Epidemiology and Control of Strawberry Bacterial Angular Leaf Spot Disease Caused by Xanthomonas fragariae
Article information
Abstract
Strawberry bacterial angular leaf spot (ALS) disease, caused by Xanthomonas fragariae has become increasingly problematic in the strawberry agro-industry. ALS causes small angular water-soaked lesions to develop on the abaxial leaf surface. Studies reported optimum temperature conditions for X. fragariae are 20°C and the pathogen suffers mortality above 32°C. However, at the nursery stage, disease symptoms have been observed under high temperature conditions. In the present study, results showed X. fragariae transmission was via infected maternal plants, precipitation, and sprinkler irrigation systems. Systemic infections were detected using X. fragariae specific primers 245A/B and 295A/B, where 300-bp and 615-bp were respectively amplified. During the nursery stage (from May to August), the pathogen was PCR detected only in maternal plants, but not in soil or irrigation water through the nursery stage. During the cultivation period, from September to March, the pathogen was detected in maternal plants, progeny, and soil, but not in water. Additionally, un-infected plants, when planted with infected plants were positive for X. fragariae via PCR at the late cultivation stage. Chemical control for X. fragariae with oxolinic acid showed 87% control effects against the disease during the nursery period, in contrast to validamycin-A, which exhibited increased efficacy against the disease during the cultivation stage (control effect 95%). To our knowledge, this is the first epidemiological study of X. fragariae in Korean strawberry fields.
Introduction
Strawberry (Fragaria × ananassa Duch.) is cultivated worldwide and is part of the major fruit industry for several countries. Various diseases cause economic damage and these disease problems have increased exponentially (Amil-Ruiz et al., 2011). Strawberry bacterial angular leaf spot (ALS) disease (Turechek et al., 2013), caused by Xanthomonas fragariae (Kennedy and King, 1962a) was first reported in Minnesota in 1959 (Roberts et al., 1996). X. fragariae can result in up to 10% loss in strawberry yield (Mass et al., 1995), but generally the disease does not result in host plant mortality. However, X. fragariae was classified as an A2 level quarantine pathogen by the European Plant Protection Organization (CABI/EPPO, 1998) and the Korean Plant Quarantine Agency. In 2010, the disease was reported in Jinju, Korea and disease incidence and severity continue to increase throughout the country (Kwon et al., 2010).
Roberts (1996) reported epidemiological and pathological characters of the ALS pathogen. ALS is a slow growing, gram-negative bacterium, possessing one flagellum (Rowhani et al., 2009). The pathogen exhibits a relatively few host range (Vandroemme et al., 2013). Early disease symptoms are caused by water soaked leaves, resulting in the development of angular spots on the abaxial leaf surface (Janse et al., 2001), and later stage symptoms appear as reddish irregular spots on the upper leaf surface (Zimmermann et al., 2004). Moist conditions are optimal for the pathogen, during which a yellow bacterial exudate is produced on the lower leaf surface and calyces (Allan-Wojtas et al., 2010).
Kennedy and King (1962b) showed the pathogen spreads through irrigation water or precipitation and is a typical soil invader. Turechek et al. (2013) reported the ALS bacteria survived during the nursery stage exhibiting symptoms or was fully asymptomatic. Furthermore, pathogen populations were maintained in maternal plant tissues, and pathogen infection was transmitted from the maternal parent to offspring during asexual clonal reproduction. The maternal plant generates nursery plants via a stoloniferous stem, where infection occurs from maternal to new nursery plants (Turechek and Peres, 2009). Zimmermann et al. (2004) indicated the widespread application of copper containing bactericides to control ALS at strawberry nurseries. Furthermore, specific primer pairs (241/241, 245A/245B and 295A/295B) were reported to detect the ALS pathogen and the PCR detection limit was 104–105 bacterial cells (Pooler et al., 1996; Stoger and Ruppitsch, 2004; Turechek et al., 2008; Vandroemme et al., 2008).
The present study served to elucidate X. fragariae transmission and survival resulting in the presence or absence of ALS development in Fragaria × ananassa. X. fragariae PCR specific primers were employed to examine ALS pathogen epidemiology in Korean strawberry agrosystems. The two PCR primer pairs 245A/B and 295A/B were designed to specifically identify X. fragariae gene regions (Pooler et al., 1996) and bacterial ALS pathogens in plants, soil, and irrigation water. Samples were collected during the 2013–2014 strawberry nursery and cultivation periods. Additionally, we tested two antibacterial agents validamycin-A and oxolinic acid, which were selected to determine bactericide efficacy against ALS disease (Kim et al., 2015). Validamycin-A is a bacteriostatic agent, which acts to arrest or cease bacterial growth. Oxolinic acid is a quinolone type bactericide, which inhibits bacterial cell division and proliferation (Shungu et al., 1983). We hypothesized that to control strawberry ALS disease, appropriate bactericides should be applied with Integrated Pest Management (IPM) level protocols, including application to disease free plants; administer soil fumigation; improve irrigation; and protect cultivation systems. These cumulative measures should result in effective strawberry X. fragariae ALS control.
Materials and Methods
Bacterial pathogen isolation from infected plants
Year of 2013–2015, cultivated strawberry fields in Jinju, South Korea, infected strawberry exhibited ALS symptoms with bacterial exudate on abaxial leaf surfaces. Infected specimens were obtained by cutting leaf pieces and placing samples in 5 ml distilled water. After 4–5 hours incubation at room temperature, the mixture was centrifuged at 10,000 rpm for 5 minutes. The pellet was re-suspended in 1 ml distilled water and subsequently diluted in 9 ml distilled water. The protocol was repeated 8 times. The diluted liquid was plated at 103, 10, 105, and 106 on yeast extract-dextrose-calcium carbonate medium (YDC media; 10 g of yeast extract, 20 g of dextrose glucose, 20 g of calcium carbonate, 15 g of agar per 1 l) and incubated at 20°C for 5 days (Zimmermann et al., 2004). Single colonies were re-streaked on YDC media and incubated at 20°C for 5 days to obtain pure cultures.
Total DNA extraction from plant and soil samples
Strawberry leaves (1 g) were placed in 9 ml distilled water and ground 5 times to extract bacterial exudate and endophytic bacteria. After 5 hours, 3 ml extracted solution was centrifuged and the pellet re-suspended by 500 μl cetyltrimethyl ammonium bromide (CTAB) buffer; 8 μl (20 mg/ml) of proteinase K buffer was subsequently added. The mixture was incubated at 65°C for 30 minutes. Samples were cooled and an equal volume of PCI (phenol:chloroform:isoamyl alcohol, 24:25:1) was added and vortexed for 1 minute, then centrifuged at 13,000 rpm at 4°C for 10 minutes. Supernatants were transferred to 1.5 ml new eppendorf tubes and 450 μl of isopropanol was added and gently mixed. The samples were centrifuged at 13,000 rpm at 4°C for 5 minutes. Supernatant was removed by washing with 500 μl of 70% ethanol (Cho et al., 2016). Ethanol was subsequently removed and pellets dried at room temperature for 30 minutes. The pellet was dissolved in 50 μl of Tris-EDTA (TE) buffer. Genomic DNA was stored at −20°C until needed for PCR amplifications (Fatima et al., 2011).
Total DNA in the soil was extracted using Fast DNA™ Spin Kit (QBiogene/MP Biomedicals, Solon, OH, USA) according to the manufacturer’s instruction. The DNA samples were stored at −80°C until needed in PCR amplifications.
PCR detection of X. fragariae from strawberry plants, soil, and water
X. fragariae PCR detection required 1 μl of total DNA (100 ng/μl) and 1 μl water for the PCR template. PCR was performed using the following 20 μl volume reaction mixture: 200 μM deoxynucleoside triphosphate, 2 μl 10 × buffer, 10 pmol forward primer (245A or 295A),10 pmol reverse primer (245B or 295B), 1 μl of DNA template, and 0.5 U of Taq DNA polymerase (Bioneer, Daejeon, Korea). PCR conditions were as follows: initial denaturation step at 95°C for 4 minutes; 95°C for 30 seconds (denaturation); 58°C for 30 seconds (annealing); 35 cycles at 72°C for 30 seconds (elongation); and 72°C for 4 minutes (final extension step). Following PCR, amplicons were loaded on 1% agarose gels for the nursery stage and 3% agarose gels for the cultivation stage samples. DNA ladders (1-kb Plus DNA Ladder Marker and 100-bp DNA Ladder Marker, DOCTOR PROTEIN; MGmed Inc., Seoul, Korea) were loaded on the left lane, and gels were run in 1 × TAE buffer at 150 V for 40 minutes. Following electrophoresis, the agarose gels were stained with 1% ethidium bromide.
Bactericide efficacy to control ALS disease
Field tests were conducted via plant bactericide application in June through August during the nursery stage and September through March during the cultivation stage in each year. The efficacy of two bactericides (validamycin-A 1 ml/l and oxolinic acid 1 g/l) was tested for both stages. In both nursery and cultivation stages, each field size was 660 m2 and each field had three blocks, which designed completely randomized block design. A single block size was 20 m length, it contained 100 strawberry plants. Each bactericide was applied to plants three times with 10 days between applications. Disease control effects were recorded 10 days after the final application. Control effects were evaluated using the following disease index: 0 = absence of symptoms; 1 = one leaf water soaked; 2 = slight chlorosis or necrosis under leaves; 3 = water soaked expansion and bacterial exudate often observed; 4 = leaf surface color change to reddish; and 5 = total necrosis (Lewers et al., 2003). Disease severities and bactericide control value were analyzed with analysis of variance (ANOVA) and means were divided by using Tukey’s honest significant difference (HSD) test (P = 0.05) (Statistix 9.0; Analytical Software, St. Paul, MN, USA).
In year of 2013, total 4 strawberry cultivation fields were designed; (i) infected maternal plants with soil fumigation, (ii) infected maternal plants without soil fumigation, (iii) healthy maternal plants with soil fumigation, and (iv) healthy maternal plants without soil fumigation. Experimental field conditions included the following four types of nursery fields, 2014: (i) field A (cultivated in soil, overhead irrigation, infected maternal plants); (ii) field B (cultivated in soil, overhead irrigation, mixed disease-free and ALS infected maternal plants); (iii) field C (rainproof, elevated production, overhead irrigation, non-infected maternal plants); and field D (rainproof, elevated production, drip irrigation, non-infected maternal plants). Three commercial greenhouse field types were used in the cultivation stage. 2014: (i) field A (infected maternal plants transferred to the field); (ii) field B (mixed disease free and ALS infected maternal plants transferred to the field); and (iii) field C (disease free maternal plants transferred to the field). All plants were cultivated with the same soil culture and drip irrigation system under greenhouse conditions. Strawberry cultivation season was September 2014 through March 2015.
To confirm disease control effect of oxolinic acid, fields were designed as following condition at nursery stage in 2015, A (cultivated in soil, overhead irrigation, infected maternal plants), B (elevated production, overhead irrigation, mixed disease-free and infected maternal plants), and C (elevated production, overhead irrigation, non-infected maternal plants).
Results
Bacterial pathogen isolation
Typical ALS disease symptoms were observed under field conditions, including water soaked lesions, leaf color change from green to reddish, and developing necrosis in severe cases (Fig. 1A, B). High relative humidity resulted in yellowish exudate from the abaxial leaf surface of infected plants (Fig. 1C). X. fragariae was isolated from infected strawberry plants and colonies showed typical morphological characteristics, including yellow color, glossy smooth surface, and sticky colonies on YDC media (Fig. 1D). The isolated pathogen exhibited typical ALS disease symptoms under laboratory conditions (data not shown). Therefore, we successfully field collected from Fragaria × ananassa and subsequently isolated pure X. fragariae cultures in the laboratory.
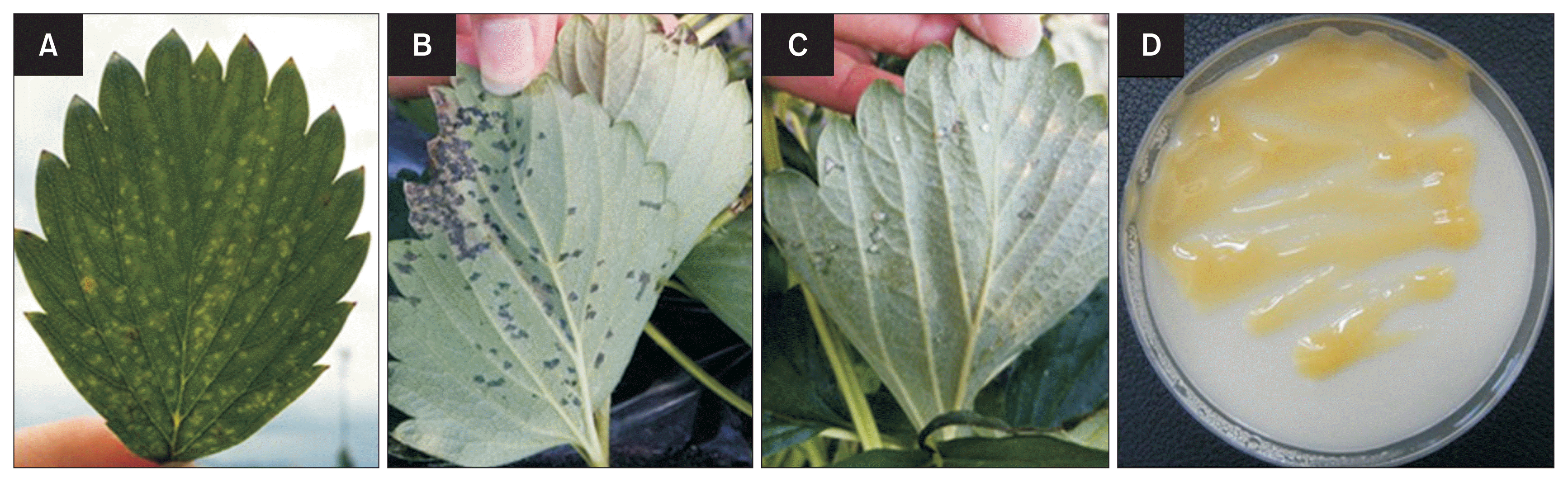
Xanthomonas fragariae colonies and angular leaf spot disease symptoms. (A) Angular leaf spot (ALS) symptoms in early stages. (B) ALS late stage disease symptoms, color change to reddish with necrosis development. (C) Pathogen exudate from abaxial leaf surface. (D) X. fragariae colony morphological characteristics on YDC media. The colony was grown at 20°C for 5 days.
Rapid PCR detection of X. fragariae in plants, soil, and water
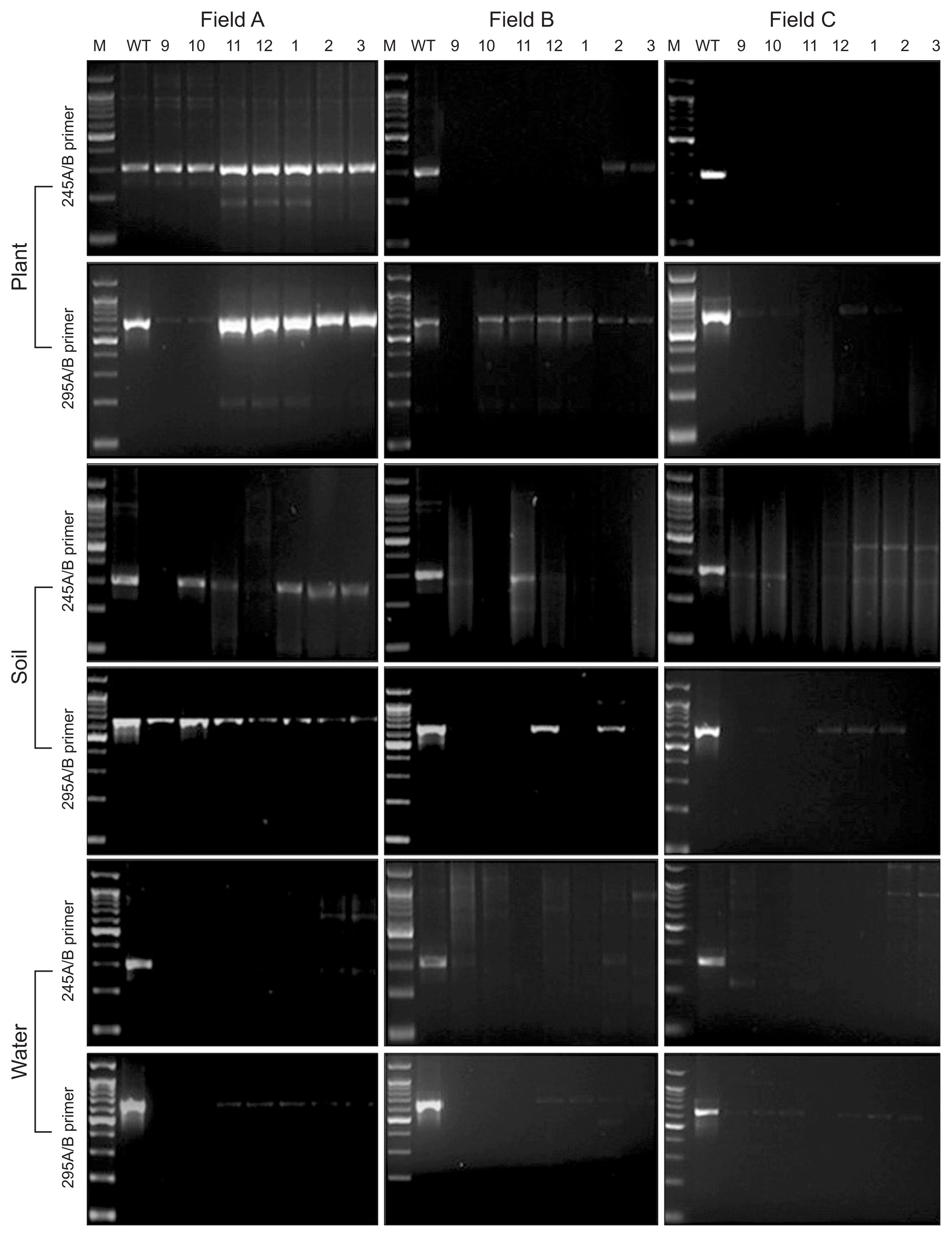
PCR amplicons 300 bp and 615 bp were generated with the standard isolate by primer sets 245A and 245B, 295A and 295B, respectively. To detect and investigate infection route the ALS pathogen, plant, irrigation water and soil samples were collected every month from September to March in 2013.
Plants in field A and B showed strong PCR reactions with the both primer set through entire season. PCR reaction with plant in field C generated relatively weak amplicon only in February and March. Field D showed also weak PCR signal from November to March (Fig. 2). However, ALS disease symptom was not observed in field C and D during the period. Soil sample in field A (soil fumigated with infected plant), C (soil fumigated with non-infected plant) and D (no-soil fumigated with no-infected plant) showed negative reaction with ALS detective PCR. However, field B (no-soil fumigated with infected plant) response to the PCR protocol in October sample (Fig. 2). In irrigation water samples, which were collected in year of 2013 (field C and D used same source of the water), unspecific bands were showed in all samples. But there was no ALS specific band detected (Fig. 2).

Xanthomonas fragariae PCR detection in the cultivation stage in 2013–2014. M, 1-kb ladder maker; WT, X. fragariae genomic DNA. Lanes 9–3 indicate sampling month from September 2013 to March 2014.
The primer pairs 245A/B and 295A/B (Table 1) were used to detect X. fragariae from strawberry plants, soil, and water. The two primer pairs were specific to amplify regions of X. fragariae genomic DNA; each amplicon was 300 bp and 615 bp. Field A used an infected maternal plant, which was supplied from an infected nursery field; fields B and C were the source of disease-free maternal plants. X. fragariae was PCR tracked throughout the entire strawberry field-growing season to elucidate the ecology and epidemiology of the strawberry ALS pathogen, including the nursery (May to August) and cultivation (September to March) periods. During the nursery period, the pathogen was not detected in soil and water, regardless of field conditions. However, strawberry plants in fields A and B showed positive PCR reactions with the pathogen detection primers during the nursery period (Fig. 3). Interestingly, plants in field B did not exhibit disease symptoms (data not shown).
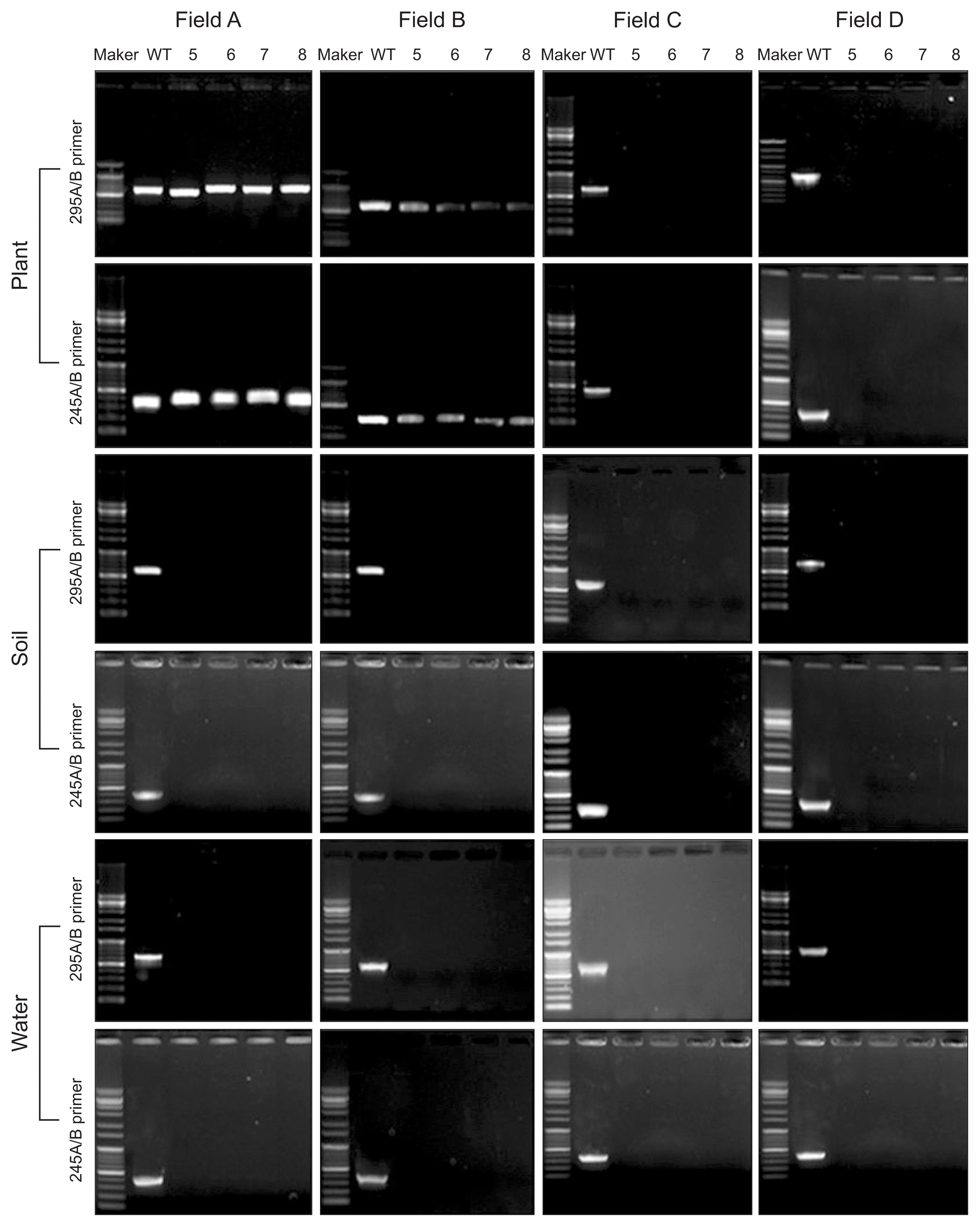
Xanthomonas fragariae PCR detection in the nursery stage in year of 2014. M, 1-kb ladder maker; WT, X. fragariae genomic DNA; PCR performed with X. fragariae genomic DNA. Lanes 5–8 indicate May through August during the nursery stage.
During the cultivation period, the pathogen was detected in fields A and B by PCR. Infected maternal plants were grown in field A, supplied from an infected nursery field; field B plants were a mixed maternal group (i.e., 50% infected and 50% disease-free); and field C were disease-free maternal plants. Primer 245A/B detected the pathogen in field A, but not B; primer 295A/B showed a positive reaction in fields A and B. In field A, the pathogen was consistently detected in soil with both primer sets. However in field B, PCR was inconsistent in pathogen recognition. Field C, a disease-free field, showed negative X. fragariae PCR results. All field PCR irrigation water samples exhibited negative results with both primers (Fig. 4).
Bactericide efficacy to control angular leaf spot disease
During cultivation period in 2013, validamycin-A only 7% and oxolinic acid showed 16% the ALS disease incidence at field A. Similar result showed at field B, validamycin-A and oxolinic acid showed 5% and 18% the disease incidences, respectively. No ALS disease was observed in both field C and D (both no-infected plant) (Fig. 5). Disease control effect of the both pesticides in the cultivation stage in 2013, 92.6–94.3% for validamycin-A and 81.6–83.6% for oxolinic acid were recorded (Fig. 5).
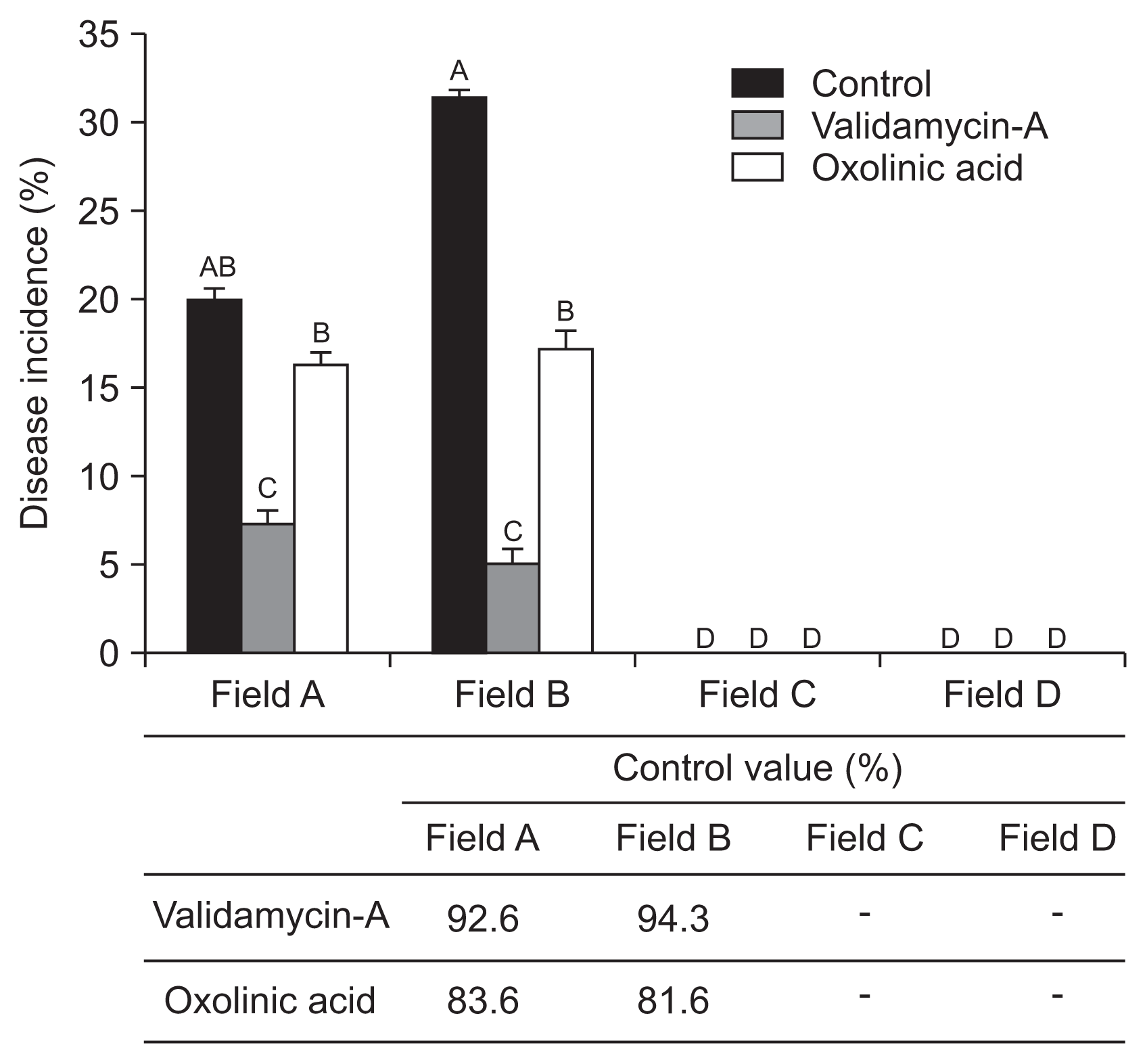
Angular leaf spot disease control efficiencies of oxolinic acid and validamycin-A at strawberry cultivation stage in 2013. The bactericides were applied in October for three times with 10-day interval. Statistical analysis performed with Tukey’s honest significant difference (HSD) test (P = 0.05) and the letter on the bar represent significant difference.
The reduction of strawberry ALS disease risk was tested by chemical application of two bactericides. Results showed notably different disease severity and disease control values depended on bactericide type and cropping stage. At the nursery stage, untreated control fields A and B showed high disease incidence, i.e., 40% and 20%, respectively. In comparison, bactricides treatments were low, under 10% and 0% respectively for fields A and B (Fig. 6). Oxolinic acid (1 g/l) showed 86%, 95%, and 80% control efficiency in fields A, B, and C, respectively. Validamycin-A exhibited 72%, 86%, and 0% disease control effects for fields A, B, and C, respectively. During the cultivation stage, field A (infected maternal plants) showed 9% disease incidence. Validamycin-A and oxolinic acid exhibited 96% and 70% control effects, respectively. In field B (with 6% overall disease incidence), validamycin-A exhibited 98% and oxolinic acid 57% control effects (Fig. 7). Interestingly, results indicated oxolinic acid was more effective at the nursery stage and validamycin-A exhibited marked effects at disease control during the cultivation stage.
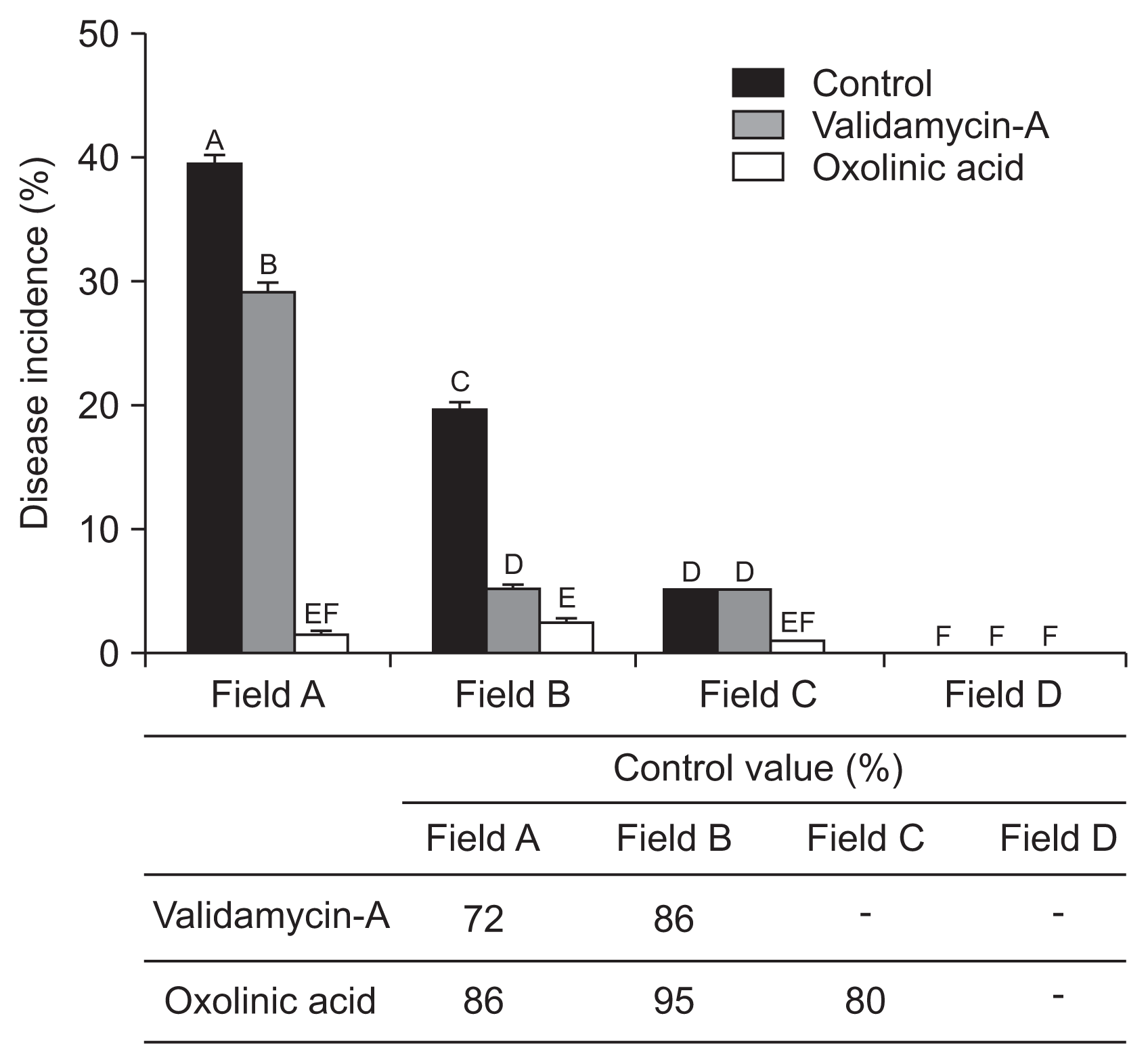
Angular leaf spot disease severity and control value in the nursery stage. Each bactericide was applied to plants three times with 10-day intervals. Disease control values were recorded 10 days after the final application. Different letters indicate statistically significant differences using Tukey’s honest significant difference (HSD) test (P = 0.05).
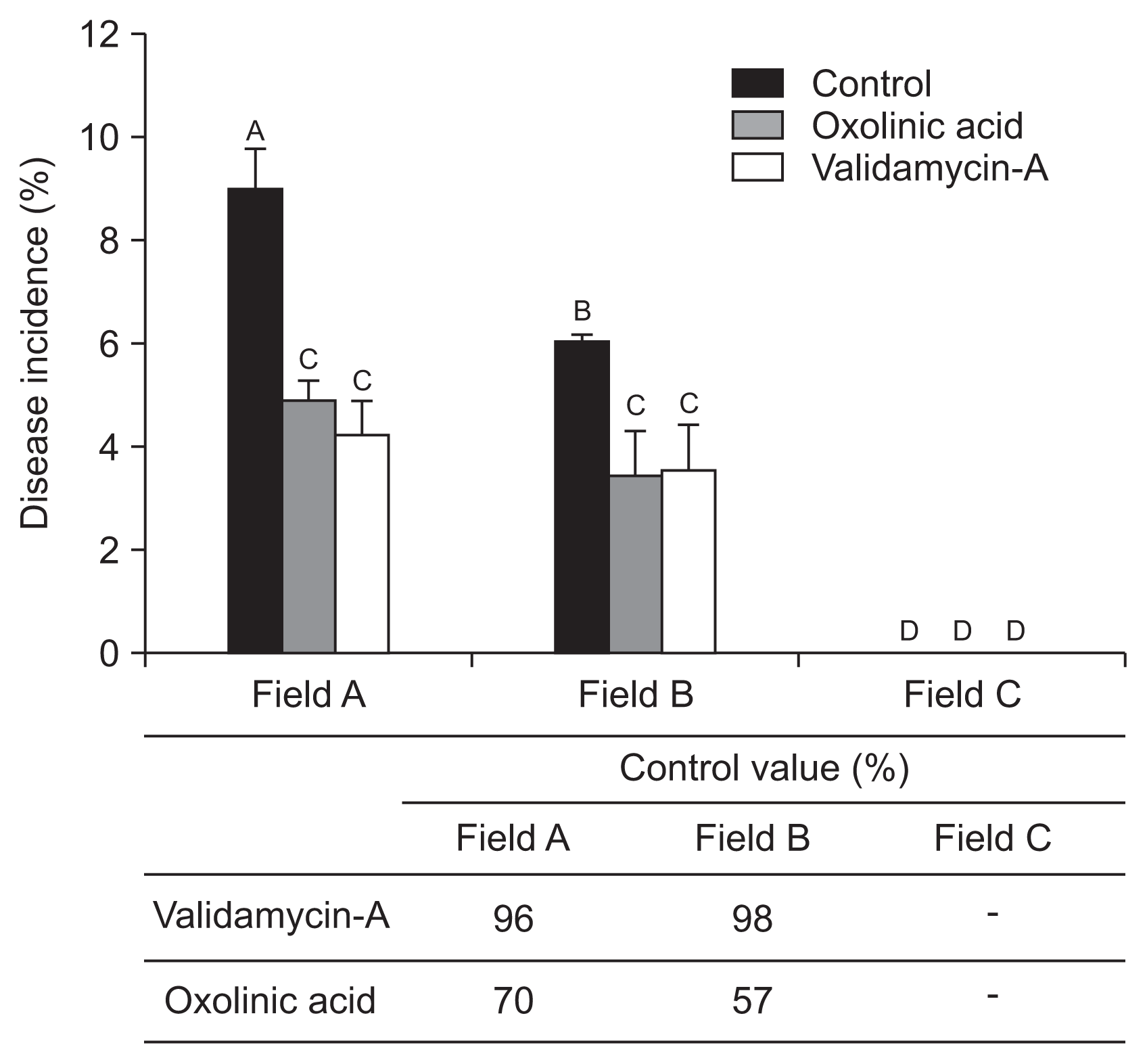
Bactericide application effects to control angular leaf spot disease during the cultivation stage. The bactericides were applied to plants three times with 10-day intervals. Different letters represent statistically significant differences using ANOVA (Tukey’s honest significant difference [HSD] test, P = 0.05).
To confirm the disease control effect of oxolinic acid in the nursery stage, in 2015 the bactericide was applied on field A and B. After 10 days of treatment, ALS disease incidences were 67% and 1.6% for untreated and treated plot in field A. In field B, 56% and 2% disease incidence for untreated and treated the bactericide were observed (Fig. 8). The ALS disease control efficiency of oxolinic acid was 97–98% in 2013 (Fig. 8).
Discussion
X. fragariae, the causal agent of strawberry bacterial ALS disease, reportedly spreads through irrigation water and precipitation and is a typical soil invasion type pathogen (Turechek and Peres, 2009). In the present study, we identified and characterized X. fragariae epidemiological traits and determined non-copper bactericides more effective in pathogen control. Epidemiologically, we found the nursery stage resulted in increased pathogen survival. Bestfleisch et al. (2015) characterized the transfer of X. fragariae from infected maternal plants to daughter plants as the primary inoculum. The infected pathogen presence in cultivation facilities is a major secondary pathogen source.
During the nursery stage (2014 year), disease-free and ALS infected plants were present in field B (mixed sample) and PCR detection results showed even disease-free maternal plants were rapidly infected by the ALS pathogen (Fig. 3). However, PCR failed to detect X. fragariae in field B soil and irrigation water. In contrast, during the cultivation stage, the ALS pathogen was PCR confirmed from plants and soils in field B (Fig. 4). The result indicated the pathogen survived as a soil inhabitant and the pathogen actively re-infected healthy plants during disease cycles. Clearly, the pathogen was not detected in water during nursery and cultivation stages, which demonstrated pathogen transmission does not occur by irrigation water itself. Therefore, the water source or disinfecting irrigation water is not a requirement to control ALS disease. During non-cultivation periods, X. fragariae’s biology does not support pathogen survival under soil conditions. The ALS pathogen is a weak competitive saprophytic bacterium and Roberts (1996) demonstrated X. fragariae can only survive short term without a host plant. However, in 2013 during the cultivation stage, field D (no-soil fumigated with no infected plants) showed weak PCR reaction since November even no ALS symptom was observed (Fig. 2). The result suggested that the pathogen may persistence or survive in soil with previous plant debris. Soil fumigation has not direct effect to control ALS disease, but it may be necessary treatment to eradication the disease in Koran strawberry fields.
In addition, fields A and B (cultivation stage, 2014) were positive for the presence of X. fragariae via PCR. This result indicated the ALS pathogen survived in strawberry field soils. We suggest pathogen survival was dependent on the agrosystem and the strawberry cultivars grown in Korea’s greenhouse systems. The conditions provided a suitable environment for the pathogen; the strawberry agrosystem from nursery to cultivation stages included an intensive agricultural system, and allowed the pathogen to survive and disseminate. In field B soil, PCR revealed an irregular X. fragariae distribution pattern, where the pathogen was not evenly distributed throughout the field (Fig. 4). Low-density X. fragariae were identified in parts of field B and other areas of the field exhibited high-density pathogen populations. This might be explained by the abaxial leaf surface infection exudate becoming a soil component. Disease-free plants were cultivated in field C and the pathogen was not observed in plants, soil, and water. The result strongly suggested the most important factor to prevent ALS disease was planting disease-free plants.
In general, copper-containing bactericides are effective against bacterial disease, however the two bactericides tested in the present study failed to control ALS disease caused by X. fragariae (Alippi et al., 1989). Recently, validamycin-A, oxolinic acid, kasugamycin, streptomycin, streptomucin/valinamycin-A, oxolinic acid/streptomycin, copper oxychloride/kasugamycin, and polyoxin D/kasugamycin were applied to test their efficacy against X. fragariae ALS (Kim et al., 2015). We applied oxolinic acid and validamycin-A to commercial strawberry nurseries and cultivation fields to examine disease control. Validamycin-A is an antibiotic and the bactericide mode of action is a bacteriostatic agent, which reduces bacterial growth (Stall and Thayer, 1962). Oxolinic acid is a quinolone type bactericide, which impedes cell division and pathogenic bacteria proliferation (Shungu et al., 1983). In the nursery stage (2014 and 2015), oxolinic acid showed promising control efficiency (~86–97% control effects compared to un-treated plants; Fig. 6, 8). Yukiko et al. (2004) first described the widespread use of oxolinic acid to control rice bacterial blight disease. In strawberry cultivation fields (2013 and 2014), validamycin-A showed increased efficacy against the disease (~92.6–98% control efficiency; Fig. 5, 7). Different disease control efficiencies between the two pesticides might be due to varied chemical properties and interactions with environmental parameters. During the nursery stage (May to August), air temperatures are relatively high compared to the cultivation period, a condition unfavorable to X. fragariae. Most bactericides showed restrictive effects against target pathogens under certain temperatures and other climatic conditions (Stall and Thayer, 1962). The strawberry cultivation period (September through April), under greenhouse conditions, is typically the most suitable for the ALS X. fragariae pathogen, i.e., X. fragariae cell division and proliferation is at a peak. For this reason, a cell division inhibitor pesticide (validamycin-A mode of action) might exhibit increased efficacy compared with other bactericides during the cultivation period.
In conclusion, to prevent or control strawberry bacterial ALS disease, the results of our study identified several critical elements: (i) non-infected maternal plants; (ii) improvement in cultivation facilities; (iii) appropriate and effective bactericides; and (iv) soil fumigation. If these measures are consistently and aggressively applied as an IPM protocol in strawberry production, ALS disease caused by the X. fragariae pathogen will be manageable.
Acknowledgments
This study was supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (Project No. 315004-5).
Notes
Articles can be freely viewed online at www.ppjonline.org.