Effect on Colony Growth Inhibition of Soil-Borne Fungal Pathogens by Available Chlorine Content in Sodium Hypochlorite
Article information
Abstract
Our study investigated the available chlorine content, contact time and difference among strains of each pathogen for sodium hypochlorite (NaOCl) to control chemically against soil-borne fungal pathogens, such as Phytophthora rot by Phytophthora cactorum, violet root rot by Helicobasidium mompa, and white root rot by Rosellinia necatrix, causing die-back symptom on apple trees. As a result, the colony growth of Phytophthora cactorum was inhibited completely by soaking over 5 s in 31.25 ml/l available chlorine content of NaOCl. Those of H. mompa and R. necatrix were inhibited entirely by soaking over 160 s in 62.5 and 125 ml/l available chlorine content in NaOCl, respectively. Also, inhibition effect on available chlorine in NaOCl among strains of each soil-borne pathogen showed no significant difference and was similar to or better than that of fungicides.
Recent changes in climate and cultivation environment have led to the problem of apple tree dieback caused by soil-borne diseases, which causes economic losses (Lee et al., 2016). The major soil-borne diseases reported in Korea are Phytophthora root rot by Phytophthora cactorum, violet root rot by Helicobasidium mompa, and white root rot by Rosellinia necatrix (KSPP, 2009; Lee et al., 2006; RDA, 1993). The apple trees infected with these diseases show remarkably weak leaf growth, poor growth of shoots, early leaf yellowing and abscission, and reduction of fruit yield with low quality. In the case of severe damage, the entire trees become dieback within 2–3 years, and the possibility of re-occurrence is high even after supplementary planting, and eventually it is easy to reach the disused orchards (Kim et al., 1995; Lee et al., 1995; Lee, 2002; Lee et al., 2016).
At present, azoxystrobin for Phytophthora root rot, tolclofos-methyl and thiophanate-methyl for violet root rot, fluazinam and benomyl, isoprothiolane for white root rot are registered as control agents (Korea Crop Protection Association). However, these control agents have been used in most farms suffering from the tree dieback, but they have not had clear control effects, and the annually repetitive supplementary planting is the only alternative to control the diseases resulted in increasing of economic losses.
A variety of chemical disinfestants can be used in disease control. Among them, NaOCl can be used in seeds (Chun et al., 1997; Sauer and Burroughs, 1986), soil (Bisessar and Mcllveen, 1992), and water systems (Santos-Rufo and Rodriguez-Jurado, 2016) because it meets the registration criteria in terms of human health and environment with wide disinfection effects and a short half-life (WHO, 2011). NaOCl was first registered in the United States in 1957 as a crop protection agent, however, NaOCl is not yet registered as a control agent in Korea, it is restricted to apply in fields. Nevertheless, because the effect of the registered control agents is insignificant, it is necessary to develop a definite control agent and method for soil-borne diseases which cause dieback in apple trees.
In this study, the inhibition available chlorine content, minimum contact time, and inhibition effect among isolates for NaOCl on colony growth of the 3 soil-born fungal pathogens, compared with registered control agents was investigated in vitro.
Materials and Methods
Phytopathogens
Six isolates of Phytophthora cactorum (KACC40166, 40174, 40175, 40176, 40183, and 40448), 2 isolates of Helicobasidium mompa (KACC40169 and 40836), 4 isolates of Rosellinia necatrix (KACC40168, 40445, 40446, and 40447) were distributed from the National Agrobiodiversity Center (https://genebank.rda.go.kr) in Rural Development Administration. Also, 1 isolate of Helicobasidium sp. (CBARES2015V1) and 2 isolates of Rosellinia sp. (CBARES2015W1 and W2) were isolated from apple trees (Lee, 1995; Shikata and Mitsueda, 1978; Singleton et al., 1992; Tadao, 1984). The used medium for culture and experiment was potato dextrose agar (PDA; Difco, Becton, Dickinson and Company, MD, USA).
Tested chemical and fungicides
The chemical used was sodium hypochlorite (NaOCl, 8.0% available chlorine content, Junsei, Japan) for growth inhibition of each pathogen. The fungicides used are as follow; azoxystrobin (WP, a.i. 10%) for Phytophthora cactorum, thiophanate-methyl (WP, a.i. 70%) and tolclofos-methyl (WP, a.i. 50%) for Helicobasidium mompa and Helicobasidium sp. and fluazinam (WP, a.i. 50%), benomyl (WP, a.i. 50%), isoprothiolane (GR, a.i. 12%) for Rosellinia necatrix and Rosellinia sp.
Experimental design
In order to determine the available chlorine content of NaOCl, cultured agar plug of each phytopathogen colony with Cork borer 2 (Ø 6.2 mm, Usbeck, Radevormwald, Germany) was placed on sterilized paper discs (Ø 8 × 1.5 mm, Advantec, Japan) on PDA medium and then a cultured agar plug on a paper disc were placed. Six available chlorine content of the chemical were chosen for the in vitro experiments: 1.25, 3.125, 12.5, 31.25, 62.5, 125 ml/l of 8.0% NaOCl in sterilized water. Each available chlorine content was injected into a paper disc by 70 μl while being kept cool on ice, and incubated at 23 ± 1°C for 5 to 15 days in MIR-154 (Panasonic, Tokyo, Japan).
To investigate the inhibitory contact time in NaOCl for each pathogen, 5 agar plugs of each pathogen were soaked in the determined available chlorine content in NaOCl for 5, 10, 20, 40, 80, 160, 320, 640, and 1,280 s, respectively. They were taken out, put on sterilized absorbent paper, cultured on PDA, and then incubated at 23 ± 1°C for 5 to 15 days.
Inhibition rate for each available chlorine content of Na-OCl was as follows; (control value – value of each available chlorine content of NaOCl) / control value × 100.
Finally, isolates of the pathogens were tested for showing the inhibition difference within the available chlorine content of NaOCl determined above as well as the currently registered fungicides.
As the medium preparation, 100 ml of the PDA medium was sterilized and cooled by 50–60°C, and then the standard or 2-fold amount of each crop protection agent were added. Especially, isoprothiolane was dispensed by 30 ml of PDA medium after adding standard amount 25.5 g or 2-fold amount 51 g in 100 × 40 mm plant culture dish (SPL Lifesciences Co., Ltd., Gyeonggi, Korea). All treatments were performed 3 times in 3 replications.
Data analysis
The colony growth diameter of each treatment was measured and the inhibition rate compared to non-treatment was calculated. The diameter of the colony growth was measured by the average of long axis and short axis and experiments were tested by 3 times with 5 repetitions per treatment.
Means could be compared to the respective control using the Duncan’s test (P = 0.05) when DMRT showed significant differences (P < 0.05) by using CoStat 6400 program (CoHort software, USA).
Results and Discussion
Available chlorine content and contact time in NaOCl for colony growth inhibition of soil-borne fungal pathogens
P. cactorum KACC40166 isolate was completely inhibited over 31.25 ml/l of NaOCl. H. mompa KACC40836 and R. necatrix KACC40168 isolate were completely inhibited over 31.25 and 62.5 ml/l of NaOCl despite difference among repetitions, respectively (Fig. 1). In addition, as shown in Table 1, P. cactorum KACC40166 isolate showed a significant inhibition rate of 66% in the colony diameter on available chlorine content of 12.5 ml/l of NaOCl, compared with the control and no colony formation over 31.25 ml/l of NaOCl was showed. H. mompa KACC40836 isolate showed significant inhibition rate of 11%, 16%, and 21% in the colony diameter on 1.25, 3.125, and 12.5 ml/l of NaOCl, respectively, compared to the control and no colony formation over 31.25 ml/l of NaOCl was showed. There was no significant difference for the colony growth diameter between 1.25 and 3.125 ml/l as well as 3.125 and 12.5 ml/l of NaOCl. R. necatrix KACC40168 isolate showed significant inhibition rate of 36% and 61% in the colony diameter on 31.25 and 62.5 ml/l of NaOCl, respectively, compared to the control. There was no statistical difference for the colony growth diameter between 31.25 and 62.5 ml/l of NaOCl and no colony formation over 125 ml/l of NaOCl was showed. From these results the colony inhibition effect of soil-borne pathogens was different according to available chlorine content of NaOCl, as if the sensitivity of NaOCl varies according to plant pathogens (Cayanan et al., 2009; Hong et al., 2003; Santos-Rufo and Rodriguez-jurado, 2016; Taylor et al., 2000; Thompson, 1965). Chun et al. (1997) reported that 2.6% of NaOCl killed most of bacteria and fungi in rice seeds. Shin et al. (2014) showed that conidia of Gibbrella fujikuroi were significantly inhibited by 0.008–0.01% of NaOCl and the control value on 0.3% and 0.5% of NaOCl in the infected rice seeds were 93.1% and 93.8%, respectively. Sauer and Burroughs (1986) reported inhibition of Aspergillus spp. at 1–5% concentration NaOCl. Therefore, this available chlorine content of NaOCl were similar range to the colony inhibition for P. cactorum, H. mompa, and R. necatrix.
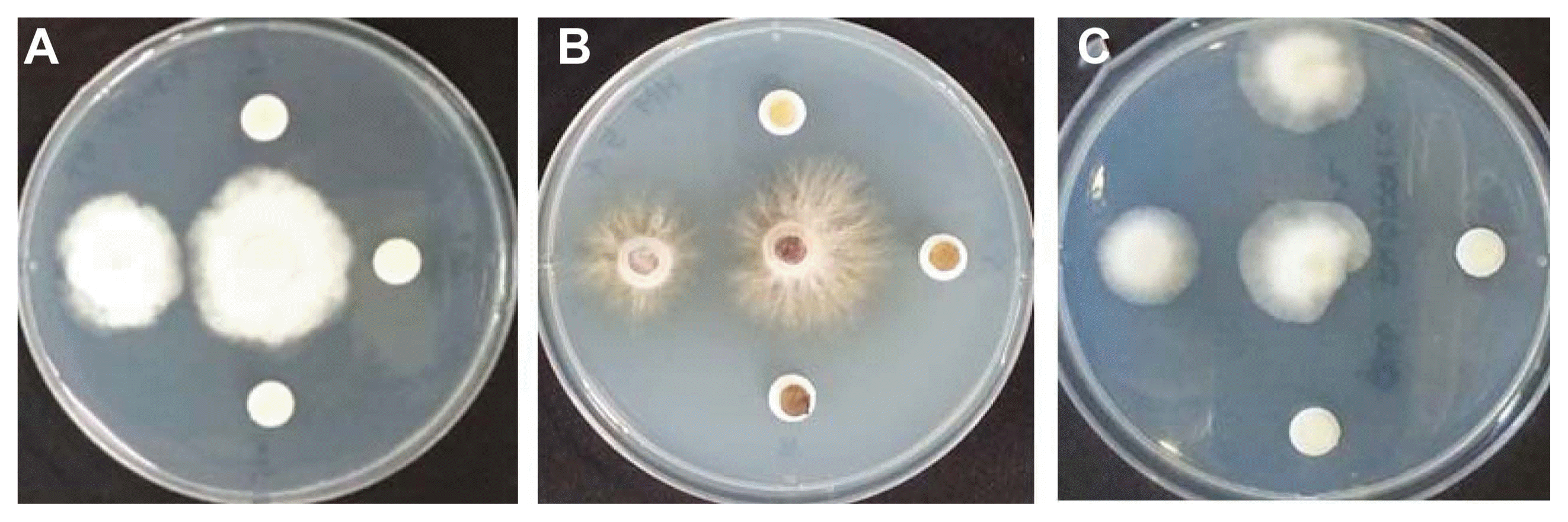
Representative colony growth inhibition of soil-borne fungal pathogens causing apple tree dieback by available chlorine content in NaOCl on PDA. Each treated available chlorine content in NaOCl was 12.5 (left), 31.25, 62.5 and 125 ml/l on plate (A), (B) and (C), clockwise. The center agar plug on all plates was a control. Test plate A, B and C were for Phytophthora cactorum KACC40166, Helicobasidium mompa KACC40836 and Rosellinia necatrix KACC40168 isolate, respectively.
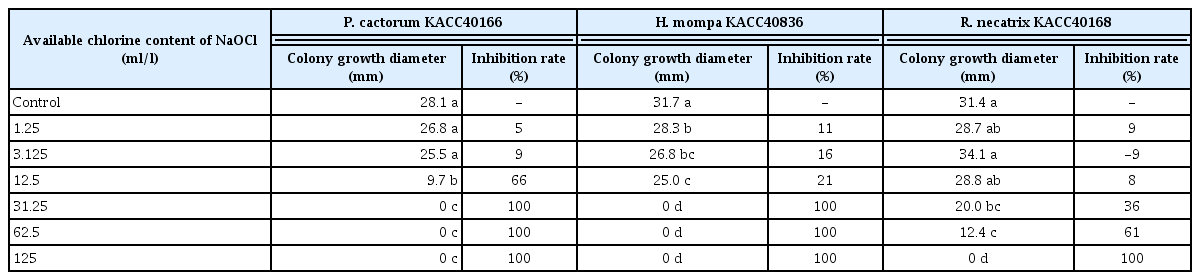
Colony inhibition rate for a typical isolate of soil-borne fungal pathogens by available chlorine content of NaOCl on PDA
The results on contact time that inhibits colony formation of each pathogen isolate by the available chlorine content determined above are shown in Table 2. Compared with the control treatment, the P. cactorum KACC 40166 isolate showed the significant colony inhibition rate of 6–25% and 100% after soaking in 12.5 ml/l of NaOCl for 5–20 s and over 40 s, respectively. In addition, the isolate incubated after soaking in 31.25 ml/l of NaOCl over 5 s. showed the significant inhibition rate of 100% without colony formation. The H. mompa KACC40836 isolate showed no significance after soaking in 31.25 ml/l of NaOCl for 5–10 s. The isolate treated for 20–160 s, whereas, showed the significant inhibition rate of 22–80% and tended toward the longer the soaking time, the higher the inhibition rate. That treated over 320 s. showed the significant inhibition rate of 100% without colony formation. No significant difference showed among the range of 5–40 s as well as between 40 and 80 s. Treatments in 62.5 ml/l of NaOCl for the 5 s showed no significant difference. On the other hand, treatments for 10–80 s showed the significant inhibition rate of 23–82% without difference among the range of either 5–20 s or 10–40 s. That treated over 320 s showed the significant inhibition rate of 100% without colony formation. The R. necatrix KACC40168 isolate incubated after soaking in 62.5 ml/l of NaOCl for 5–320 s showed significant inhibition rate of 35–85, compared to control, with no significant difference among treatments for 5–160 s. Thus the isolate showed significant inhibition rate of 100% over 640 s without colony formation. That treated in 125 ml/l of NaOCl for 5–80 s showed significant inhibition rate of 34–49%, compared to control, with no significant difference between 5 and 10 s. Also, that showed significant inhibition rate of 100% over 160 s without colony formation. From these results, the sensitivity of phytopathogens for NaOCl concentration is consistent with the report that they have different inhibition degree depending on contact time in NaOCl (Copes et al., 2001; Datnoff et al., 1987; Johnson et al., 1997; Santos-Rufo and Rodriguez-jurado, 2016; Segall, 1968).
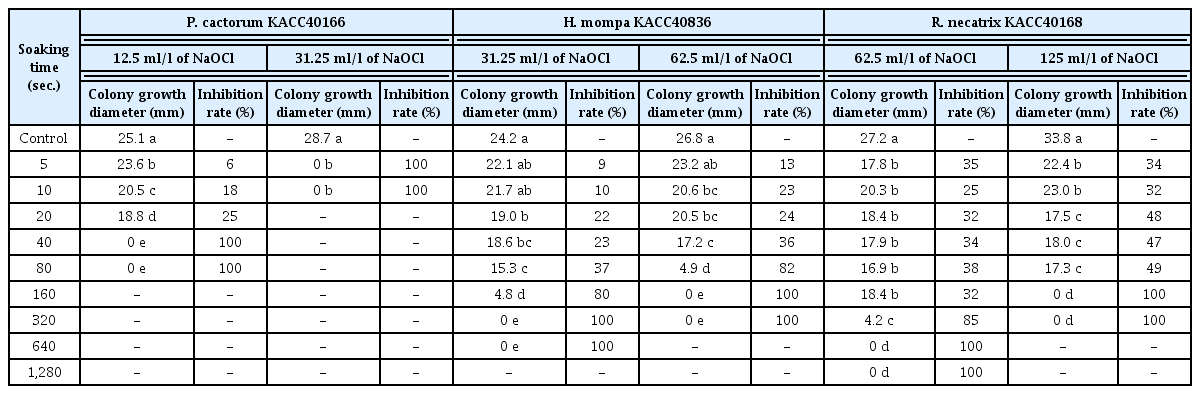
Colony inhibition rate for a typical isolate of soil-borne fungal pathogens by soaking time from the determined available chlorine content of NaOCl
Shin et al. (2014) reported that the conidia of Fusarium fujikuroi showed significant inhibition of the colony formation after treatment in 0.006% of NaOCl for 120 min. Cayanan et al. (2009) reported that the zoospores of P. cactorum showed complete inhibition after treatment in free chlorine of 0.3 ml/l for 6 min. Meanwhile, Jeffers (1992) reported that the apple root stock MM.106 treated in 1.05% of NaOCl for 10 min. were fewer plants diseased by Phytophthora root rot as well as increased significantly both shoot length and fresh weight of root than the control that soaked in water. Thus, the root stock treated for 60 min. showed no significance with phytotoxic, compared to the control soaked in water.
Effect on colony growth inhibition of soil-borne pathogens by available chlorine content in NaOCl
On the basis of above determined available chlorine content in NaOCl, Table 3 to 5 showed the inhibition effect of colony growth for the isolates of each pathogen with control fungicides. From the results of Table 3, P. cactorum KACC40175, 40176, 40183, and 40448 isolate showed the significant inhibition rate of 45, 80, 76, and 18% on 12.5 ml/l of NaOCl, respectively. On 31.25 ml/l of NaOCl the 6 isolates showed significant inhibition rate of 100% without colony growth. On the standard dose of a control fungicide, azoxystrobin WP, P. cactorum KACC40175, 40183, and 40448 isolate showed significant inhibition rate of 29, 45, and 42%, respectively. On the double dose, P. cactorum KACC40174, 40175, 40183, and 40448 isolate showed significant inhibition rate of 29, 53, and 45%, respectively. Also, P. cactorum KACC40166, 40174, 40175, and 40183 isolate showed no significant difference between 12.5 ml/l of NaOCl and the fungicide without distinction of treatment dose. P. cactorum KACC40176 isolate was inhibited significantly by rather 12.5 ml/l of NaOCl than the fungicide and P. cactorum KACC40448 isolate showed the opposite. P. cactorum KACC40174 isolate showed no significant difference between 31.25 ml/l of NaOCl and 2-fold dose treatment of the fungicide and both P. cactorum KACC40176 and 40183 isolate showed no significance between 12.5 and 31.25 ml/l of NaOCl treatment (Table 3). As only azoxystrobin WP was registered as a control agent and possible the occurrence of the fungicide-resistant isolate with annual use (2015 APS Annual Meeting, http://www.apsnet.org/meetings/Documents/2015_meeting_abstracts/aps2015abP667.htm), therefore, we suggest that 12.5–31.25 ml/l of NaOCl treatment would be effective to control the Phytophthora root rot without the concern for resistant isolate.

Colony inhibition rate of 6 isolates for Phytophthora cactorum by available chlorine content of NaOCl and a fungicide
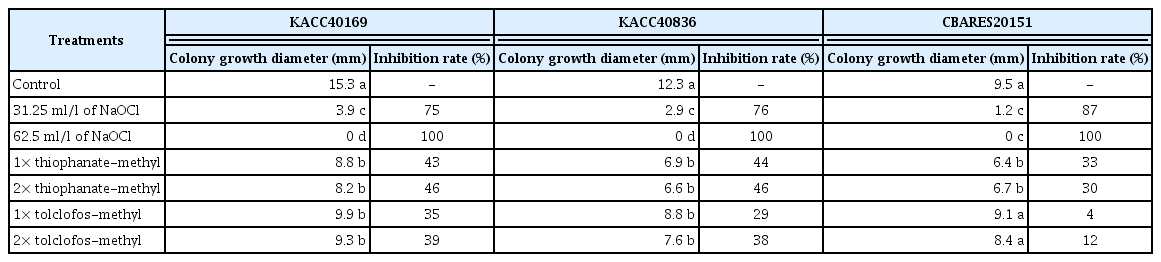
Colony inhibition rate of 3 isolates for Helicobasidium mompa and Helicobasidium sp. by available chlorine content of NaOCl and 2 fungicides
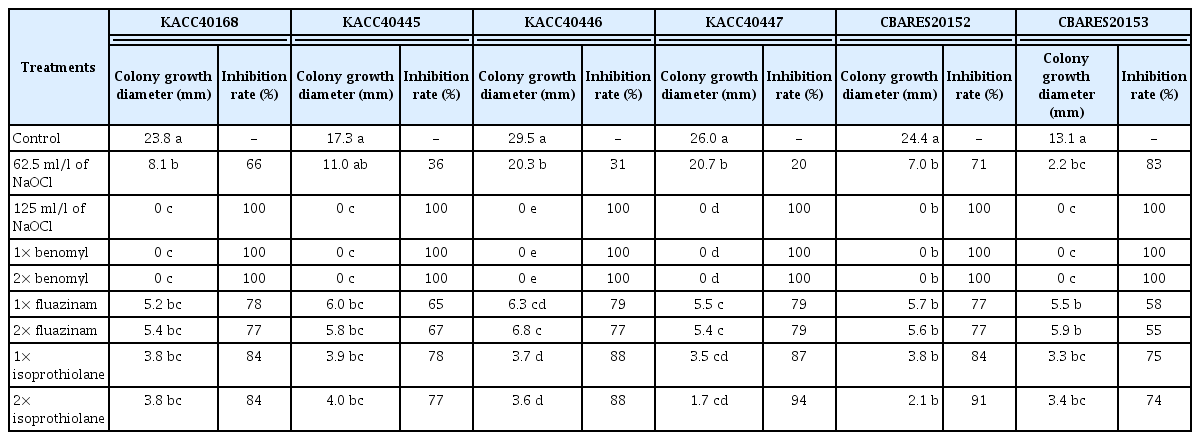
Colony inhibition rate of 6 isolates for Rosellinia necatrix and Rosellinia sp. by available chlorine content of NaOCl and 3 fungicides
From the results of colony inhibition rate for Helicobasidium sp. CBARES20151, H. mompa KACC40169, and KACC40836 isolate, the 3 isolates showed significant inhibition rate of 75–87% and all 100% in 31.25 and 62.5 ml/l of NaOCl treatment, respectively, compared to control. Thus, those treated by each available chlorine content of NaOCl showed significant difference compared to standard and double dose of 2 control fungicides, thiophanatemethyl and tolclofos-methyl. Compared to control, the 3 isolates showed significant inhibition rate of 30–46%, regardless of thiophanate-methyl treatment dose. Also, H. mompa KACC40169 and KACC40836 isolate showed significant inhibition rate of 29–39%, but Helicobasidium sp. CBARES20151 isolate showed no significant difference, regardless of tolclofos-methyl treatment dose (Table 4). As reports of FRAC Code list 1 (2017), these results indicate that the resistant isolates for thiophanate-methyl and tolclofos-methyl were possible to occur, according to use the fungicides over 20 years. In fact, Fernández-Ortuño and Schnabel (2012) reported Botrytis cinerea that showed the resistance for thiophanate-methy as well as Van Bruggen and Arneson (1984) reported Rhizoctonia solani that showed the resistance for tolclofos-methyl. Therefore, we suggest that 31.25–62.5 ml/l of NaOCl treatment would be effective to control the violet root rot diseased by H. mompa.
From the results of colony inhibition rate for Rosellinia sp. CBARES20152 and CBARES20153, R. necatrix KACC40168, KACC40445, KACC40446, and KACC40447 isolate, the other isolates except for R. necatrix KACC40445 showed significant inhibition rate of 20–83% in 62.5 ml/l of NaOCl treatment. All of the 6 isolates showed significant inhibition rate of 100% in 125 ml/l of NaOCl treatment. For standard dose and 2-fold dose of the fungicides, such as benomyl, fluazinam and isoprothiolane, all of the 6 isolates showed significant inhibition rate of 100% in benomyl treatments. However, the other isolates except for R. necatrix KACC40446 showed significant inhibition rate of 55–79% and 74–94% in fluazinam and isoprothiolane treatment, respectively. Difference between standard and 2-fold treatments in the each fungicide showed no significance (Table 5). From this result, the fungicide, benomyl, was the most effective fungicide to inhibit the colony of white root rot. Yukita (2003) reported that soaking treatment for 20 min in 500-fold diluted solution of a fungicide, Fluazinam (a.i. 39.5%), showed high control value at both laboratory and field experiments against H. mompa and R. necatrix. Nevertheless, Eguchi et al. (2008) reported that white root rot of pear trees re-occurred 2 years after fluazinam treatment in Japan. Meanwhile, Takaya et al. (1976) reported that soaking treatment for 24 h in 1,000-fold diluted solution of a fungicide, benomyl (a.i. 50%), showed significant control effect against white root rot as well as Komori and Nakamura (1985) reported that 1,000 ppm of benomyl or isoprothiolane inhibited completely the isolate of R. necatrix.
Recently, FRAC Code list 1 (2017) reported that all of the tested fungicides were concerned by occurrence of resistant isolates. In fact, there were reported that a resistance isolate for benomyl in Rosellinia necatrix occurred (López-Herrera and Zea-Bonilla, 2007), a cross-resistance isolate for isoprothiolane in Magnaporthe oryzae occurred (Ishii and Hollomon, 2015), a resistance mutant isolate for fluazinam in Botrytis cinerea induced (Shao et al., 2015).
In conclusion, our study showed that pathogens caused soil-borne diseases, Phytophthora root rot, violet root rot, and white root rot, were effectively inhibited by 12.5–31.25 ml/l, 31.25–62.5 ml/l, and 62.5–125 ml/l of NaOCl, respectively.
Acknowledgments
This work was carried out with the support of Chungcheongbuk-do Agricultural Research and Extension Services (Project No. LP0035762017), funded by Governor of Chungcheongbuk-do as well as ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ0100142016)’ Rural Development Administration, Republic of Korea.