Biocontrol of Citrus Canker Disease Caused by Xanthomonas citri subsp. citri Using an Endophytic Bacillus thuringiensis
Article information
Abstract
Citrus canker is a devastating disease of citrus caused by Xanthomonas citri subsp. citri (Xcc). A total of 134 endophytic bacteria were isolated from various gymnospermic and angiospermic plants. They were screened for their antagonistic activities against three wild-type and six streptomycin-resistant Xcc strains. TbL-22 and TbL-26, both later identified as Bacillus thuringiensis, inhibited all the wild and resistant Xcc strains. TbL-22 exerted the highest antagonistic activity against XccW3 and XccM6 with inhibition zones of 20.64 ± 0.69 and 19.91 ± 0.87 mm, respectively. Similarly ethyl acetate extract of TbL-22 showed highest inhibition zones 15.31 ± 2.08 and 19.37 ± 3.17 mm against XccW3 and XccM6, respectively. TbL-22 reduced canker incidence on infected leaves by 64.05% relative to positive controls. Scanning electron microscopy revealed that the cell membranes of Xcc treated with ethyl acetate extract of TbL-22 were ruptured, lysed, and swollen. B. thuringiensis TbL-22 can effectively and sustainably controls streptomycin-resistant citrus canker.
Citrus is a genus of flowering plants belonging to the family Rutaceae. It originated in Australia, New Caledonia, and New Guinea (Liu et al., 2012). Citrus fruits provide an ample supply of vitamin C, folic acid, minerals, fiber, and various phytochemicals such as carotenoids, flavonoids, and limonoids which have tremendous health benefit. They contain no fat or cholesterol and have low sodium levels (Paul and Shaha, 2004; Ramful et al., 2010). The production of citrus fruits, however, is threatened by bacterial canker disease (Gottwald et al., 2001). The causative agent is Xanthomonas citri subsp. citri (Xcc). Symptoms include leaf spotting, fruit rind blemishing, defoliation, shoot dieback, and fruit drop in favorable environmental conditions conducive to pathogen proliferation (Das, 2003). This disease has a serious economic impact on citrus production worldwide (Jalan et al., 2013). Citrus canker outbreaks occurred in the state of Florida in 1910, 1984, and 1995. Many controlling methods were attempted to control the disease by uprooting and burning all citrus trees known or suspected to be infected with the pathogen (Gottwald et al., 2001).
Integrated management practices can reduce citrus canker incidence by (1) using canker disease-free nursery plants, (2) pruning and burning infected twigs, (3) spraying copper-based bactericides, and (4) developing canker-resistant varieties (Das and Singh, 1999, 2001). The integrated application of copper oxychloride (0.3%), streptocycline (100 ppm), and neem cake suspension on infected twigs was very effective in controlling the disease (Das and Singh, 2000). Since 1955, streptomycin has been used as the main antibiotic to control various bacterial diseases of Citrus spp. as well as fire blight, soft rot, bacterial spot, and wildfire diseases on other crops (McManus et al., 2002). Nevertheless, pathogen resistance to streptomycin has developed due to its prolonged and excessive use (Carter et al., 2000; Hyun et al., 2012). In a recent study, 72% of the Xcc strains isolated from Jeju Island, Republic of Korea were found to be resistant to streptomycin (Hyun et al., 2012).
The cultivation of citrus varieties resistant to citrus canker is an effective and eco-friendly means of controlling the disease. The development of disease-resistant varieties, however, is time-consuming, laborious, and not widely available. Therefore, it is neither practical nor convenient as a citrus canker control method. For sustainable citrus production, more effective and sustainable techniques are required to control citrus canker.
Endophytes are important components in plant-microbe ecosystems (Li et al., 2012) and may serve as environmentally sustainable resources for citrus canker control. Endophytic microorganisms are bacteria or fungi that colonize the internal host tissues in high numbers but do not harm the host or induce plant disease symptoms (Quispel, 1992). Endophytic bacteria (EB) are endosymbiotic microorganisms that colonize the same ecological niches as phytopathogens, so they can function as biocontrol agents and coexist safely with plant tissues (Berg et al., 2005; Kloepper and Beauchamp, 1992). The application of EB as biocontrol agents enhances disease resistance (Van Loon et al., 1998) and provides a safe alternative to chemical control products (Backman and Sikora, 2008). Several EB used as biocontrol agents have been isolated from various plants, e.g., Burkholderia cepacia from citrus (Tan et al., 2007), Aureobacterium saperdae, Bacillus pumilus, Phyllobacterium rubiacearum, and Pseudomonas putida from cotton (Chen et al., 1995), and Enterobacter cloacae from rice (Yang et al., 2001).
In this study, EB isolated from different gymnosperm and angiosperm tissues were evaluated for their efficacy against citrus canker disease. Antibacterial efficacy was tested using three wild-type and six streptomycin-resistant mutant Xcc strains. The objectives of this research were to (1) screen for effective EB against Xcc strains and (2) determine the efficacies of the best EB able to control citrus canker through both in vitro and in vivo assays.
Materials and Methods
Collection of plant materials and isolation of EB
Various gymnosperms and angiosperms, including Metasequoia glyptostroboides, Ginkgo biloba, Taxus brevifolia, Pinus densiflora, Salix babylonica, and Salix chaenomeloides were sampled at Yeungnam University, Gyeongsan, Republic of Korea in October 2014. Leaves, fruit, seeds, and cones (Table 1) were collected, placed in plastic bags, and brought to the laboratory for EB isolation.
EB were isolated according to standard procedures (De Oliveira Costa et al., 2012) with minor modifications. Plant parts were washed under running tap water. Visibly injured organs were discarded. The samples were dried and weighed on a digital balance. Tissues were sterilized by exposure to 70% v/v alcohol for 60 s, 2% sodium hypochlorite for 180 s, and 100% ethanol for 30 s. The tissues were then rinsed several times with sterile distilled water (SDW) and dried with sterile filter papers. The plant parts were then ground with sterile 3 ml/g of aqueous NaCl solution (0.9% w/v) using a sterile mortar and pestle. The tissue extracts were incubated at 28°C for 3 h to release all of the EB.
The tissue extracts were diluted to 10−1 and 10−2 with sterile NaCl solution. They were then applied with a stainless steel spreader onto Petri dishes containing 25% yeast extract, nutrient broth and agar (YNA), 25% nutrient agar, de Man, Rogosa and Sharpe agar, or Luria-Bertani agar media to obtain single bacterial colonies. The plates were incubated for up to 15 days at 28°C. All colonies were counted and reported as the number of colony-forming units per gram fresh tissue [cfu/g fresh weight (FW)]. Single colony isolation was repeated at least three times to obtain pure EB isolates. Colony form, margin, color, and elevation were recorded. The selected EB were cultured in various media broths and stored in 50% glycerol at −80°C until use.
The isolated EB were identified with 16S rRNA gene sequencing. The sequencing data, alignment, and phylogenetic trees were analyzed by Molecular Evolutionary Genetics Analysis (MEGA) 6 software version 6.0. The phylogenetic trees were also analyzed by the neighbor-joining method (Susilowati et al., 2015; Tamura et al., 2013).
Antagonistic assay of EB against citrus canker pathogen Xcc
Nine Xcc strains, including three wild-type and six streptomycin-resistant mutants (Hyun et al., 2012) were used in this study. The wild-type (XccW1 [Xcc 19-18], XccW2 [Xcc 10-5], and XccW3 [Xcc 53-4]) and streptomycin-resistant mutant (XccM4 [Xcc 27-9], XccM5 [Xcc 8-4], XccM6 [Xcc 57-2], XccM7 [Xcc 57-5], XccM8 [Xcc 27-13], and XccM9 [Xcc 27-12]) strains were kindly provided by Dr. Hyun of the Citrus Research Station, National Institute of Horticultural and Herbal Science, Rural Development Administration, Jeju, Korea. The wild-type Xcc were grown in yeast extract-nutrient broth (YNB) medium. The streptomycin-resistant Xcc strains were incubated in YNB supplemented with 50 mg/ml streptomycin. All Xcc were stored in 50% glycerol at −80°C until use.
The EB isolates were screened for antagonistic activity against the nine strains of Xcc following standard procedures (Roh et al., 2009) with minor modifications. Screening was performed by culturing 134 EB isolates in 96-well plates with 10 μl individual bacterial strain grown in YNB and the plates were incubated for 36 h at 28°C. Then the cultured bacteria were again grown on square plates (size as 127.94 × 85.50 × 16.25 mm, SPL Life Sciences Co., Ltd., Pocheon, Korea) using 25% YNA (1.5% agar) media following incubation for 36 h at 28°C. The EB were then killed by placing 1 ml chloroform on the square plate lids and inverting the plates for 15 min. The lids were then removed and the plates were left to stand for 20 min to vent any chloroform residue, then the plates were treated with UV light for 15 min to kill the bacteria completely. Simultaneously, 30 μl aliquots of Xcc cultures that had been grown overnight in YNB media at 28°C were suspended in 10-ml fresh YNB media (0.75% agar) at 55°C, mixed gently, then poured slowly and uniformly on the square plates. After the agar solidified, all the plates were sealed and incubated at 28°C for 24 h. Inhibition zone diameters were measured using an electronic digital caliper (M500-182M, Hando, Cheonan, Korea) with a 1/100 mm precision.
Further analysis for the antagonistic activity was done using TbL-22 (B. thuringiensis) and TbL-26 (B. thuringiensis) as the isolates with the highest antagonistic activity along with TbS-8 (B. altitudinis) and TbL-19 (B. altitudinis) as the isolates of another Bacillus species against all of Xcc strains. The above-described method was used the same way using all Xcc strains and using Petri dish (90 mm diameter) instead of square plates.
Antagonistic activity of the extracted EB against Xcc
After being sub-cultured on YNA plates for 24 h, single colonies of the antagonistic EB were added to 200 ml YNB in 1-liter Erlenmeyer flasks with continuous shaking at 180 rpm at 28°C for 48 h. The culture broths were centrifuged at 3,500 rpm for 15 min at 4°C. The supernatant was collected, mixed with equal volumes of ethyl acetate and incubated overnight. Ethyl acetate was selected for the metabolite extraction process for its low boiling point and moderate polarity. Simultaneously, the bacterial pellets were suspended in 20 ml methanol, placed in a sonicating bath (WUC-A03H, Daihan Scientific Co., Wonju, Korea) for 15 min to break the cells and incubated overnight. The ethyl acetate layer was then collected in a separating funnel. The methanol extract was separated from the bacterial debris by centrifugation at 3,500 rpm for 15 min at 4°C. Both extracts were dried at 50°C in a rotary evaporator (A-1000S, EYELA, Tokyo, Japan). The dried extracts were then removed from the evaporation flask, dissolved in 2 ml methanol, and air-dried in tubular glass vials. The ethyl acetate extract was measured and dissolved in 5% dimethyl sulfoxide (DMSO) in methanol to make 0.1 g/ml. Total 50-μl aliquots of this dilution were used to the well diffusion assay. The antagonistic activities of the EB against the Xcc strains were determined by measuring the inhibition zone diameters.
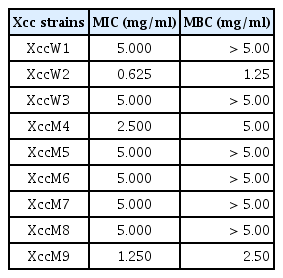
Determination of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC)
The MIC and MBC of the ethyl acetate extract of TbL-22 against Xcc were determined by the two-fold dilution method (Patra et al., 2015) with minor modifications. Five percent DMSO was used both as a control and a solvent to homogenize the ethyl acetate extract for the MIC and MBC tests. Various dilutions of the ethyl acetate extract of TbL-22 in YNB media were prepared. The lowest concentration of TbL-22 at which there was zero visible Xcc growth was taken to be the MIC and was recorded in mg/ml. The MIC and the next higher concentration were selected, spread on YNA plates and incubated at 28°C for 24 h. The lowest concentration of TbL-22 at which no pathogenic bacteria grew on the 25% YNA plates was considered the MBC and was expressed in mg/ml.
Analysis using scanning electron microscopy (SEM)
The effects of the ethyl acetate extract of TbL-22 on Xcc cell morphology were determined by SEM analysis. Both the 5% DMSO control and the samples treated with the ethyl acetate extract of TbL-22 were applied to the four Xcc strains and observed under SEM using a slightly modified version of the procedure described by Bajpai et al. (2009). The four different Xcc strains used for SEM analysis were XccW1, XccM4, XccM6, and XccM8, which were selected based on their genotypic classification. Overnight bacterial culture samples were washed gently with 0.05 M phosphate buffer solution (pH 7.4) and fixed with 2.5% v/v glutaraldehyde in SDW. The specimens were dehydrated with sequential exposures to ethanol ranging from 50 to 100%. The ethanol was then replaced with tert-butyl alcohol. The specimens were sputter-coated with platinum in an ion coater for 120 s then examined under an SEM (S-4100, Hitachi, Tokyo, Japan).
Biocontrol efficacy of the antagonistic EB
Pathogenic wild-type XccW2 were grown at 28°C in YNB media. Overnight bacterial cell cultures were harvested at 4°C, centrifuged at 3,500 rpm for 15 min, and suspended in SDW to make a concentration of 0.4 OD600nm determined by spectrophotometer (ASP-3700, ACTGene, Piscataway, NJ, USA). For the detached leaf assay (Brown and Soepena, 1994), fully expanded immature (4-week-old) leaves were excised from Hwanggeum hyang, a highly canker-susceptible cultivar generated from the cross between Citrus unshiu × C. sinensis and C. unshius. The leaves were infiltrated with a 0.1 ml bacterial suspension using a 1-ml needleless syringe. The bacterial suspensions were prepared by mixing 0.4 OD600nm XccW2 either with 0.4 OD600nm EB suspension or with SDW. The infiltrated leaves were placed on three layers of sterile tissue paper moistened with SDW in a humid box and incubated in a growth chamber at 28°C. The infiltrated leaves were photographed, and any canker lesions that developed were measured 7 days post-infiltration (dpi) (Martínez-Medina et al., 2013).
Pathogenicity test of the isolated EB
To determine whether TbL-22 and TbL-26 could cause disease in citrus plants, bacterial suspensions at 0.4 OD600nm concentration were infiltrated into Hwanggeum hyang leaves as described by Brown and Soepena (1994). The pathogenic bacterium XccW2 and SDW were used as a positive and negative control, respectively. Citrus canker disease symptoms were assessed for up to 7 dpi.
Statistical analysis
All data were expressed as the mean ± standard deviation by measuring four independent replicates per experiment. Statistical analysis of the results was conducted by one-way ANOVA followed by the Duncan’s multiple range test at P < 0.05 using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Isolation and identification of EB
A total of 134 morphologically distinct EB were isolated from M. glyptostrobodies, G. biloba, T. brevifolia, P. densiflora, S. babylonica, and S. chaenomeloides. They were obtained from the leaves (73 isolates, 54.48%), fruit (49 isolates, 36.57%), seeds (8 isolates, 5.97%), and cones (4 isolates, 2.98%) (Table 1). The tissue-specific cell counts (CFU) per gram FW were in the range of 1.8 × 102–6.0 × 104 cfu/g in the fruits, 1.8 × 103–1.5 × 105 cfu/g in the leaves, 7.2 × 103 cfu/g in the seeds, and 1.2 × 103 cfu/g in the cones (Table 1).
Antagonistic activity of EB against Xcc
In the primary screening, all EB isolates were tested for antagonistic activity against XccW1. Eighteen of the EB efficacious against XccW1 were identified by 16S rRNA gene sequencing analysis (Table 2). Among them, TbL-22 and TbL-26 exerted the highest antagonistic activity against all Xcc, so they were selected for further study (Table 3). Phylogenetic trees were constructed using the 16S rRNA sequences of TbL-22 and TbL-26 and those of their closest relatives. The phylogenetic tree analysis revealed that TbL-22 and TbL-26 had 99% and 100% identity with the 16S rRNA gene sequences of B. thuringiensis Bt 407 and B. thuringiensis IAM12077, respectively (Fig. 1A and B).
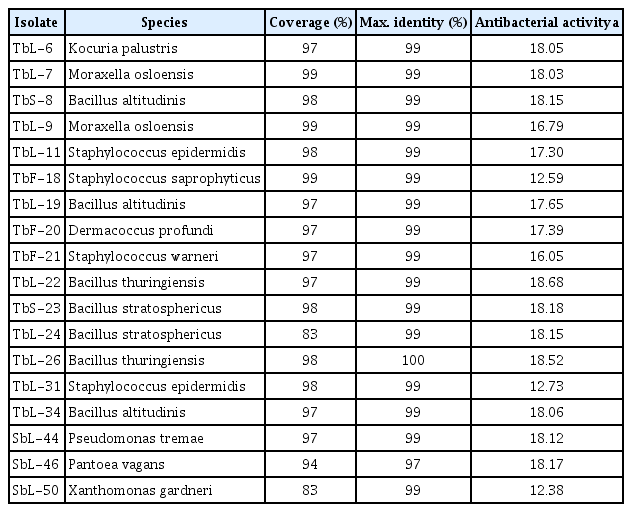
Screening and identification of antibacterial activity of endophytic bacteria isolated from gymnosperms and angiosperms against XccW1

Neighbor-joining phylogenetic tree based on 16S rRNA gene sequence analyses showing the relationships among the selected strains TbL-22 (A), TbL-26 (B), and the standard strains. Evolutionary distances were computed using the maximum composite likelihood method. The numbers at the nodes are bootstrap values % (based on 1,000 samples). GenBank accession numbers are shown in parentheses.
In Xcc cultures, TbL-22 and TbL-26 exhibited the highest antagonistic activity with inhibition zones of 20.64-15.33 and 17.34-11.65 mm, respectively (Table 3, Fig. 2). TbL-22 had greater activity than TbL-26 against all Xcc. Only the ethyl acetate extract of TbL-22 was antagonistic against all Xcc strains (Table 4, Fig. 3). The extract of TbL-22 exerted the highest antagonistic activity of all, and produced inhibition zones of 15.31 ± 2.08 mm and 19.37 ± 3.17 mm against XccW3 and XccM6, respectively. The extract of TbL-26 produced inhibition zones of 13.81 ± 1.05 mm and 18.08 ± 4.69 mm against XccW3 and XccM6, respectively, but had no antagonistic activity against XccW2 (Table 4, Fig. 3).
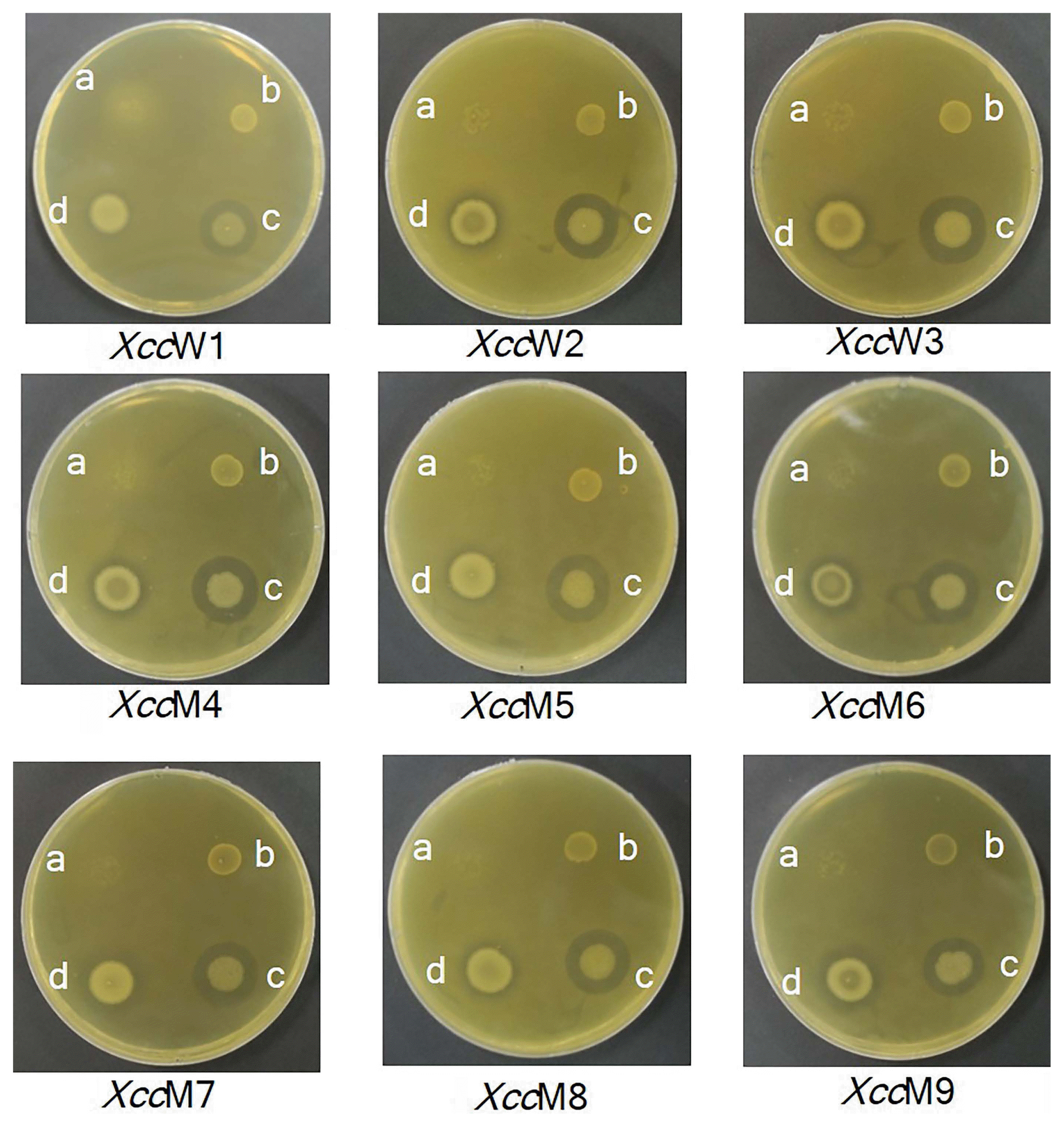
Antagonistic activity of isolated endophytic bacteria against Xanthomonas citri subsp. citri (XccW1–XccW3 [wild-type strains] and XccM4–XccM9 [streptomycin-resistant mutant type strains]). a, TbS-8 (Bacillus altitudinis); b, TbL-19 (B. altitudinis); c, TbL-22 (B. thuringiensis); d, TbL-26 (B. thuringiensis).
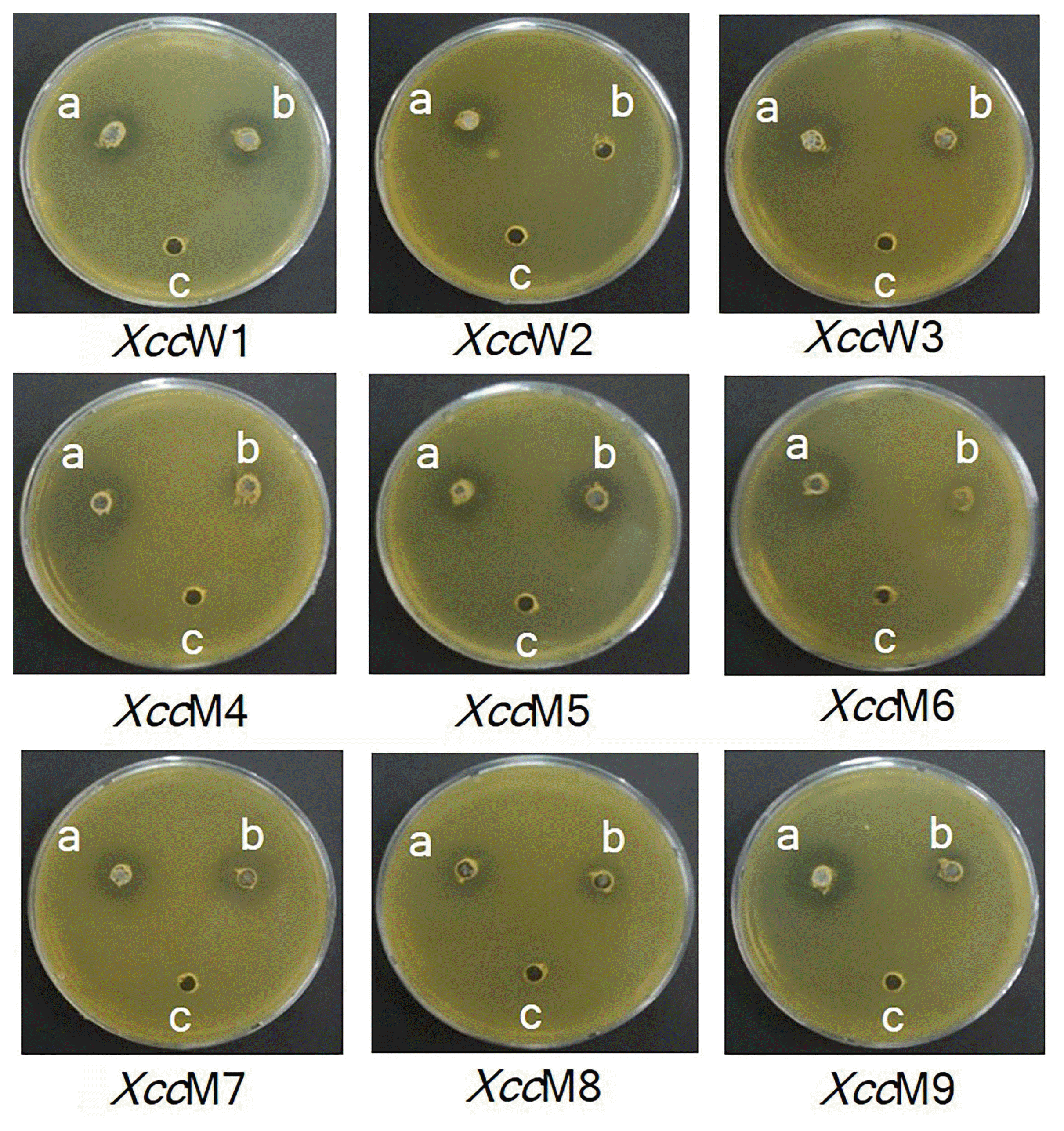
Antagonistic activity of ethyl acetate extracts of endophytic bacteria against Xanthomonas citri subsp. citri (XccW1–XccW3 [wild-type strains] and XccM4–XccM9 [streptomycin-resistant mutant type strains]) determined by well diffusion assay. a, TbL-22 (Bacillus thuringiensis); b, TbL-26 (B. thuringiensis); c, 5% dimethyl sulfoxide control.
Determination of MIC and MBC
Since the extract of TbL-22 had the highest antagonistic activity against all Xcc, it was selected for the determination of MIC and MBC. The values of MIC and MBC for the ethyl acetate extract of TbL-22 ranged from 0.625 to 5.00 mg/ml and from 1.25 to >5.00 mg/ml, respectively (Table 5). The lowest MIC and MBC values were found for XccW2 (0.625 mg/ml and 1.25 mg/ml, respectively) (Table 5).
SEM analysis with the extract of TbL-22
The effects of the ethyl acetate extract of TbL-22 on cell morphology were observed for XccW1, XccM4, XccM6, and XccM8 (Fig. 4). XccW1 was selected to represent the wild-type, and XccM4, XccM6, and XccM8 were selected as representatives of each mutant on the basis of their genotypic classification. All Xcc treated with the ethyl acetate extract presented with ruptured cell surfaces. They were elongated, shrunken, lysed, and swollen. In contrast, the control Xcc cell surfaces were regular, uniform, and smooth (Fig. 4).
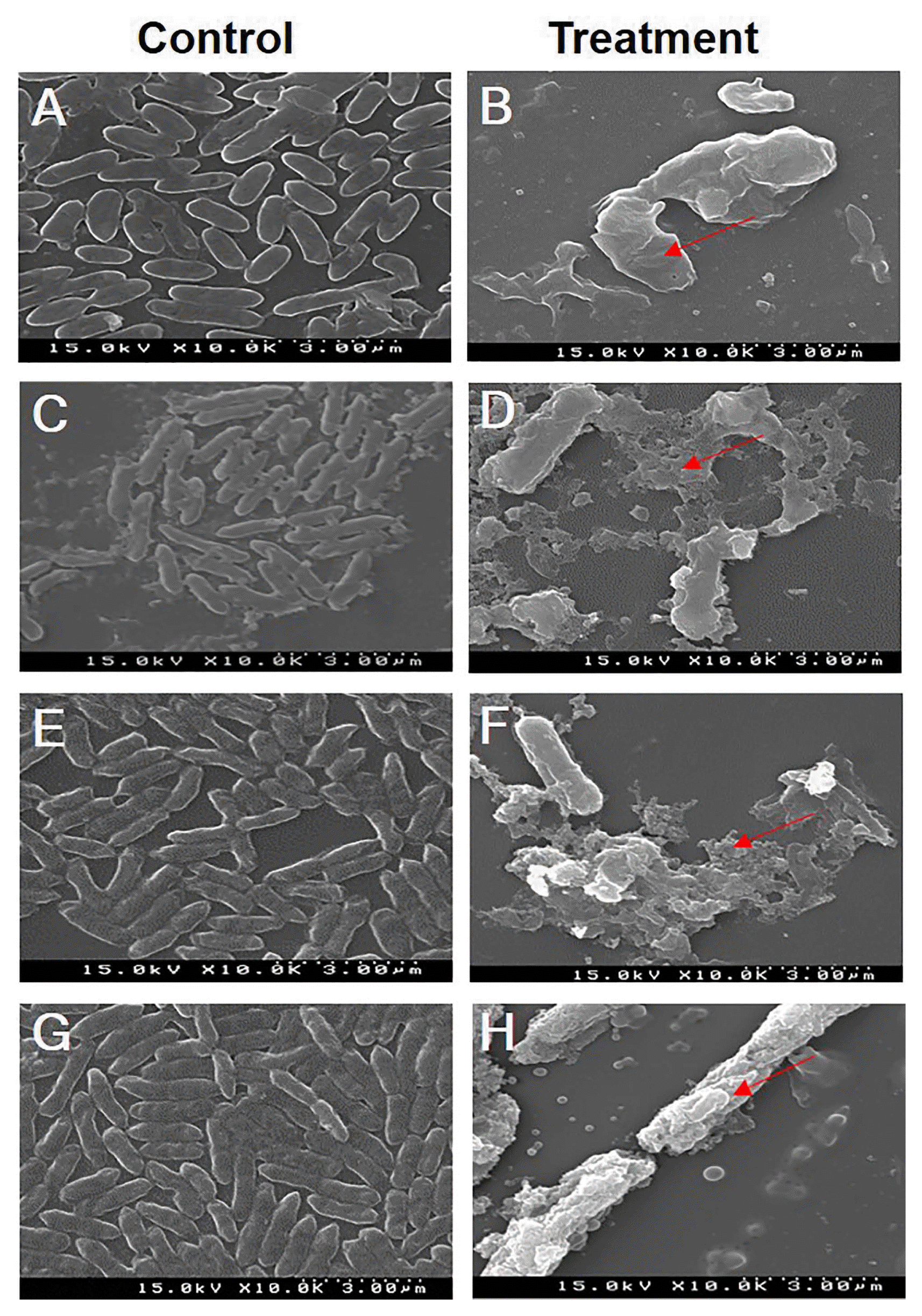
Photograph of scanning electron microscopy images of Xanthomonas citri subsp. citri (Xcc) strains treated with 5% dimethyl sulfoxide as control (A, C, E, G) and those treated with the ethyl acetate extract of TbL-22 as treatment (B, D, F, H). (A, B) XccW1. (C, D) XccM4. (E, F) XccM6. (G, H) XccM8. The collapsed and lysed cells are indicated by red arrows.
In vivo biocontrol efficacy of the antagonistic endophytes
TbL-22 and TbL-26 were tested for in vivo biocontrol efficacy against Xcc. In the detached leaf assay, all citrus leaves infiltrated with XccW2, TbL-22, and TbL-26 showed disease symptoms. Nevertheless, the infected areas of the leaves infiltrated with TbL-22 and TbL-26 were smaller than those of the leaves treated with XccW2 alone by 64.05% and 47.76%, respectively (Fig. 5A and B). After 7 dpi, XccW2 infiltration alone caused the widest necrotic lesion (10.68 ± 1.34 mm), followed by XccW2 with TbL-26 (5.58 ± 0.51 mm) and XccW2 with TbL-22 (3.84 ± 0.27 mm) (Fig. 5C).

Disease symptom development at 7 days post-infiltration (dpi) in leaves of Citrus spp. inoculated with Xanthomonas citri subsp. citri (XccW2) and the endophytic bacteria Bacillus thuringiensis (TbL-22 and TbL-26). In all cases, the leaves were infiltrated with 0.1-ml aliquots of OD600nm 0.4 XccW2 mixed with sterile distilled water (W2) or OD600nm 0.4 XccW2 mixed with OD600nm 0.4 TbL-22 and TbL-26 (22 and 26). (A) Adaxial leaf surface. C, control (sterile distilled water); W2, XccW2; 22, XccW2 with TbL-22; 26, XccW2 with TbL-26. (B) Abaxial leaf surface. (C) Disease lesion diameter measured 7 dpi. The means ± standard deviation from four independent replicates are presented for each treatment. Means with different letters are significantly different at P < 0.05 according to Duncan’s test.
Pathogenicity test for the TbL-22 and TbL-26
The lesions that developed on the leaves infiltrated with XccW2 were usually visible on both surfaces and appeared in the form of water-soaked margins surrounded by yellow rings or haloes (Fig. 6A). Leaves infiltrated with TbL-22 and TbL-26 alone, however, did not display any visible toxic effects or canker symptoms.
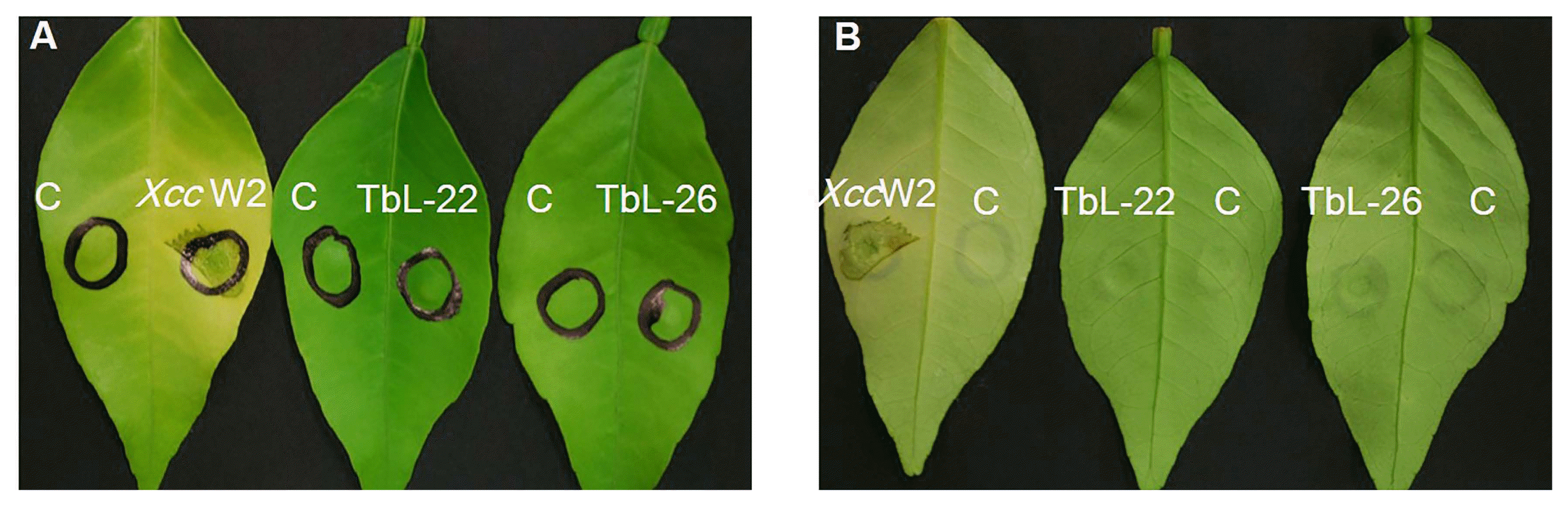
Pathogenicity test determined by disease symptom development in the leaves of Citrus spp. at 7 days post-infiltration (dpi). Detached fresh leaves were infiltrated with 0.1-ml aliquots of OD600nm 0.4 Xanthomonas citri subsp. citri (XccW2) and OD600nm 0.4 endophytic bacteria (TbL-22 and TbL-26). (A) Adaxial leaf surface. C, control (sterile distilled water); XccW2, TbL-22, TbL-26. (B) Abaxial leaf surface.
Discussion
Citrus trees are cultivated throughout the tropical and subtropical regions of the world (Webber, 1967). Citrus canker, which is caused by the bacterial pathogen Xcc, is particularly destructive to citrus. Copper-based bactericides and streptomycin antibiotics have been used in the attempt to control this disease (Behlau et al., 2011; Graham et al., 2008). Nevertheless, they are relatively ineffective on certain Xcc strains (Voloudakis et al., 2005) which have developed resistance to these disease control products (Hyun et al., 2012). Therefore, our aim was to find efficacious alternative citrus canker control measures. EB may serve as powerful canker biocontrol agents.
A total of 134 EB were isolated from the leaves, fruit, seeds, and cones of six different plant species (Table 1). The EB population densities (in cfu/g FW) varied depending on the plant species and tissue (Table 1). Similar findings were published elsewhere (Hallmann et al., 2002; Quadt-Hallman et al., 1997; Sturz and Nowak, 2000). In the present study, the highest EB densities were found in the leaves of all species tested except for G. biloba. The EB density in its fruit was 33 times higher than that in its leaves (Table 1).
Based on the relative diameters of the inhibition zones they formed on Xcc plates, 18 EB isolates exhibited varying degrees of positive antibacterial activity (Table 2). In addition, solvent extracts of these isolates were evaluated for their antagonistic activity against XccW1 (data not shown). Based on the screening data using the EB and the extracts, the endophytes TbL-22 and TbL-26 were selected for their antibacterial activities against all Xcc strains (Table 3, Fig. 2). Both the endophytes and their ethyl acetate extracts created inhibition zones on Xcc culture media. Therefore, it may be the metabolites of TbL-22 and TbL-26 that are antagonistic to Xcc. Nevertheless, the inhibition zones produced by these two EB differed in size. This discrepancy may be explained by differences in the amount, stability, and diffusion rate of the metabolites secreted by TbL-22 and TbL-26 (Kim et al., 1999). Previous studies reported on the antimicrobial activity of Bacillus spp. like B. subtilis, B. pumilus, B. amyloliquefaciens, B. pasteurii, B. cereus, B. mycoides, and B. sphaericus. These endophytes were isolated from various plants and suppressed the growth of microbial plant pathogens including X. oryzae pv. oryzae (Chithrashree et al., 2011; Lin et al., 2001), P. syringae (Bais et al., 2004; Wei et al., 1996), Phytophthora medicaginis (Silo-Suh et al., 1994), Pythium torulosum (Shang et al., 1999), and Botrytis cinerea (Touré et al., 2004). Endophytic B. thuringiensis was used as a biocontrol agent against the phytopathogens Ciboria shiraiana, Urocystis agropyri, and Sclerotinia sclerotiorum, and against insects such as silkworms (Bombyx mori) (De Goes et al., 2012; Roh et al., 2007; Schnepf et al., 1998; Sultana and Kim, 2016; Tao et al., 2014; Zhou et al., 2008). B. thuringiensis is a Gram-positive, soil-borne bacterium. It has been widely used as a biocontrol agent and bioinsecticide (Chattopadhyay et al., 2004; Jouzani et al., 2017).
The ethyl acetate extract of B. thuringiensis TbL-22 exhibited the highest antagonistic activity against all Xcc (Table 4, Fig. 3). Therefore, it is a promising candidate as a biocontrol agent against the streptomycin-resistant strains XccM4-9 (Hyun et al., 2012). The ethyl acetate extract may have contained potent secondary metabolites which suppressed the growth of Xcc. Many lipopeptides and fengycin-like substances isolated and purified from B. thuringiensis CMB26 were found to be fungicidal, bactericidal, and insecticidal (Kim et al., 2004). B. thuringiensis suppresses plant diseases by producing antimicrobial compounds (Cherif et al., 2008; Dong et al., 2002; Raddadi et al., 2009; Reyes-Ramírez et al., 2004). The TbL-26 extract exerted no antibiotic activity against XccW2 but did suppress both XccW1 and XccW3. This observation suggests that the three pathogen strains may differ in terms of genotype and metabolite susceptibility (Fig. 3). When the Xcc strains were subjected to ethyl acetate extracts of TbL-22, SEM analysis indicated that the cells became irregular, rough, and deformed. They eventually collapsed and lysed (Fig. 4). Similar morphological deformities have been observed in X. campestris pv. glycine and Pythium myriotylum treated with the metabolites of endophytic Actinomycetes spp. and Fusarium spp. (Keerthi et al., 2016; Mingma et al., 2014).
EB isolates are known for their beneficial relationships with host plants and their antagonistic activity against plant pathogens (Rosenblueth and Martínez-Romero, 2006). The biocontrol approach is an eco-friendly alternative to chemical pest control products for the suppression of plant diseases. Both in vivo and in vitro tests with EB and/or their extracts can be used to screen for efficacy. Detached citrus leaves infiltrated with XccW2, TbL-22, and TbL-26 showed significantly reduced disease symptoms in comparison to XccW2 alone infected leaves (Fig. 5), but the two EB did not induce any disease symptoms themselves (Fig. 6). Reductions in citrus canker severity and incidence were also reported for citrus species treated with the Bacillus strains WG6-14 and TKS1-1 and with B. subtilis (S-12) (Das et al., 2014; Huang et al., 2012). B. subtilis, B. amyloliquefaciens, B. pumilus, B. mycoides, B. pasteurii, and B. cereus induce plant disease resistance and are commercially available as biocontrol agents (Kloepper et al., 2004; Nam et al., 2016).
In summary, the endophytic Bacillus thuringiensis strains TbL-22 and TbL-26 were determined to be prospective antagonists against both wild-type and streptomycin-resistant Xcc. Both EB were highly active against Xcc, but TbL-22 was the more potent antibacterial and may serve either as a biocontrol agent or a source of biocontrol compounds. The purification and identification of the antimicrobial metabolites of TbL-22 will be necessary in order to formulate them for agricultural applications. Further in vitro and in vivo research with TbL-22 and/or its extracts may help develop sustainable control measures which could replace the synthetic agrochemicals currently being used to manage Xcc.
Acknowledgments
This work was supported by a grant from Yeungnam University, Gyeongsan, Korea.