Distribution of Pectobacterium Species Isolated in South Korea and Comparison of Temperature Effects on Pathogenicity
Article information
Abstract
Pectobacterium, which causes soft rot disease, is divided into 18 species based on the current classification. A total of 225 Pectobacterium strains were isolated from 10 main cultivation regions of potato (Solanum tuberosum), napa cabbage (Brassica rapa subsp. pekinensis), and radish (Raphanus sativus) in South Korea; 202 isolates (90%) were from potato, 18 from napa cabbage, and five from radish. Strains were identified using the Biolog test and phylogenetic analysis. The pathogenicity and swimming motility were tested at four different temperatures. Pectolytic activity and plant cell-wall degrading enzyme (PCWDE) activity were evaluated for six species (P. carotovorum subsp. carotovorum, Pcc; P. odoriferum, Pod; P. brasiliense, Pbr; P. versatile, Pve; P. polaris, Ppo; P. parmentieri, Ppa). Pod, Pcc, Pbr, and Pve were the most prevalent species. Although P. atrosepticum is a widespread pathogen in other countries, it was not found here. This is the first report of Ppo, Ppa, and Pve in South Korea. Pectobacterium species showed stronger activity at 28°C and 32°C than at 24°C, and showed weak activity at 37°C. Pectolytic activity decreased with increasing temperature. Activity of pectate lyase was not significantly affected by temperature. Activity of protease, cellulase, and polygalacturonase decreased with increasing temperature. The inability of isolated Pectobacterium to soften host tissues at 37°C may be a consequence of decreased motility and PCWDE activity. These data suggest that future increases in temperature as a result of climate change may affect the population dynamics of Pectobacterium.
Pectobacterium species is one of the most important plant pathogenic bacteria and the causal agent of soft rot disease. Mansfield et al. (2012) placed P. carotovorum and P. atrosepticum (Pa) at the tenth position in the top ten plant pathogenic bacteria based on economic loss. Four species of Erwinia, including E. chrysanthemi, and five subspecies of E. carotovorum were transferred to the Pectobacterium genus by Hauben et al. (1998). Pectobacterium carotovorum subsp. carotovorum (Pcc) is considered as a complex species because its strains show divergent characteristics (Li et al., 2019). Additionally, Pectobacterium is a genus of the soft rot disease complex that also includes Dickeya (Charkowski, 2018). The taxonomic structure of Pectobacterium has been revised with several subspecies being elevated to the species level and new species derived from Pcc and P. wasabiae (Pw) (Gardan et al., 2003; Khayi et al., 2016; Oulghazi et al., 2019; Portier et al., 2019; Shirshikov et al., 2018; Waleron et al., 2018). According to the current classification, Pectobacterium is divided into 18 species: Pcc, Pa, P. aroidearum, P. aquaticum, P. betavasculorum, P. cacticidum, P. fontis, P. parmentieri (Ppa), P. polonicum, P. polaris (Ppo), P. peruviense, P. punjabense, Pw, P. zantedeschiae, P. versatile (Pve), P. odoriferum (Pod), P. brasiliense (Pbr), and P. actinidiae (Brady et al., 2010; Dees et al., 2017; Duarte et al., 2004; Gardan et al., 2003; Khayi et al., 2016; Koh et al., 2012; Nabhan et al., 2012, 2013; Oulghazi et al., 2019; Pédron et al., 2019; Portier et al., 2019; Sarfraz et al., 2018; Waleron et al., 2018, 2019b, 2019c). Of the 18 species, P. fontis, P. aquaticum, P. polonicum, and Pve were isolated from aquatic ecosystems including, groundwater, waterways, waterfalls, and streams.
In South Korea, napa cabbage (Brassica rapa subsp. pekinensis) and potato (Solanum tuberosum) are cultivated in highlands during summer and in lowlands during fall to avoid high temperatures and outbreak of disease. In addition, there is an annual rotation of potato cultivation with napa cabbage cultivation to avoid replant failure as a result of continuous cropping in highland. Recently, the temperature of highlands has been slowly increasing, and soft rot disease incidence is an emerging problem in highland potato cultivation. No et al. (2009) reported a 130 ha field loss of napa cabbage cultivation as a result of Pectobacterium infection. In South Korea, Pcc, Pbr, Pod, and Pa have been reported to cause soft rot disease in various crops including potato and napa cabbage (Lee et al., 2014; Park et al., 1999; Roh et al., 2009; Seo et al., 2004). P. actinidiae was confirmed as a pathogen of canker in kiwifruit (Koh et al., 2012). Of the Pectobacterium species, Pcc is known to be the predominant cause of soft rot disease in South Korea (Kang et al., 2003). However, the taxonomy of Pectobacterium has changed dramatically in recent studies, and the species formerly identified as Pcc and Pw have been reclassified as new species (Khayi et al., 2016; Oulghazi et al., 2019; Waleron et al., 2018). The objective of this study was to identify the Pectobacterium species collected from South Korea based on the current taxonomy.
Materials and Methods
Sample collection
During 2016-2017, disease surveys were conducted to estimate the incidence of soft rot disease in potato, napa cabbage, and radish (Raphanus sativus). Field selection was based on the major area of cultivation for the three crops. For sample collection, infected plant tissues were randomly collected during the growing season from ten growing areas in South Korea: Hongcheon, Gangneung, Pyeongchang, Jeongseon, Yeongwol, Muju, Geochang, Miryang, Boseong, and Jeju. The infected plant tissues were first sterilized with 1% hypochlorite solution for 90 s, then rinsed in distilled water, and then macerated with 1 ml of distilled water. The suspension was streaked on Luria-Bertani (LB; BD, Franklin Lakes, NJ, USA) agar medium to isolate soft rot pathogens. The isolated strains were further cultured in LB broth with shaking at 28°C overnight (16 h). Strains were preserved in 20% glycerol (v/v) at –72°C. For comparison and identification of these isolated strains, we obtained 35 strains from the Microbial Safety Team (MST) and two strains from the National Agrobiodiversity Center (NAC), National Institute of Agricultural Sciences (NAS), Rural Development Administration (RDA).
Isolation of genomic DNA and phylogenetic analysis
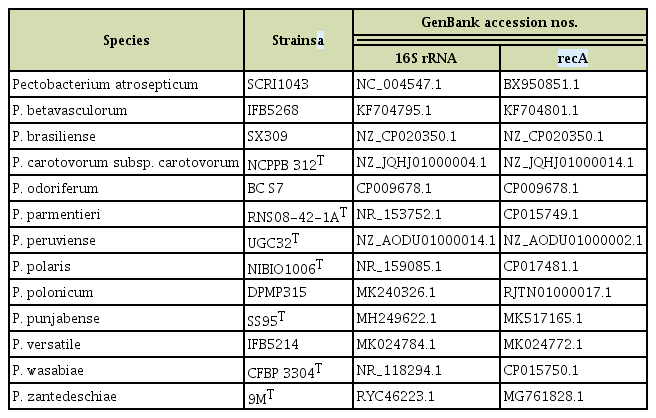
Genomic DNA of all strains was extracted using the Gspin Genomic DNA Extraction Kit (Intron Biotechnology, Seongnam, Korea) according to the manufacturer’s instructions. To identify the strains, the 16S rRNA and recombinase A (recA) gene were sequenced using Macrogen (Seongnam, Korea) with the following primers: 27F, 5′-AGAGTTTGATCMTGGCTCAG-3′; 1492R, 5′-TACGGYTACCTTGTTACGACTT-3′; Ec_recA_F, 5′-GGTAAAGGGTCTATCATGCG-3′; and Ec_recA_ R, 5′-CCTTCACCATACATAATTTGGA-3′ (Lee et al., 2014; Waleron et al., 2002). A multilocus sequence analysis was performed on concatenated sequences of two genes with alignment by ClustalW and 1,000 bootstraps in the MEGA7 program (Kumar et al., 2016; Larkin et al., 2007). Phylogenetic analysis was performed by the neighbor-joining method (Saitou and Nei, 1987). Twenty-four sequences of 13 reference strains were retrieved from NCBI (Table 1).
Biolog and pathogenicity tests
Carbon source usage of the isolated strains was evaluated using Biolog GENIII MicroPlate (Biolog, Hayward, CA, USA), according to the manufacturer’s instructions. To compare changes in pathogenicity by temperature, 40 μl of bacterial suspension was inoculated into holes made using a cork-borer on potato tuber slices and napa cabbage stems. Each plant tissue was incubated for 16 h at four different temperatures—20°C, 28°C, 32°C, and 37°C. The diameter of the macerated tissue was measured.
Determination of cardinal temperatures and motility tests
To confirm cardinal temperatures of Pectobacterium species, the growth of the isolated strains and reference strains were tested over a range of eight temperatures from 10°C to 45°C on tryptic soy agar (BD) media. Bacterial growth was evaluated at 24 h after inoculation, and for two temperatures (10°C and 15°C), growth was evaluated at 48 h. Motility was measured on LB medium with 0.3% agar according to Lee et al. (2013). To test the effect of temperature on motility, each strain was incubated at 24°C, 28°C, 32°C, and 37°C for 24 h.
Pectolytic activity on semi-selective media
To test pectolytic activity at different temperatures, isolated bacteria were cultured in LB media for 16 h at 28°C, and bacterial suspension was obtained by diluting with LB to an optical density of 0.5 at 600 nm, measured using a spectrophotometer (DS-11, DeNovix, Wilmington, DE, USA). As the semi-selective medium, we used a single layer-crystal violet pectate (SL-CVP) medium made using pectin from citrus peel (P9135, Sigma-Aldrich, St. Louis, MO, USA) according to Hélias et al. (2012). Five microliters of each bacterial suspension were inoculated on potato tuber slices and SL-CVP media. The inoculated SL-CVP media were incubated at 28°C for 16 h. The dilution solution was used as the negative control.
Plant cell-wall degrading enzyme (PCWDE) assay
Extracellular enzyme assays including cellulase (Cel), polygalacturonase (Peh), pectate lyase (Pel), and protease (Prt) were performed according to Lee et al. (2013). To compare the effect of temperature on the enzymes, bacterial cells were grown in LB broth at 24°C, 28°C, and 37°C. Supernatants were collected by centrifuging the bacterial cells at 5,800 ×g for 5 min and were used for enzyme assays. Peh, Pel, and Cel activities were measured after 16-18 h of inoculation, and Prt activity was measured after 36 h of inoculation.
Results and Discussion
Disease incidence and isolation of Pectobacterium species
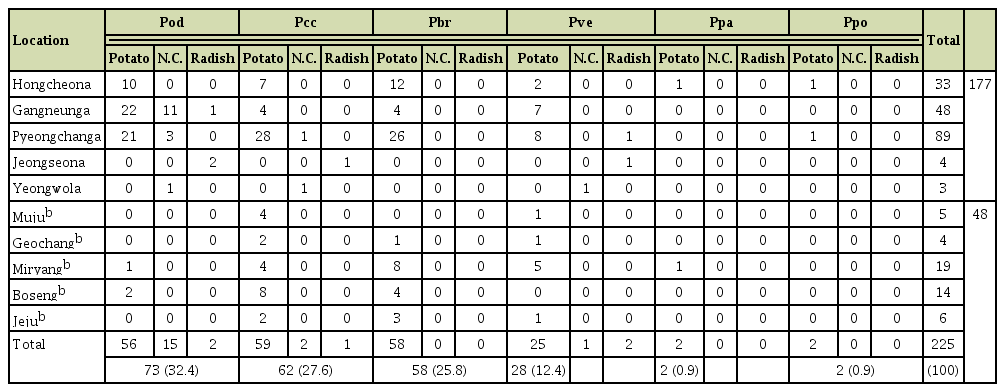
Our nationwide survey of soft rot disease incidence showed higher disease incidence in the highland cultivation during summer than in the lowland cultivation during fall (Fig. 1). Since potato and napa cabbage are cultivated by rotation, leaf and stem samples were collected from both crops to isolate Pectobacterium spp. A total of 225 strains were isolated and analyzed. Based on the 16S rRNA and recA gene analyses, 73 isolates (32.4%) were Pod, 62 (27.6%) were Pcc, 58 (25.8%) were Pbr, and 28 (12.4%) were Pve (Fig. 2). Additionally, two isolates each of Ppo and Ppa were obtained. This is the first report of Ppo in South Korea. In total, four Ppa isolates were identified—one each from the samples collected in Hongcheon and Miryang, respectively, and two from the strains obtained from the MST. Given that the MST samples were collected during 1997-2000 and Ppa strains were identified in this collection, it can be assumed that Ppa isolates have existed in South Korea for at least 20 years. Eight known species of the soft rot disease complex were not present among the 225 isolated strains: Pa, P. betavasculorum, P. peruviense, P. polonicum, P. punjabense, Pw, P. zantedeschiae, and Dickeya sp. All 225 isolates grew well at 37°C in LB medium.
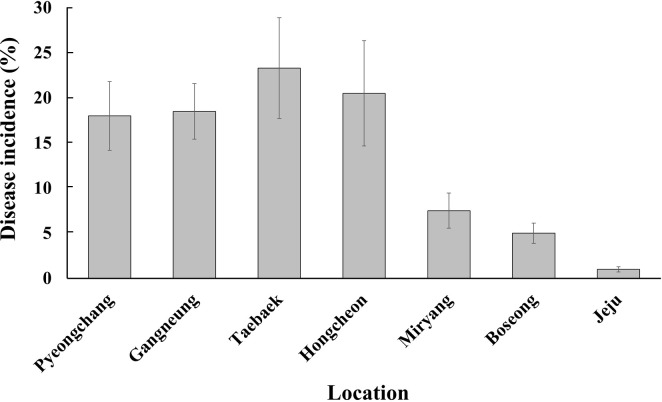
Incidence of soft rot on potato and napa cabbage in seven areas of Korea during 2016-2017. Incidence of soft rot on potato and napa cabbage in Pyeongchang, Gangneung, Taebaek and Hongcheon was evaluated in the summer; in Miryang, Boseong, and Jeju, evaluation was done in the fall.
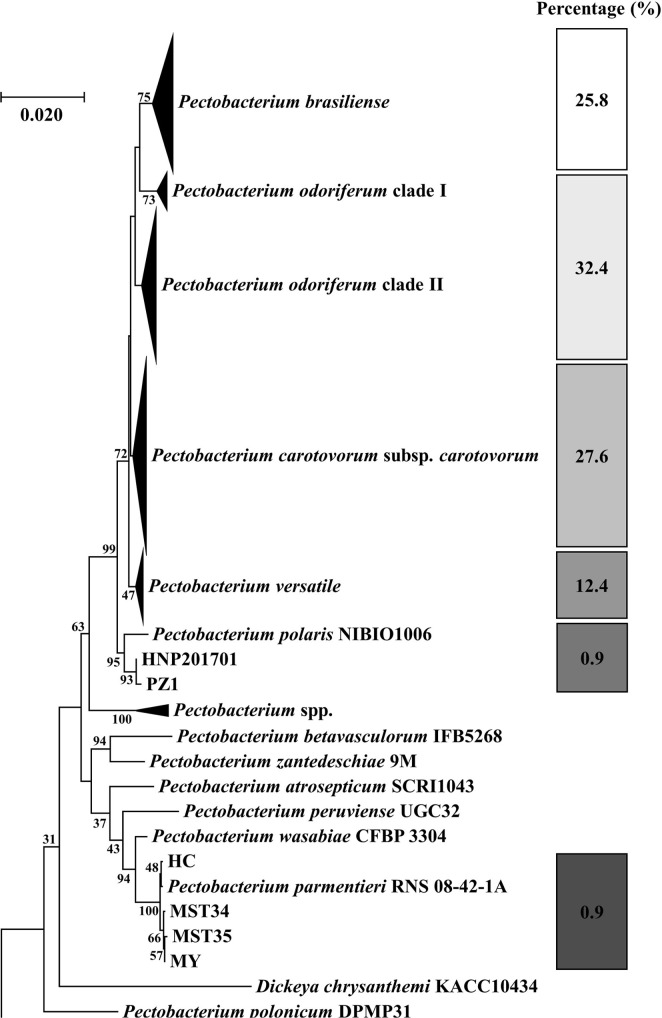
A concatenated neighbor-joining phylogenetic analysis of 225 Pectobacterium strains based on 16S rRNA and recA gene sequences. This analysis was performed with 225 isolated strains and 48 reference strains. This collection was divided into 6 clades including P. odoriferum, P. carotovorum subsp. carotovorum, P. brasiliense, P. versatile, P. parmentieri, and P. polaris.
It is interesting to note that Pa was not isolated from any sample obtained from potato fields in South Korea in this study. Park et al. (1999) reported isolating Pa in South Korea, but we were unable to identify this species in this study. Pa had been considered as a major agent of potato blackleg and soft rot in Europe including Great Britain (Skelsey et al., 2018). However, the prevalence of Pa had decreased by the climate change during two decades except Scotland (van der Wolf et al., 2017). Skelsey et al. (2018) reported that three species including Ppa, Pbr, and Dickeya solani were predominant in Great Britain. The climate of South Korea is known to be a mixed region with temperate regions and subtropical regions (Kwon et al., 2007). Even in temperate climates, it is difficult to isolate Pa (Toth et al., 2003).
Distribution of Pectobacterium species
Pectobacterium spp. were collected from 10 of the main potato, napa cabbage, and radish cultivation areas in South Korea (Table 2, Fig. 1). Of the 225 identified strains, 174 isolates (77.3%) were from summer cultivation and only 48 (21.3%) isolates were from fall cultivation areas. Further, 202 isolates (89.8%) of the 225 strains were from potato plants, and only 18 isolates were from napa cabbage, and 5 were from the radish. However, the potato was the only host from which Pectobacterium was isolated during fall. Temperature may be crucial to the success of Pectobacterium infection. In South Korea, summer cultivation starts from May and ends around August. From the end of June, when the potato plants start vegetative growth, temperature increases to above 25°C and reaches more than 30°C (Supplementary Fig. 1). However, during the fall cultivation of potato plants, the temperature at the start of the cultivation period is above 30°C but decreases to 10°C (Supplementary Fig. 2). Therefore, the temperature during the three months of summer cultivation is good for Pectobacterium activity. However, the decreasing temperature during fall cultivation may be too low for Pectobacterium activity.
Pod, Pcc, Pbr, and Pve were the most commonly found Pectobacterium spp. in samples from potato fields in South Korea. It is interesting to note that although Pod has mostly been considered to be primarily isolated from vegetables including napa cabbage in other countries (Charkowski, 2018), it has been isolated from potatoes in South Korea (Thapa et al., 2011), Algeria (Yahiaoui-Zaidi et al., 2010), and the United States (Ahmed et al., 2018). Also, Waleron et al. (2014) showed that Pod could induce soft rot symptoms on potato in laboratory conditions. In our study, 73 of the 225 isolates were identified as Pod, of which 56 of the 73 isolates were isolated from potato. A biochemical test was performed to verify this, as Pod can ferment sorbitol (Supplementary Table 1). The high population of Pod in potato fields in South Korea may be because of the crop rotation with napa cabbage. Many farmers rotate potato and napa cabbage cultivation to avoid gradual yield decline by continuous cultivation. Residues in the soil from the previous napa cabbage cultivation after tillage could be the inoculum source for the next potato crop. Pve has been reclassified in recent studies, and it has been reported in Europe, North America, North Africa, and Syria (Portier et al., 2019; Shirshikov et al., 2018; Waleron et al., 2019a). This is the first report of Pve in South Korea.
Effects of temperature on pathogenicity of Pectobacterium
At 20°C, very little maceration of potato was observed, whereas no maceration was observed in napa cabbage (Fig. 3). The maceration of potato and napa cabbage varied slightly but was not significantly different between 28°C and 32°C. Interestingly, no maceration was observed on either potato tuber slices or napa cabbage stems at 37°C. No difference in maceration was noted with respect to the host plant of the isolates. The strains isolated from potato or napa cabbage showed the same maceration ability on potato and napa cabbage at 28°C. In addition, pathogenicity was found to differ significantly: Pod showed the strongest activity in the maceration of potato and napa cabbage, and Ppa showed the weakest activity. Little is known about Pectobacterium response to environmental factors such as temperature. Pa, Ppa, and Pw mainly cause disease at 18-22°C and cannot grow at 37°C (Charkowski, 2018). In this study, Ppa showed a similar response at 37°C (Fig. 3, Supplementary Fig. 3). In a growth test on media, multiple comparisons between Pcc, Pa, and D. solani at four different temperatures (18°C, 22°C, 26°C, and 30°C) revealed a slower Pa growth in 26°C and 30°C treatment conditions; in contrast, the growth of Pcc and D. solani increased as the temperature increased (Lebecka et al., 2018). Czajkowski et al. (2017) suggested that several genes in D. solani are temperature-regulated, perhaps more are upregulated at higher temperatures than at lower temperatures, and affect the expression of the virulence factors, which differs from previous results. This is thought to be a result of adaptation of D. solani to higher temperatures in Europe (Czajkowski et al., 2017). This study suggests that Pectobacterium species could also have such temperature-regulated genes that affect multiplication; the thermal response could, hence, be different among different species.
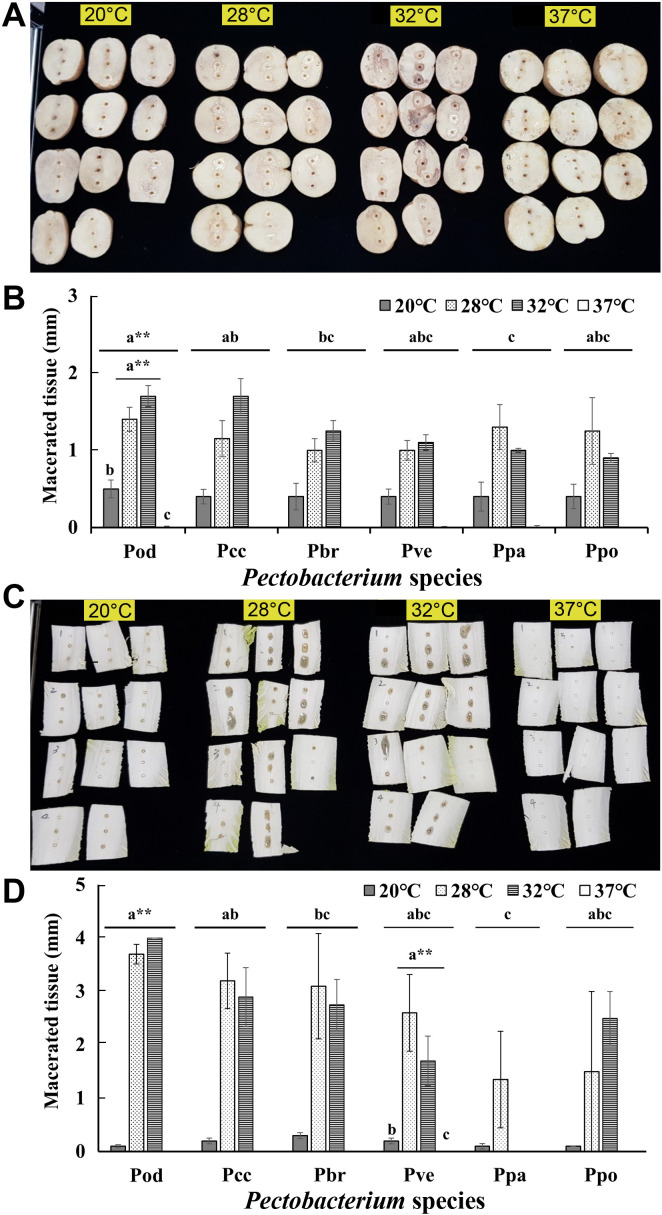
The diameter of macerated tissue in potato and napa cabbage inoculated with Pectobacterium species. The inoculated tissues of potato (A, B) and napa cabbage (C, D) were incubated at four different temperatures: 20°C, 28°C, 32°C, and 37°C. Pod, Pectobacterium odoriferum; Pcc, P. carotovorum subsp. carotovorum; Pbr, P. brasiliense; Pve, P. versatile; Ppa, P. parmentieri; Ppo, P. polaris; Pa, P. atrosepticum. Different letters indicate significant difference according to ANOVA with Duncan test at **P < 0.01.
Effects of temperature on motility and pectolytic activity of Pectobacterium
The pathogenicity was highest at 28°C and 32°C, and no pathogenicity was observed at 37°C. In order to investigate whether temperature affected the viability of the bacterium or its pectolytic activity as the key factor for soft rot pathogenicity, we assessed the swimming motility and pectolytic activity of the isolated bacteria. The swimming motility of Pod, Pcc, and Pbr was stable up to 32°C but markedly reduced at 37°C; however, the motility of Pve, Ppa, and Ppo reduced gradually with increasing temperature (Fig. 4). Therefore, the reduced swimming motility of Pectobacterium at 37°C may be why pathogenicity was not observed at 37°C. The swimming motility of the reference Pa strain was low even at 24°C and almost disappeared at 28°C, which is the optimum temperature for the other six Pectobacterium spp. isolated from potato in this study. This may explain why Pa is rarely isolated from potato fields in South Korea. Comparison of swimming motility between three Dickeya species also showed higher impact of temperature than species and geographical origin (Golanowska et al., 2017). This study, in addition to Golanowska et al. (2017), has identified that temperature plays an important role in swimming motility.

Comparison of swimming motility of Pectobacterium species at four different temperatures. Pod, Pectobacterium odoriferum; Pcc, P. carotovorum subsp. carotovorum; Pbr, P. brasiliense; Pve, P. versatile; Ppa, P. parmentieri; Ppo, P. polaris; Pa, P. atrosepticum. Different letters indicate significant difference according to ANOVA with Duncan test at **P < 0.01. This test was performed with 7 Pectobacterium species including a reference strain of P. atrosepticum.
The effect of temperature on the pectolytic activity was investigated using SL-CVP medium (Fig. 5). In contrast to the swimming motility, the pectolytic activity of Pectobacterium decreased at 37°C but not markedly, except for Ppo. The pectolytic activity at 37°C of all Pectobacterium isolates was 20-30% lower than that at 28°C. Pectolytic activity is defined as the maceration ability involving several pectolytic enzymes. The Prt activity of the six Pectobacterium species decreased gradually as temperature increased (Fig. 6). None of the species showed Prt activity at 37°C. All tested bacteria, except Pod, lost their Cel activity at 37°C. The activities of Cel, Pel, and Peh were not significantly different between 24°C and 28°C. The Peh activity of Pcc, Pbr, and Ppo at 37°C was half of that at 28°C, but Pel activity showed no change except for Ppo. It is well known that PCWDEs are controlled by quorum sensing (QS), and the QS signal molecule, N-(3-oxohexanoyl)-lhomoserine lactone, is not detectable at 37°C (Hasegawa et al., 2005; Põllumaa et al., 2012; Saha et al., 2015). Golanowska et al. (2017) showed that Cel activity of Dickeya species was mainly regulated by temperature, but there was a larger effect of species on pectinase and Prt activities. In this study, Cel and Prt appeared to be strongly affected by high temperature, but Pel and Peh were only slightly affected by both species and temperature.
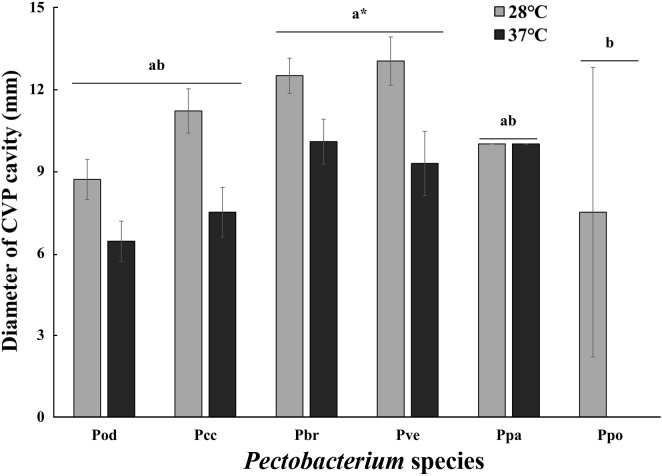
Comparison of pectolytic activity with Pectobacterium species at 28°C and 37°C. The assessment of pectolytic activity was performed with diameter of a cavity on crystal violet pectate (CVP) medium. Different letters indicate significant difference according to ANOVA with Duncan test at *P < 0.05.
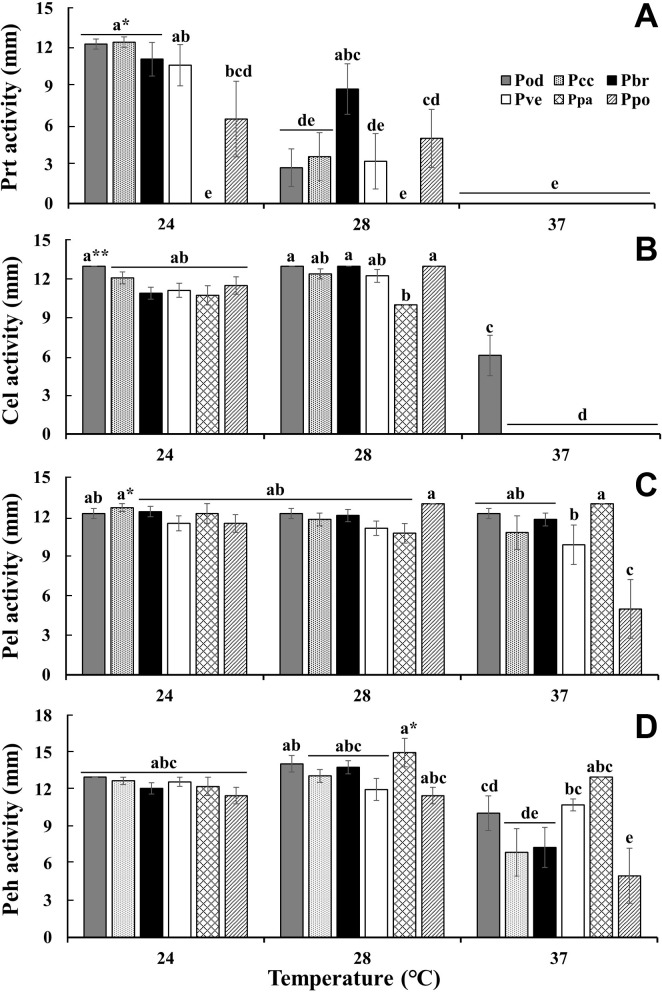
Comparison of plant cell-wall degrading enzyme activity of Pectobacterium species at 24°C, 28°C and 37°C. The diameter of halo response was measured for each enzyme activity: protease (Prt) (A), cellulase (Cel) (B), pectate lyase (Pel) (C), and polygalacturonase (Peh) (D). Pod, P. odoriferum; Pcc, P. carotovorum subsp. carotovorum; Pbr, P. brasiliense; Pve, P. versatile; Ppa, P. parmentieri; Ppo, P. polaris. Different letters indicate significant difference between species in an enzyme activity test according to ANOVA with Duncan test at *P < 0.05 or **P < 0.01.
Our results showed that Pod, Pcc, Pbr, and Pve were the most prevalent Pectobacterium species isolated from potato and napa cabbage fields in South Korea. In addition to these four species, two isolates each of Ppa and Ppo were isolated. Although Pod is usually isolated from vegetables in South Korea, the most isolated Pectobacterium strains in potato fields were identified as Pod rather than Pcc or Pbr as in other countries (Duarte et al., 2004). The high prevalence of Pod may be caused by crop rotation of potato with napa cabbage in South Korea; further studies are required to verify this. The high potato cultivation temperature may limit the growth of P. atrosepticum in Korea. In a previous study, pathogenicity was lost in both the mutants of flagella, the major organs associated with motility, and the mutants of the QS related gene (Lee et al., 2013). Considering this, it is suggested that the pathogenicity loss of isolated Pectobacterium at 37°C was caused by loss of motility and PCWDE activity at 37°C. These data suggest that future increases in temperature as a result of climate change can change the population dynamics of Pectobacterium.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Acknowledgments
This research was supported by the Rural Development Administration (RDA) fund PJ01127802.