Acknowledgments
This research project was financially supported by Mahasarakham University (MSU). The authors also thank the Department of Agriculture and Resources, Faculty of Natural Resources and Agro-Industry, Kasetsart University Chalermphrakiat Sakon Nakhon Province Campus, and Mahasarakham University, Faculty of Science for supporting equipment and space for this study.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.