Stemphylium Leaf Blight of Garlic (Allium sativum ) in Spain: Taxonomy and In Vitro Fungicide Response
Article information
Abstract
The most serious aerial disease of garlic is leaf blight caused by Stemphylium spp. Geographical variation in the causal agent of this disease is indicated. Stemphylium vesicarium has been reported in Spain, whereas S. solani is the most prevalent species recorded in China. In this study, Stemphylium isolates were obtained from symptomatic garlic plants sampled from the main Spanish production areas. Sequence data for the ITS1–5.8S–ITS2 region enabled assignation of the isolates to the Pleospora herbarum complex and clearly distinguished the isolates from S. solani. Conidial morphology of the isolates corresponded to that of S. vesicarium and clearly discriminated them from S. alfalfae and S. herbarum on the basis of the size and septation pattern of mature conidia. Conidial morphology as well as conidial length, width and length:width ratio also allowed the Spanish isolates to be distinguished from S. botryosum and S. herbarum. Control of leaf blight of garlic is not well established. Few studies are available regarding the effectiveness of chemical treatments to reduce Stemphylium spp. incidence on garlic. The effectiveness of nine fungicides of different chemical groups to reduce Stemphylium mycelial growth in vitro was tested. Boscalid + pyraclostrobin (group name, succinate dehydrogenase inhibitors + quinone outside inhibitors), iprodione (dicar-boximide), and prochloraz (demethylation inhibitors) were highly effective at reducing mycelial growth in S. vesicarium with EC50 values less than 5 ppm. In general, the effectiveness of the fungicide was enhanced with increasing dosage.
Introduction
Garlic (Allium sativum L.) is a crop cultivated worldwide. According to the Food and Agriculture Organization of the United Nations (FAO), the world production in 2013 consisted of 24.3 millions tonnes. Within the European Union, Spain is the largest producer with approximately 173,600,000 kg harvested in 2013 (FAOSTAT, 2014). Garlic is affected by several diseases caused by different types of microorganisms of which fungi are the most important pathogens. Diseases affecting the root system include those caused by fungi Sclerotium cepivorum, Fusarium spp. and Penicillium (Dugan et al., 2007; Schwartz and Mohan, 2008). As for the aerial part, together with rust, the key aerial disease is leaf blight caused by Stemphylium spp. (Basallote et al., 1993), which has been described in many countries worldwide, including India (Raghayendra and Pavgi, 1975), South Africa (Aveling and Naude, 1992), Spain (Basallote et al., 1993), Australia (Suheri and Price, 2000), China (Zheng et al., 2008), and Turkey (Polat et al., 2012). The occurrence of this disease drastically reduces garlic yield every year by as much as 70% in some fields (Zheng et al., 2010).
The causal agent of leaf blight of garlic seems to be different depending on the growing area. Stemphylium vesicarium has been reported in Spain (Basallote et al., 1993), and in Australia (Susheri and Price, 2000), whereas S. solani is the most prevalent species recorded in China (Zheng et al., 2008). Stemphylium spp. also have been identified as important pathogens of other crops such as onion, pear, asparagus, alfalfa, parsley and a variety of horticultural crops (Falloon et al., 1987; Koike et al., 2013; Lamprecht et al., 1984; Llorente and Montesinos, 2006; Mehta, 1998; Miller et al., 1978; Nasehi et al., 2013a, 2013b; Reis et al., 2011; Vakalounakis and Markakis, 2013).
Early symptoms of Stemphylium spp. infection in garlic leaves consist of small white spots and apical necrosis. These lesions soon develop into larger, elongate white spots that eventually become purple and water soaked (Basallote et al., 1993). Fungal pseudothecia are able to persist on crop residues and ascospores are the primary inoculum in the following season (Basallote et al., 1998, 1999). Once the disease is established, conidia form in primary lesions and rapidly disseminate to infect healthy plants, resulting in an important decrease in photosynthesis and therefore in bulb yield reduction (Zheng et al., 2010). It is well known that pseudothecia maturation and ascospore release are closely associated with high relative humidity and mild temperature (Prados-Ligero et al., 1998).
Control of leaf blight of garlic is not well established. Johnson (1990) reported that burying garlic residues prevents the release of ascospores and, therefore, the spread of the primary inoculum. Basallote et al. (1999) reported differences in the incidence and severity of this disease among garlic cultivars. In general, white cultivars were more susceptible to Stemphylium spp. infection than purple cultivars. In vitro experiments show that tebuconazole, procymidone and chlorothalonil are ineffective in controlling growth of S. vesicarium isolated from onion and asparagus (Aveling et al., 1993; Grinstein et al., 1988).
On the contrary field trials indicate that chlorothalonil and procymidone reduce at some extent the occurrence of Stemphylium leaf spot of onion and asparagus, respectively (Grinstein et al., 1988). At high concentrations, other chemicals such as mancozeb, azoxystrobin, propiconazole and propineb allow in vitro fungal growth inhibition of S. vesicarium isolated from onion (Mishra and Gupta, 2012). However, few studies on the effectiveness of chemical treatments to control Stemphylium spp. on garlic are available in the literature. Basallote et al. (1998) reported that regular application of tebuconazole or procymidone during vegetative growth of garlic controlled the occurrence of leaf spots caused by S. vesicarium in Europe. Moreover, Zheng et al. (2008) indicated that the most effective fungicides with which to control S. solani in Chinese garlic fields were mancozeb and flusilazole.
This study aimed to clearly identify the causal agent of leaf blight in the main Spanish production areas of garlic. In view of the scanty information on chemical control of Stemphylium leaf spot of garlic, this paper also tries to identify the effectiveness of nine fungicides representing different chemical groups to reduce Stemphylium mycelial growth.
Materials and Methods
Pathogen isolation from diseased garlic leaves
Samples were taken from a commercial plot in Albacete (Spain) during the 2012 season. Fifty randomized symptomatic leaf samples were taken from different garlic symptomatic plants. cv. Morado de Cuenca. Symptomatic portions of garlic leaves were surface disinfected in 2% sodium hypochlorite solution, rinsed twice in sterilized distilled water and placed on potato dextrose agar (PDA) plates. The plates were incubated for 5 days at 25 ± 1°C. Single-spore cultures were obtained and the fungal isolates were identified using morphological and molecular methods.
Morphological characterization of the isolates
Twenty-five fungal strains were isolated from foliar samples and seven were randomly selected for a deeper taxonomical analysis and in vitro test. A total of 25 conidiophores and 100 conidia from each isolate were examined. Length and width of conidia, number of transverse septa, constrictions, and series of longitudinal septa were compared with descriptions of Stemphylium spp. provided by Simmons (1969). Mature conidia and conidiophores were mounted in a drop of blue cotton and observed with a light microscope coupled to a digital camera (Nikon Eclipse 80i; Nikon, Utrecht, The Netherlands). Pictures taken at 1,000× magnification were evaluated and processed using Axio Vision 4.7 software (Axio Systems NV, Wetteren, Belgium).
Molecular identification of the isolates
Fungal mycelia were removed from a 4-day-old PDA culture and genomic DNA was extracted following the method described by Querol et al. (1992). The DNA concentration was determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, NC, USA). The nucleotide sequence of the complete ITS1–5.8S–ITS2 region was obtained using the primer pair ITS1/ITS4 described by White et al. (1990).
PCR reactions were performed in 25 μl volumes containing 1 μl of each primer (20 μM), 2.5 μl of 10× PCR buffer, 1 μl MgCl2 (50 mM), 0.5 μl dNTPs mix (40 mM; Biotools, Madrid, Spain), 0.15 μl Taq DNA polymerase (5 U/μl) (Biotools), and 2 μl (100 ng) template DNA. The cycling conditions for ITS1 and ITS4 primer pair used were in accordance with those described by Henry et al. (2000). PCR products were detected in 1% agarose ethidium bromide gel in 1× TAE buffer (40 mM Tris-acetate and 1 mM ethylenediaminetetraacetic acid).
PCR products were purified with the UltraClean™ PCR Clean-Up Purification kit (Mobio, Carlsbard, CA, USA) following the manufacturer’s instructions. Both strands were sequenced using an ABI 3730xl genetic analyzer (Applied Biosystems, Carlsbard, CA, USA) by Stab Vida Ltd. (Sintra, Portugal). Sequences were processed and edited using CLC 5.0 software (CLC Bio, Boston, MA, USA) and deposited in the GenBank database.
Fungicide evaluation
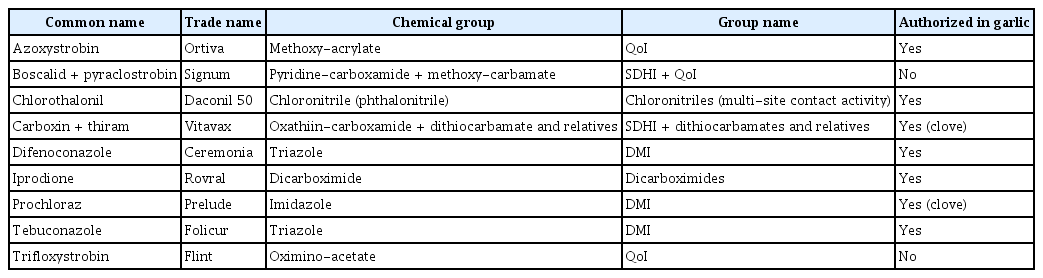
Seven isolates of S. vesicarium identified as described above were selected for subsequent studies. The effectiveness of nine fungicides was evaluated (Table 1) in in vitro assays on PDA medium supplemented with the corresponding concentration of the chemical (1, 10, 100, and 1,000 ppm). Doses were selected according to Alberoni et al. (2005) and Avenot and Michailides (2007). Control assays were also included using PDA medium not supplemented with fungicides.
All the plates (including the control) were inoculated with a 1-cm-diameter agar plug excised from the actively growing front of 7-week-old colonies of each isolate. Inoculated plates were incubated for 7 days at 25 ± 1°C in obscurity. Each combination of isolate, fungicide, and concentration was evaluated in six replicate plates. Radial growth was determined using a digital scalimeter at the end of the incubation period by calculating the mean of two colony diameters and then used to determine the percentage inhibition in comparison to the control assays. All data analyses were conducted with IBM SPSS Statistics version 21.0 (IBM Co., Armonk, NY, USA). Data were tested for normality, homogeneity of variances, independence and linearity. The data were also tested for significance of the main effects and interactions. The data were analyzed statistically using analysis of variance (ANOVA) and regression analyses. Post-hoc analyses were performed using Tukey test. In all cases, P < 0.05 was applied as the significance level.
Percentage inhibition was plotted against
Results
Identification of Stemphylium isolates
For all portions of symptomatic garlic leaves evaluated, a brown-colored fungal colony was consistently obtained. Morphological characteristics of the conidiophores and conidia of pure cultures of Stemphylium isolates were similar to those described by Simmons (1969) for S. vesicarium. Conidiophores were cylindrical, unbranched, light brown with dark brown apical swelling in the site of conidium production. Mature conidia were brown, oblong or broadly oval, with 1–3 transverse constrictions, 1–3 complete or nearly complete series of longitudinal septa and 1–5 transverse septa. The conidial length and width ratio were 17.96–31.07 × 8.89–17.25 μm (average 25.93 × 12.56 μm) and the length:width ratio was 1.61–3.03 (average 2.09) (Fig. 1).
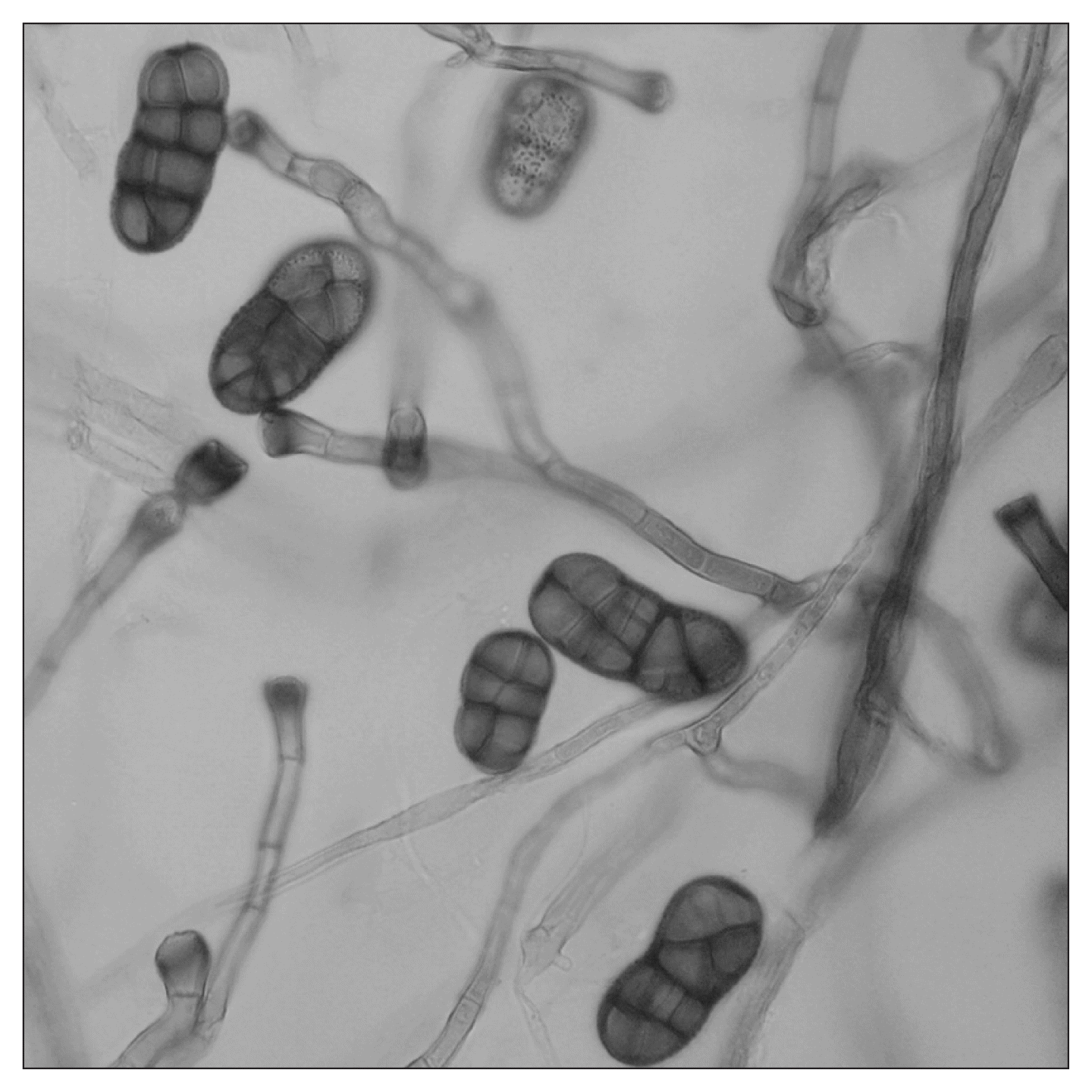
Photography of bright field microscopy of conidiophores and conidia of one of the Spanish isolates of Stemphylium vesicarium stained with lactophenol cotton blue in gray scale (×600).
Molecular identification by sequencing the ITS1–5.8S–ITS2 region enabled classification of the isolates into the Stemphylium genus, although it was not possible to assign the isolates to a species solely on molecular basis. The seven sequences showed 100% similarity and three of the sequences were deposited in the NCBI database (accession Nos. KT778794, KT778795, and KT778796). Similar identity and coverage values (100% in both cases) were obtained with the same region of S. alfalfae (accession No. AF442776), S. herbarum (AB979878), and S. vesicarium (JX424812). However, when the sequences obtained were compared with those of S. solani (accession Nos. KF534501 or KF999031) identity values were lower (98% with 100% coverage).
Effects of fungicides on mycelial growth of Stemphylium vesicarium
Screening of seven S. vesicarium isolates for sensitivity to nine different fungicides was performed. The effective concentration of each active ingredient that inhibited 50% of the radial mycelial growth (EC50) is shown in Table 2.
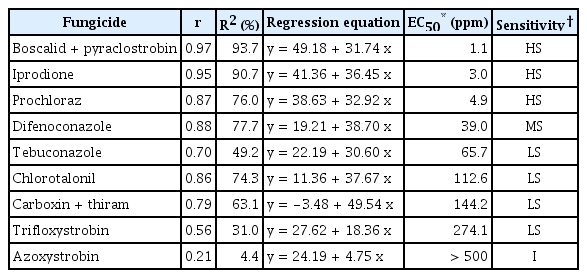
EC50 values (ppm) of Stemphylium vesicarium isolates on potato dextrose agar media supplemented with the nine fungicides evaluated
The S. vesicarium isolates showed markedly different sensitivities depending on the fungicide evaluated. Only boscalid + pyraclostrobin (pyridine-carboxamide + methoxy-carbamate), iprodione (dicarboximide) and prochloraz (imidazole) were highly effective at low concentrations with EC50 values less than 5 ppm. By contrast, S. vesicarium isolates showed moderate sensitivity to difenoconazole (EC50 = 39.0 ppm) and low sensitivity to tebuconazole, chlorotalonil, carboxin+thiram and trifloxystrobin (EC50 values ranged from 65.7 to 274.1 ppm). Isolates were insensitive to azoxystrobin (EC50 value higher than 500 ppm).
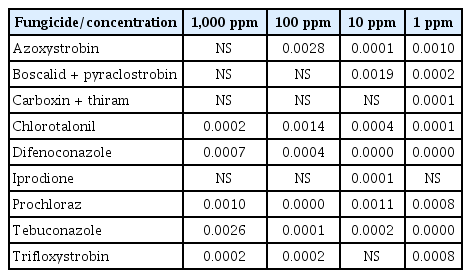
Statistical analysis revealed that concentration itself had a significant effect on the reduction of S. vesicarium growth. The concentration-dependent effects of the fungicides evaluated on the percentage inhibition of mycelial growth are presented in Table 3. No active ingredient reduced fungal growth more than 50% with respect to the control assays at 1 ppm. The most effective active compound at 1 ppm was boscalid + pyraclostrobin with 48.55% inhibition followed by prochloraz (39.08%) and iprodione (38.78%). The results obtained at 10 ppm showed that boscalid + pyraclostrobin and iprodione inhibited fungal growth by almost 80% followed by prochloraz and difenoconazole with percentage inhibition of 65.98% and 54.55%, respectively. Boscalid + pyraclostrobin completely inhibited S. vesicarium growth at 100 ppm, and prochloraz and iprodione achieved 95% inhibition, followed by difenoconazole and carboxin + thiram with 71.25% and 69.71% growth reduction at 100 ppm respectively. The least effective fungicide at 100 ppm was azoxistrobin (27.30% inhibition).

Growth inhibition (%) of Stemphylium vesicarium on potato dextrose agar plates supplemented with different concentrations of the nine fungicides
S. vesicarium isolates were extremely sensitive to boscalid + pyraclostrobin, carboxin + thiram, and iprodione at 1,000 ppm, which completely or almost completely inhibited mycelial growth. The second group was composed of tebuconaloze, difenoconazole and prochloraz, which reduced fungal growth by approximately 90%. Trifloxystrobin and azoxystrobin were the least effective compounds with growth reduction of 59.27% and 34.27%, respectively.
Isolate response to fungicides
Stemphylium isolates showed statistically significant differences among them in respect to their fungal growth when high concentrations (1,000 ppm) of chlorotalonil, difenoconazole, prochloraz, tebuconazole and trifloxystrobin are tested (Table 4). At 100 ppm, azoxystrobin also induced different response in growth among isolates. At 10 ppm, only trifloxistrobin and carboxin + thiram did not show differences among the tested isolates. Conversely, significant differences among isolates were observed in the presence of all fungicides at 1 ppm except for iprodione (Table 4).
Discussion
Stemphylium leaf blight is one of the most serious diseases of cultivated garlic (Basallote et al., 1993). The causal agent is reported to be a different species depending on the region (Basallote et al., 2004; Zheng et al., 2008). The taxonomy of this genus has always been controversial and the classification has been revised based on molecular and morphological methods (Inderbitzin et al., 2009; Simmons, 1969, 1985). In the present work, Stemphylium isolates were obtained from infected garlic leaves from the main Spanish cultivated region. Molecular evidence only allowed assignation of the isolates to the Pleospora herbarum complex (Simmons, 1985). These results clearly distinguished the isolates from S. solani, although it was impossible to discriminate among S. vesicarium, S. alfalfae and S. herbarum. Inderbitzin et al. (2009) published an extensive phylogenetic study incorporating the main species included in the Stemphylium genus. Despite using sequence data for four different regions (ITS1–5.8S–ITS2, glyceraldehyde-3-phosphate dehydrogenase, elongation factor 1-alpha and vmaA–vpsA intergenic spacer), these authors were not able to discriminate among these species; therefore, in this case, morphological characterization is indicated to be compulsory to achieve reliable taxonomic identification of the isolates.
With respect to conidial morphology the studied isolates clearly correspond to S. vesicarium on the basis of the size and septation pattern of mature conidia. The length and width of conidia as well as the length:width ratio of the Spanish isolates were consistent with the description of S. vesicarium by Simmons (1969). Conidial morphology allowed distinction of the isolates from S. botryosum (conidia length and width around 34 and 25 μm, respectively), and from S. herbarum (which produces even larger mature conidia) (Simmons, 1969, 1985). This conclusion was also supported by conidia length:width ratios, which are always between 1 and 1.5 in S. botryosum and S. herbarum, and around 2 in S. vesicarium.
Little previous information is available on effective control of S. vesicarium isolates from garlic either in vitro or in the field. The present results showed that boscalid + pyraclostrobin (group name, succinate dehydrogenase inhibitors [SDHI] + quinone outside inhibitors [QoI]), iprodione (dicarboximide) and prochloraz (demethylation inhibitors, DMI) are highly effective fungicides that reduce mycelial growth of S. vesicarium with EC50 values less than 5 ppm. The three active ingredients of these fungicides belong to different chemical groups and, therefore, it might be advantageous to plan their rotation in the field to prevent resistance or selection pressure for tolerant isolates. Hosen et al. (2009) and Rahman et al. (2010) observed that iprodione was highly effective to control growth of S. botryosum isolated from lentil in vitro, a species closely related to S. vesicarium.
Difenoconazole (DMI), tebuconazole (DMI) and chlorotalonil (multi-site), with EC50 values between 39 and 118 ppm, might be considered as a second option to be applied in a fungicide calendar in the field, mainly if their chemical family differs from the most effective fungicides. It is important to highlight that it is strongly recommended to rotate active ingredients included in the same chemical group. The last option would be the use of carboxin + thiram (SDHI + dithiocarbamate), trifloxystrobin (QoI) and azoxystrobin (QoI). Trifloxistrobin is not authorized for garlic crop, in addition, although the doses usually recommended for vegetables are around 250 ppm, our results show a low efficiency even at four times higher concentrations.
These results also agree with those obtained by Basallote et al. (2004), who observed the low efficiency of chlorothalonil to reduce S. vesicarium growth, as well as those reported by Aveling et al. (1993), who indicated that thiram was ineffective at controlling fungal growth even at high concentrations (more than 900 ppm).
However, it is also important to analyze the most effective concentration to evaluate the most appropriate dose to be applied. In general, the higher the concentration, the higher the effectiveness. The fungicides evaluated were generally ineffective at the lowest concentration tested (1 ppm). At this concentration, growth inhibition of Stemphylium isolates approached 50%. The particular case of carboxin + thiram was notable because this compound is only effective at a high concentration, which supports the importance of applying the fungicides at the doses suggested by manufacturers. Moreover, it is also important to highlight that the use of carboxin + thiram is restricted to pre-sowing treatments and, therefore, it could not be applied to garlic leaves during crop growth.
Results obtained with a concentration of 10 ppm revealed that boscalid + pyraclostrobin, iprodione and prochloraz were the most effective active ingredients to reduce Stemphylium growth followed by tebuconazole, with percentage inhibition of approximately 50% achieved. The present results are similar to those previously obtained by Basallote et al. (2004), who performed an in vitro experiment with five active ingredients and noted that tebuconazole was an effective fungicide to control S. vesicarium in vitro with similar inhibition rates (50% at 10 ppm). Moreover, Aveling et al. (1993) observed that tebuconazole was effective at a concentration of 50 ppm at reducing growth of S. vesicarium isolated from onion.
Some of the fungicides tested did not exert the same effect on the isolates and different inhibition percentages were observed in some cases. These differences might be associated with differences in susceptibility to the fungicides in some isolates at some extent. In some cases, this reduced sensitivity may have little to no impact on fungicide usage in the field, and save the term “resistance” for large reductions in sensitivity of individual isolates which are likely to affect efficacy of a specific fungicide under field conditions if the resistant isolates become widespread in the pathogen population. At higher doses the differences in susceptibility to the fungicides decrease significantly. At the highest concentration evaluated (1,000 ppm), azoxystrobin, boscalid + pyraclostrobin, carboxin + thiram and iprodione did not show differences among isolates but several responses were observed at decreased concentrations. These results agree with the field doses recommended by manufactures (always higher to 800 ppm for those four fungicides). The use of these chemical compounds at the suggested concentrations may prevent resistance appearance in S. vesicarium isolates. The fungicides that have shown major differences among isolates belong to the DMI fungicides group, which have shown resistance problems with their use against several fungi and a range of different resistance mechanisms have been identified (Gisi et al., 2000; Ma and Michailides, 2005). Other fungicides, such as the highly effective prochloraz, were indicated to promote decreased sensitivity susceptibility in some isolates at all concentrations tested. The application in the field of these compounds (carboxin + thiram and prochloraz) are now only permitted as preplanting dips treatment, so its use in field should be carefully controlled, the effective dose determined, and it is extremely important to alternate them with other fungicides belonging to different chemical groups because they could create a high risk of resistance appearance in S. vesicarium in the field. This fact has been reported previously by Alberoni et al. (2005) in the control of S. vesicarium from pear. These authors observed that repetitive application of procymidone favors resistance development, and, in fact, this fungicide is no longer effective to control brown spot caused by S. vesicarium. Therefore, it is very important to establish an effective schedule of preventive treatments with fungicides to control S. vesicarium. Alberoni et al. (2008) reported the high effectiveness of QoI fungicides to control S. vesicarium brown spot of pear (of which trifloxystrobin and pyraclostrobin were used in the present study). However, the risk of resistance evolution in this group is estimated to be high according to the Fungicide Resistance Action Committee (FRAC, 2016) and, after several years of treatment, S. vesicarium has already developed resistance to these fungicides (Alberoni et al., 2010).
The present data should be taken into account to design an effective fungicide calendar to control S. vesicarium leaf blight of garlic. Following FRAC recommendations, it is essential to apply a combination of fungicides of different types or alternate different fungicide treatments, always following national requirements, and being careful to adhere to the dose recommended by the manufacturer.
Acknowledgments
The authors gratefully acknowledge the technical assistance and the use of the facilities of Coopaman SCL.
Notes
Articles can be freely viewed online at www.ppjonline.org.